Abstract
Background
Aphasia is a disabling chronic stroke symptom but the prognosis for patients presenting with aphasia in the hyperacute window has not been well characterized. The purpose of this study is to assess the prognosis for recovery of language function in subjects presenting with aphasia due to ischemic stroke within 12 hours of symptom onset.
Methods
Subjects presenting with aphasia were identified from a prospective cohort study of 669 subjects presenting emergently with acute stroke. Subjects were characterized by demographics, serial clinical exams, unenhanced CT and CT angiography. Aphasia severity was assessed by NIHSS exams performed at baseline, discharge and 6 months. Demographic, clinical and imaging factors were assessed for prognostic impact.
Results
Aphasia was present in 30% of subjects (n=204). Of the 166 aphasic patients alive at discharge (median 5 days), aphasia improved in 57% and resolved in 38%. In the 102 aphasic subjects evaluated at 6 months, aphasia improved in 86% and completely resolved in 74% of subjects. Among aphasic subjects with “mild” stroke (initial NIHSS<5), aphasia resolved in 90% of subjects by 6 months. Factors significantly associated with better outcome included clinically and radiographically smaller strokes and lower pre-stroke disability.
Conclusions
The prognosis for full recovery of aphasia present in the hyperacute window is good. Radiographic and clinical markers indicating lesser extent of ischemia correlated to greater recovery. Given the excellent prognosis for language recovery in mild stroke, the net benefit of thrombolysis in such cases is uncertain.
Key Words for Indexing: aphasia, prognosis, stroke, recovery
Introduction
The assessment of a patient with presumed ischemic stroke in the emergency setting inevitably leads to decisions weighing risks and benefits of potential therapies. The potential long term disability faced by a patient due to their stroke symptoms becomes clearer as time goes on, but in the hyperacute treatment window must be estimated from basic demographic data, history of significant medical comorbidities and the extent of their presenting neurological deficits. Aphasia causes substantial functional disability and figures prominently into treatment decisions. Our knowledge about the prognosis for aphasia is based mostly on natural history studies from the 1970s and 80s showing approximately 40% of patients completely recovering within 1 year.(1) These studies are limited in that they studied patients in the subacute to chronic stage. To our knowledge, no study has assessed the long term prognosis of aphasia identified in the hyperacute window, and thus a study is needed to adequately inform treatment decisions within the relevant window. The primary goal of this study is to assess the prognosis for aphasia identified in the hyperacute window and to identify characteristics that are associated with favorable outcomes. The secondary goals of this study are to assess the prognosis for aphasia in the hyperacute window for patients with mild strokes (NIHSS<5) and isolated aphasia.
Materials and Methods
Patient Selection and Clinical Data
We analyzed data from 741 consecutive patients enrolled between March 2003 and January 2006 in a prospective cohort study at two university-based hospitals, part of the Screening Technology and Outcomes Project in Stroke (STOPStroke). All patients presenting with symptoms consistent with acute cerebral ischemia within 24 hours of onset were considered eligible for enrollment in STOPStroke, although subjects presenting beyond 12 hours from onset were excluded for this analysis. Admission nonenhanced CT scans were obtained, followed by CT angiograms. Institutional protocol calls for all patients presenting with signs of acute cerebral ischemia to undergo nonenhanced head CT and CT angiographic imaging unless specific contraindications are present. Patients were excluded from enrollment for contraindication to iodinated contrast agent administration (history of contrast agent allergy, pregnancy, congestive heart failure, renal insufficiency) or if the nonenhanced CT scan showed evidence of intracranial hemorrhage. NIH Stroke Scale (NIHSS) scores were obtained by direct examination of subjects at the prespecified time points of their initial emergent evaluation at baseline, discharge and at a 6 month follow up. All study staff performing examinations to obtain NIHSS scores were certified through the American Stroke Association's NIH Stroke Scale training program. Item 9 of the NIHSS identifies language impairment as absent, mild-to-moderate, severe or mute.(2) In this study, aphasia was defined as a score >0 on item 9 of the NIHSS, improved aphasia as any decrease in that score, and resolved aphasia as a decreased to a score of 0. The study received institutional review board approval at both participating institutions and was Health Insurance Portability and Accountability Act compliant. At enrollment, all subjects gave informed consent for participation.
Neuroimaging Protocol
Nonenhanced CT and CT angiographic acquisitions were performed according to standard departmental protocols with 8- or 16-section multidetector CT scanners (LightSpeed; GE Healthcare, Milwaukee, Wis) (7,8). Nonenhanced CT was performed in the transverse plane with the patient's head in a stabilizing head holder to minimize motion during scanning. Representative sample parameters, with minimal variations between scanners and sites shown as ranges, were as follows: 120–140 kVp, 170 mA, 2-second scan time, and 5-mm section thickness. Imaging with these parameters was immediately followed by biphasic helical scanning, performed at the same head tilt as was nonenhanced CT. CT angiography was performed after a 25-second delay (40 seconds for patients in atrial fibrillation) and administration of 100–140 mL of a nonionic contrast agent (Isovue; Bracco Diagnostics, Princeton, NJ) at an injection rate of 3 mL/sec by using a power injector (Medrad Power Injector; Medrad, Indianola, Pa) via an 18-gauge intravenous catheter. Parameters were 140 kVp, 220–250 mA, 0.8–1.0-second rotation time, 2.5-mm section thickness, 1.25-mm reconstruction interval, 3.75 mm per rotation table speed, and 0.75:1 pitch. Images were obtained from the C6 vertebral body level through the circle of Willis. Immediately afterward, a second set of images was obtained from the aortic arch to the skull base.
Image Review
Image review was independently performed on a picture archiving and communication system workstation (Impax; Agfa Technical Imaging Systems, Richfield Park, NJ) by a board-certified neuroradiologist and a clinical neurologist experienced in stroke imaging (M.H.L. and W.J.K., 15 and 25 years of neuroimaging review experience, respectively). Reviewers were blinded to follow-up clinical and imaging findings but had information in regard to the patients' age, sex, and presenting clinical symptoms. Neither of the reviewers had participated in the selection of the patients.
Variable window width and center level settings were used for optimal ischemic hypoattenuation detection with nonenhanced CT and CT angiographic source images. In all cases, nonenhanced CT images obtained for acute stroke were reviewed first, followed by CT angiographic source images, and finally, follow-up images for confirmation of true-positive or true-negative infarct regions. Disagreements in readings were resolved in consensus.
Reviewers rated the ischemic lesion on the nonenhanced CT scans, CT angiographic source images, and follow-up images according to ASPECTS.(3) These regions included the insula (I), caudate nucleus (C), lentiform nucleus (L), internal capsule (IC), superior parietal lobe (M6), precentral and superior frontal lobe (M5), anterior superior frontal lobe (M4), inferior parietal and posterior temporal lobe (M3), temporal lobe (M2), and anterior inferior frontal lobe (M1). In addition, other regions were defined for the remainder of the brain including the anterior portion of the anterior cerebral artery territory (ACA), middle portion of the ACA, posterior portion of the ACA, corpus callosum genu, corpus callosum body, corpus collosum splenium, occipital lobe, hippocampus, thalamus, medulla, pons, midbrain, and superior cerebellar artery territory, anterior inferior cerebellar artery territory, and the posterior inferior cerebellar artery territory in the cerebellum. Therefore, for every image, each region was reviewed for the presence or absence of ischemic lesions according to a five-point level of certainty score (score 5, definitely present; 4, probably present; 3, equivocal; 2, probably absent; 1, definitely absent).
Analysis
Patients lacking requisite data for analysis were excluded as well as patients ultimately judged to have had transient ischemic attacks or symptoms not due to stroke. The evaluation of prognosis at discharge necessarily excluded subjects who died prior to discharge, and the analysis at 6 month follow up excluded subjects who died or were lost to follow up. A univariate analysis was performed to identify demographic, clinical and radiographic factors that may be useful in predicting aphasia prognosis during the period of initial evaluation. Significance for differences between continuous variables (age, body mass index, pack-years of smoking history) were evaluated using t-tests, ordinal variables (pre-stroke modified Rankin scale (mRS), initial unenhanced CT (UECT) and CT-angiogram source image (CTA-SI) ASPECTS scores, number of hypodense regions on UECT and CTA-SI outside the middle cerebral artery (MCA) distribution covered by ASPECTS regions) were evaluated using the Wilcoxon Rank Sum test, and dichotomized variables (sex, pre-stroke mRS>0 and mRS>1, initial NIHSS<5, presentation with isolated aphasia, initial presentation included right arm weakness, history of smoking, consumption of alcohol more than occasionally, sedentary activity level, working full time, completed education beyond high school, taking antiplatelet agent, taking warfarin, history of prior stroke, history of cardiovascular disease, and received either intravenous tPA or intra-arterial therapy) were evaluated using Fisher's Exact test. Finally, the variables were entered into a logistic regression model and a backward, stepwise process was employed to identify the set of variables that significantly predicted improvement at the time of discharge and follow up.
Results
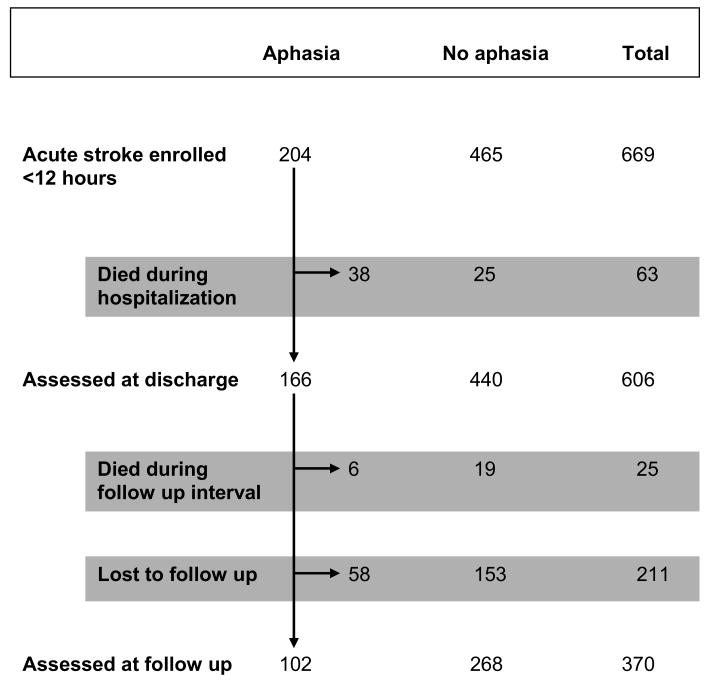
The study enrolled 741 subjects and sufficient data were available to analyze 669. Figure 1 summarizes the flow of patients through the study intervals. Of the 669 subjects, 204 initially presented with aphasia. Administration of the baseline NIHSS to the aphasia cohort occurred at a median of 2.3 hours after symptom onset (interquartile range 0.7-5.3 hours). Intravenous thrombolysis or intra-arterial therapy was given to 116 subjects, including 60 who presented with aphasia. Subjects with aphasia were more likely to receive those acute treatments compared to non-aphasics (29% versus 12%, p<0.001). There were 317 patients who presented with NIHSS scores less than 5. Within the aphasic cohort were 41 patients whose NIHSS<5, 17 of whom had isolated aphasia. Twenty (49%) of the aphasia patients with NIHSS<5 presented within 4.5 hours of symptom onset. At discharge 606 subjects were alive including 166 from the aphasia cohort, and 370 participated in follow up at 6 month including 102 initially aphasic subjects. There were 88 documented deaths overall in the study, 63 prior to hospital discharge. From the aphasic NIHSS<5 group, 2 patients received intravenous thrombolysis or intra-arterial therapy, 2 died and 14 were lost to follow up. Among the original 17 subjects with isolated aphasia, none received intravenous thrombolysis or intra-arterial therapy, none died and 1 was lost to follow up. Survival was the same for the initially aphasic cohort as the overall study group, the aphasia cohort representing a consistent proportion at each time point (32% at baseline, 30% at discharge and 31% at follow up, p=0.72).
Figure 1.
Study Flow Diagram
The baseline characteristics of the subjects assessed at discharge and at 6 month follow up are shown in Table 1. The proportion of subjects who received acute IV or IA therapy in the discharge and follow up assessment groups was the same as in the initially aphasic cohort of 204 (27% and 29% versus 29%, p=1). Only 3 subjects were classified as having lacunar stroke by CCS criteria.(4,5) There were no significant differences found between the group assessed at discharge and the portion of that group that was assessed at 6 months.
Table 1. Baseline Characteristics of Subjects.
| Subject Characteristics | Cohort Followed Until Discharge (n=166) | Cohort Followed Until 6 Months (n=102) |
|---|---|---|
| Time between onset and initial assessment (median [IQR] in hours) | 2.3 [0.7-5.3] | |
| Time between baseline and outcome assessment (median in days) | 5 | 192 |
| Clinical Variables | ||
| Age (mean in years) | 68.2 | 67.7 |
| Male (n,%) | 83 (50.0%) | 55 (53.9%) |
| BMI (mean in kg/m2) | 26.9 | 26.8 |
| Prestroke mRS (median (mean)) | 0 (0.55) | 0 (0.48) |
| Prestroke mRS>0 (n,%) | 46 (27.7%) | 25 (24.5%) |
| Prestroke mRS>1 (n,%) | 26 (15.7%) | 13 (12.7%) |
| Initial NIHSS<5 (n,%) | 41 (24.7%) | 29 (28.4%) |
| Isolated aphasia (n,%) | 17 (10.2%) | 14 (13.7%) |
| Mild to moderate aphasia (n,%) | 71 (42.8%) | 51 (50.0%) |
| Severe aphasia to mute (n,%) | 95 (57.2%) | 51 (50.0%) |
| R arm weakness (n,%) | 87 (52.4%) | 51 (50.0%) |
| Received IV tPA or intra-arterial therapy (n,%) | 47 (28.3%) | 30 (29.4%) |
| Imaging Variables | ||
| UECT ASPECTS score (median) | 9 (7.98) | 9 (8.09) |
| CTA-SI ASPECTS score (median) | 8 (7.57) | 8 (7.75) |
| Other UECT hypodense regions (median) | 0 (0.34) | 0 (0.37) |
| Other CTA-SI hypodense regions (median) | 0 (0.52) | 0 (0.48) |
| Medical and Social History Variables | (n=151) | (n=90) |
| History of smoking (n,%) | 73 (48.3%) | 40 (44.4%) |
| Consumes alcohol more than occasionally (n,%) | 31 (20.5%) | 16 (17.8%) |
| Sedentary activity level (n,%) | 54 (35.8%) | 31 (34.4%) |
| Working full time (n,%) | 42 (27.8%) | 24 (26.7%) |
| Completed education beyond high school (n,%) | 58 (38.4%) | 38 (42.2%) |
| Taking antiplatelet agent (n,%) | 62 (41.1%) | 37 (41.1%) |
| Taking warfarin (n,%) | 16 (10.6%) | 13 (14.4%) |
| History of prior stroke (n,%) | 10 (6.6%) | 5 (5.6%) |
| History of cardiovascular disease (n,%) | 12 (7.9%) | 8 (8.9%) |
There were no significant differences between the baseline characteristics of the cohort followed until discharge and the cohort followed until 6 months.
Table 2 summarizes the outcome of aphasia symptoms according to whether the subject showed improvement or complete resolution of aphasia compared to the baseline assessment. Among all surviving subject who initially presented with aphasia, 57% had improvement by the time of discharge and 38% showed no residual signs of language impairment. The median time between symptom onset and discharge was 5 days. By 6 months (median 192 days), 86% had improvement in language function and 74% had no residual aphasia. Of the 24 demographic, clinical and radiographic variables assessed, univariate analysis identified several factors were significant predictors of favorable outcome. Table 3 summarizes the result of the logistic regression analysis. Advanced age, the presence of hypodense regions on UECT both within and outside of the ASPECTS region, a history of prior stroke and sedentary activity level significantly predicted diminished likelihood of improvement by the time of discharge. At 6 month follow up, only the pre-stroke mRS significantly impacted likelihood of improvement. Among patients with NIHSS<5, 90% achieved full recovery by 6 months, or 89% excluding the 2 patients who had received thrombolysis.
Table 2. Subject Outcomes According to Baseline Predictors.
| Cohort Followed Until Discharge | Cohort Followed Until 6 Months | |||||
|---|---|---|---|---|---|---|
| Subject Characteristics | All (n=166) | Improved (n=94) | Resolved (n=63) | All (n=102) | Improved (n=88) | Resolved (n=75) |
| All patients | 56.6% | 38.0% | 86.3% | 73.5% | ||
| Time between onset and initial assessment (median [IQR] in hours) | 2.3 [0.7-5.3] | |||||
| Time between baseline and outcome assessment (median in days) | 5 | 192 | ||||
| Clinical Variables | ||||||
| Age (mean in years) | 68.2 | 66.7 | 65.1* | 67.7 | 67.5 | 66.8 |
| Male (n,%) | 83 | 50 (60.2%) | 33 (39.8%) | 55 | 48 (87.3%) | 41(74.5%) |
| BMI (mean in kg/m2) | 26.9 | 26.7 | 27.1 | 26.8 | 26.8 | 26.8 |
| Prestroke mRS (median (mean)) | 0 (0.55) | 0 (0.39)† | 0 (0.32)* | 0 (0.48) | 0 (0.40) | 0 (0.33)* |
| Prestroke mRS>0 (n,%) | 46 | 20 (43.5%)* | 11 (23.9%)* | 25 | 20 (80.0%) | 15 (60.0%) |
| Prestroke mRS>1 (n,%) | 26 | 9 (34.6%)* | 4 (15.4%)* | 13 | 9 (69.2%)† | 6 (46.2%)* |
| Initial NIHSS<5 (n,%) | 41 | 26 (63.4%) | 24 (58.5%)* | 29 | 26 (89.7%) | 26 (89.7%)* |
| Isolated aphasia (n,%) | 17 | 12 (70.6%) | 10 (58.8%)† | 14 | 12 (85.7%) | 12 (85.7%) |
| Mild to moderate aphasia (n,%) | 71 | 35 (49.3%) | 35 (49.3%)* | 51 | 40 (78.4%)* | 40 (78.4%) |
| Severe aphasia to mute (n,%) | 95 | 59 (62.1%) | 28 (29.5%)* | 51 | 48 (94.1%)* | 40 (68.6%) |
| R arm weakness (n,%) | 87 | 43 (49.4%)† | 25 (28.7%)* | 51 | 41 (80.4%) | 31 (60.8%)* |
| Received IV tPA or intra-arterial therapy (n,%) | 47 | 28 (59.6%) | 15 (31.9%) | 30 | 26 (86.7%) | 24 (80.0%) |
| Imaging Variables | ||||||
| UECT ASPECTS score (median) | 9 (7.98) | 9 (8.21)* | 9 (8.68)* | 9 (8.09) | 9 (8.18)† | 9 (8.52)* |
| CTA-SI ASPECTS score (median) | 8 (7.57) | 8 (7.90)* | 9 (8.40)* | 8 (7.75) | 8 (7.86)† | 9 (8.19)* |
| Other UECT hypodense regions (median) | 0 (0.34) | 0 (0.20) | 0 (0.19) | 0 (0.37) | 0 (0.39) | 0 (0.39) |
| Other CTA-SI hypodense regions (median) | 0 (0.52) | 0 (0.43) | 0 (0.46) | 0 (0.48) | 0 (0.53) | 0 (0.57) |
| Medical and Social History Variables | (n=151) | (n=85) | (n=57) | (n=90) | (n=77) | (n=66) |
| History of smoking (n,%) | 73 | 45 (61.6%)* | 30 (41.1%)† | 40 | 40 (100.0%)* | 37 (92.5%)* |
| Consumes alcohol more than occasionally (n,%) | 31 | 22 (71.0%)* | 13 (41.9%) | 16 | 13 (81.3%) | 12 (75.0%) |
| Sedentary activity level (n,%) | 54 | 25 (46.3%) | 20 (37.0%) | 31 | 25 (80.6%) | 23 (74.2%) |
| Working full time (n,%) | 42 | 26 (61.9%) | 17 (40.5%) | 24 | 21 (87.5%) | 17 (70.8%) |
| Completed education beyond high school (n,%) | 58 | 37 (63.8%)* | 28 (48.3%)* | 38 | 33 (86.8%) | 28 (73.7%) |
| Taking antiplatelet agent (n,%) | 62 | 34 (54.8%) | 21 (33.9%) | 37 | 28 (75.7%)* | 26 (70.3%) |
| Taking warfarin (n,%) | 16 | 10 (62.5%) | 7 (43.8%) | 13 | 11 (84.6%) | 9 (69.2%) |
| History of prior stroke (n,%) | 10 | 2 (20.0%)* | 2 (20.0%) | 5 | 3 (60.0%) | 3 (60.0%) |
| History of cardiovascular disease (n,%) | 12 | 6 (50.0%) | 3 (25.0%) | 8 | 7 (87.5%) | 6 (75.0%) |
The mean is reported for continuous data, the median and mean for ordinal data. For binary characteristics the total number of patients with that characteristic is reported along with the percentage of all subjects with that characteristic whose aphasia improved or resolved.
p ≤ 0.05,
p ≤ 0.10
Table 3. Significant Predictors of Aphasia Improvement by Logistic Regression.
| Predictor | Odds Ratio for Improvement by Discharge (95% Confidence Interval) | Odds Ratio for Improvement by Follow Up (95% Confidence Interval) |
|---|---|---|
| Age | 0.96 (0.93-0.99) | |
| ASPECTS score on UECT | 1.25 (1.02-1.53) | |
| Hypodense regions on UECT outside of ASPECTS territory | 0.34 (0.17-0.67) | |
| History of prior stroke | 0.08 (0.02-0.47) | |
| Sedentary activity level | 0.32 (0.14-0.72) | |
| Pre-stroke mRS | 0.58 (0.35-0.96) |
Discussion
This study has found that 74% patients initially presenting with symptoms of aphasia in the emergent setting experience full remission of their language impairments by 6 months, with partial improvement in 86% of cases. This excellent prognosis stands in stark contrast to the 40% recovery rate reported in the literature.(1) Past studies enrolled subacute-to-chronically symptomatic subjects, usually from the population referred to speech therapy. A study of aphasia outcome by Pedersen from 1995 in which baseline assessments occurred relatively early found that half of patients with mild aphasia completely recovered within 1 week and that only 28% of patients were referred to speech therapy, whether due to medical comorbidities or resolution of symptoms.(6) A second study on aphasia by the same group found that 19% of patients reported to have aphasia at the time of admission had experienced full resolution of symptoms by the time they could be formally evaluated by study staff at a median of 4 days after admission.(7) These findings strongly suggest that a significant number of patients with early aphasia were not adequately captured in these early reports.
More recently, a series of studies have attempted to capture aphasia subjects within an earlier timeframe, but were limited by methodological issues which resulted in bias. One study sample was selected from patients referred for speech therapy at a mean of 11 days after stroke,(8) another depended on speech pathology assessments at a median of 5 days from onset.(9) A study by Lazar and colleagues was limited to first-time stroke and assessed its subjects up to 72 hours from onset.(10) The most recent study by Inatomi, et al used NIHSS examinations to study 130 consecutive stroke patients within 48 hours of symptom onset and again at day 8-10, finding that 46% improved and 21% fully recovered.(11)
The NIHSS was utilized to assess aphasia in this study as well. While the NIHSS exam is a relatively crude instrument for language assessment, it is a standardized instrument that has demonstrated reliability in the setting of emergent stroke evaluation. Furthermore, to score 0 points for language function at follow up in this study a subject would have to complete an interview with study staff in which their medical history and medications were reviewed, and a modified Rankin Scale, Barthel Index, Life Quality Scale and repeat NIHSS exam without any evidence of language impairment. This rigorous methodology provides a high level of reassurance that subjects deemed fully recovered were in fact without impairment. Use of a battery of language assessment instruments would allow for separate analysis by various aphasia features, but obtaining a baseline in the hyperacute window is not feasible. This study addresses a question pertaining to emergent management decisions in stroke where use of the standard NIHSS exam is consistent with standard practice, making our conclusions easily understood and applied to clinical practice.
It is important to note that this study continues the analytic approach established in prior studies of aphasia prognosis and excludes patients who died between interval assessments or were lost to follow up.(7-12) In this study the death rate was equivalent between subjects who initially presented with aphasia and those who did not. Compared with other major studies in the literature, our data show better short term improvement rates than previously reported. Whereas the Inatomi study found that 15% of patients initially presented with aphasia, aphasia was identified in 32% of our population.(11) This disparity may be due to the earlier assessments done in our study (median 2.3 hours and all <12 hours versus <48 hours) that would capture symptoms before resolution occurs. In Inatomi's study, only hypercholesterolemia and higher initial NIHSS score were significantly associated with early improved outcomes. In our current study, with nearly twice the number of aphasia subjects, we identified several clinical, demographic and neuroimaging characteristics predictive of improved early recovery. Regardless of those findings, the most important outcome is the long term outcome at 6 months. Studies have repeatedly shown that nearly all language recovery after stroke occurs within the first 3 months; therefore, 6 months should have been an adequate amount of time to allow for a plateau in recovery and provide a reliable measure of chronic language function.(9,12) As expected, early evidence of more extensive infarction portended less early recovery. On univariate analysis this was found in a variety of markers for extensive volume of infarction: radiographically by UECT and CTA-SI, clinically by the presence of concurrent right arm weakness (a highly reliable and reproducible NIHSS exam finding) and by higher overall NIHSS score. The variables that were found to most significantly capture this phenomenon were the extent of CT hypodensity and the clinical history of prior stroke injury. For long term outcome, the presence of pre-stroke disability (by mRS) was a clear predictor of lower likelihood of improvement. Finally, a beneficial effect of smoking in terms of recovery is observed in this cohort in the univariate analysis. This apparent smoking paradox has been observed in other stroke outcome studies and is not fully understood, although the effect became insignificant when adjustment were made for other clinical variables in the logistic regression model.(13,14)
The unique case of isolated aphasia and aphasia in patients with low total NIHSS scores is worth considering separately. These patients are often considered for thrombolysis or intra-arterial procedures on the basis that their aphasia symptoms represent a threat of significant long term disability. These data would suggest otherwise. Patients with isolated aphasia experienced complete recovery in 86% of cases (none received thrombolysis or intra-arterial treatment), and in 90% of all subjects with NIHSS<5 (p=0.02; 2 of the 41 subjects received tPA although 20 presented within 4.5 hours). Although this analysis included only cases of confirmed stroke, a recent study reviewing stroke mimics in patients who had received thrombolysis found that 3 of 11 cases (27%) of isolated aphasia were stroke mimics.(15) Köhrmanm et al also reported on the safety and outcome after thrombolysis in stroke patients with mild symptoms (NIHSS<5) and argued that thrombolysis was appropriate in such cases because of the observed favorable outcomes in treated, versus untreated, patients with mild deficits. In their study of 32 subjects, with aphasia the most frequent symptom, 94% of subjects achieved favorable outcome with 47% fully recovering.(16) Based on our data that included substantially more patients, only 5% treated with tPA, such outcomes would be expected by natural history alone.
Conclusion
The existing body of literature on aphasia prognosis, based on subacute-to-chronically affected subjects, estimates that 40% go on to achieve full recovery. Studies on acute stroke patients have demonstrated a high rate of early symptom recovery that suggests those earlier long term outcome studies cannot be applied to the population of patients presenting with aphasia in the hyperacute period. These data from a representative sample of subjects presenting with stroke in the hyperacute period showed that 38% of subjects with aphasia demonstrated complete recovery by discharge (median of 5 days), and complete recovery in 74% of subjects at 6 months. The presence of a low NIHSS score was associated with a more favorable outcome, and nearly 90% of aphasic subjects with NIHSS<5 fully recover without receiving thrombolysis. In addition to low NIHSS score, the absence of right arm weakness and pre-stroke disability are also manifestations of the aggregate effects of many relevant clinical variables and thus may serve as simple but useful predictors of favorable outcome for making decisions in the acute setting. Given the excellent prognosis of aphasia symptoms in patients with mild strokes, the net benefit of thrombolysis or other recanalization therapies is unclear.
Acknowledgments
Funding:
Supported by Agency for Healthcare Research and Quality grant AHRQ R01 HS11392. Matthew B. Maas supported by National Institutes of Health grant P50NS051343. We gratefully acknowledge the support of the Deane Institute for Integrative research in Stroke and Atrial Fibrillation and the Lakeside Foundation.
Footnotes
Conflicts of Interest/Disclosures:
Matthew B. Maas, MD- none.
Michael H. Lev, MD- is a speaker for GE Healthcare, Waukesha, WI; receives research support from GE Healthcare; and serves as a consultant for CoAxia, Maple Grove, MN, GE Healthcare, Waukesha, WI and Millennium Pharmaceuticals, Cambridge, MA.
Hakan Ay, MD- none.
Aneesh B. Singhal, MD- none.
David M. Greer, MD, MA- has served on the speaker's bureau for Boehringer-Ingelheim Pharmaceuticals, Ridgefield, CT.
Wade S. Smith, MD, PhD- owns stock and has stock options in Concentric Medical, Inc., Mountain View, CA, is a paid consultant for Concentric Medical, Inc., and has a research grant from Boerhinger-Ingelheim Pharmaceuticals, Ridgefield, CT.
Gordon J. Harris, PhD- none.
Elkan F. Halpern, PhD- none.
Walter J. Koroshetz, MD- none.
Karen L. Furie, MD, MPH- none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferro JM, Mariano G, Madureira S. Recovery from aphasia and neglect. Cerebrovasc Dis. 1999;9 5:6–22. doi: 10.1159/000047571. [DOI] [PubMed] [Google Scholar]
- 2.Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–70. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 3.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–4. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 4.Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol. 2005;58:688–97. doi: 10.1002/ana.20617. [DOI] [PubMed] [Google Scholar]
- 5.Ay H, Benner T, Arsava EM, et al. A computerized algorithm for etiologic classification of ischemic stroke: the Causative Classification of Stroke System. Stroke. 2007;38:2979–84. doi: 10.1161/STROKEAHA.107.490896. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen PM, Jørgensen H, Nakayama H, Raaschou HO, Olsen TS. Aphasia in acute stroke: incidence, determinants, and recovery. Ann Neurol. 1995;38:659–66. doi: 10.1002/ana.410380416. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen PM, Vinter K, Olsen TS. Aphasia after stroke: type, severity and prognosis. The Copenhagen aphasia study. Cerebrovasc Dis. 2004;17:35–43. doi: 10.1159/000073896. [DOI] [PubMed] [Google Scholar]
- 8.Godefroy O, Dubois C, Debachy B, Leclerc M, Kreisler A, Lille SP. Vascular aphasias: main characteristics of patients hospitalized in acute stroke units. Stroke. 2002;33:702–5. doi: 10.1161/hs0302.103653. [DOI] [PubMed] [Google Scholar]
- 9.Laska AC, Hellblom A, Murray V, Kahan T, Von Arbin M. Aphasia in acute stroke and relation to outcome. J Intern Med. 2001;249:413–22. doi: 10.1046/j.1365-2796.2001.00812.x. [DOI] [PubMed] [Google Scholar]
- 10.Lazar RM, Speizer AE, Festa JR, Krakauer JW, Marshall RS. Variability in language recovery after first-time stroke. J Neurol Neurosurg Psychiatr. 2008;79:530–4. doi: 10.1136/jnnp.2007.122457. [DOI] [PubMed] [Google Scholar]
- 11.Inatomi Y, Yonehara T, Omiya S, Hashimoto Y, Hirano T, Uchino M. Aphasia during the acute phase in ischemic stroke. Cerebrovasc Dis. 2008;25:316–23. doi: 10.1159/000118376. [DOI] [PubMed] [Google Scholar]
- 12.Demeurisse G, Demol O, Derouck M, de Beuckelaer R, Coekaerts MJ, Capon A. Quantitative study of the rate of recovery from aphasia due to ischemic stroke. Stroke. 1980;11:455–8. doi: 10.1161/01.str.11.5.455. [DOI] [PubMed] [Google Scholar]
- 13.Ovbiagele B, Saver JL. The smoking-thrombolysis paradox and acute ischemic stroke. Neurology. 2005;65:293–5. doi: 10.1212/01.wnl.0000168163.72351.f3. [DOI] [PubMed] [Google Scholar]
- 14.Wahlgren N, Ahmed N, Eriksson N, et al. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: Safe Implementation of Thrombolysis in Stroke-MOnitoring STudy (SITS-MOST) Stroke. 2008;39:3316–22. doi: 10.1161/STROKEAHA.107.510768. [DOI] [PubMed] [Google Scholar]
- 15.Winkler DT, Fluri F, Fuhr P, et al. Thrombolysis in stroke mimics: frequency, clinical characteristics, and outcome. Stroke. 2009;40:1522–5. doi: 10.1161/STROKEAHA.108.530352. [DOI] [PubMed] [Google Scholar]
- 16.Köhrmann M, Nowe T, Huttner HB, et al. Safety and outcome after thrombolysis in stroke patients with mild symptoms. Cerebrovasc Dis. 2009;27:160–6. doi: 10.1159/000185607. [DOI] [PubMed] [Google Scholar]