Abstract
Background
According to a recent consensus, the cachectic syndrome is defined as: “… a complex metabolic syndrome associated with underlying illness and characterized by loss of muscle with or without loss of fat mass. The prominent clinical feature of cachexia is weight loss in adults (corrected for fluid retention) or growth failure in children (excluding endocrine disorders). Anorexia, inflammation, insulin resistance, and increased muscle protein breakdown are frequently associated with cachexia.” Although this definition is accompanied by diagnostic criteria, it does not consider the problem of staging. Stratification of patients is important when considering therapy. The very first stage of the wasting syndrome does not necessarily involve body weight loss—a state known as pre-cachexia.
Methods and Results
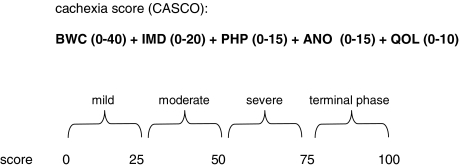
The aim of the present score was to overcome the problem of patient staging in cancer. This score considers five main different factors: body weight and lean body mass loss; anorexia; inflammatory, immunological, and metabolic disturbances; physical performance; and quality of life. The scoring scale goes from 0 to 100: mild cachexia (less than 25), moderate (more than 26 and less than 50), severe (more than 51 and less than 75), and terminal phase (more than 76 and up to 100). The score also takes into consideration the condition known as pre-cachexia.
Conclusion
The present score will facilitate cachexia staging and will therefore allow for a more adequate therapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s13539-011-0027-5) contains supplementary material, which is available to authorized users.
Keywords: Cachexia, Wasting score, Anorexia, Weight loss, Physical performance, Quality of life
Introduction: defining cachexia
Cachexia is a term originating from the Greek: “kakos” and “hexis” meaning “bad condition”. The cachectic state is observed in many pathological conditions such as cancer, chronic obstructive pulmonary disease (COPD), sepsis, or chronic heart failure. For several years, it has been considered essential to develop a standardized definition of cachexia [1]. This represents a key issue for treatment, for reimbursement, and for inclusion and exclusion from clinical trials. The definition and diagnostic criteria must be clear on the identification of the early signs. Also a key point, the definition must include an appreciation that cachexia is found in a number of life-limiting illnesses that the management of this syndrome in end-of-life must be facilitated, albeit that this is not done the same way as in earlier stages.
A group of international experts who met in Washington DC (USA) in December 2006 for an international consensus meeting organized by the Society for Cachexia and Wasting Disorders stated: “cachexia is a complex metabolic syndrome associated with underlying illness and characterized by loss of muscle with or without loss of fat mass. The prominent clinical feature of cachexia is weight loss in adults (corrected for fluid retention) or growth failure in children (excluding endocrine disorders). Anorexia, inflammation, insulin resistance and increased muscle protein breakdown are frequently associated with wasting disease. Wasting disease is distinct from starvation, age-related loss of muscle mass, primary depression, malabsorption and hyperthyroidism and is associated with increased morbidity” [2].
Very recently, a consensus on this one and other cachexia definitions [3, 4] has been reached and published.
The Consensus group reached two basic conclusions: (1) there is a need to incorporate the term “pre-cachexia” as a condition associated with no or very small weight loss (less than 5% of body weight loss in 6 months) which is associated with underlying chronic disease and characterized by anorexia, inflammation, and/or metabolic alterations. Clearly, the pre-cachectic population will be very heterogeneous, with some patients progressing rapidly but others remaining weight-stable. The development of suitable clinical identifiers or biomarkers to map out the different cohorts will be an area of intense interest; (2) some type of cachexia “staging” or “score” is essential to be able to classify patients according to the severity of the syndrome. The classification domains (phenotyping) of patients could comprise of measures of whole body and lean body mass loss, catabolic drivers (including immune and inflammatory response), anorexia, quality of life, and physical performance.
Bearing all this in mind, the object of the present study is to fulfill the existing gap in the classification of cachectic cancer patients by introducing a new score that takes into consideration the stated parameters.
Diagnostic tools
In spite of the existence of different cachexia definitions and consensus, cachexia is infrequently diagnosed. This is, in part, due to the lack of clear standardized cachectic markers. Some of the criteria used in the past include weight loss, decreased physical performance, fatigue, anorexia, and metabolic alterations. The group of investigators who met in Washington [2] concluded that cachexia can be diagnosed in the following way: a weight loss of at least 5% or more in 12 months or less in the presence of underlying illness, plus three of the following criteria: decreased muscle strength, fatigue, anorexia, low fat-free mass index, abnormal biochemistry (increased inflammatory markers (C-reactive protein >5.0 mg/l), IL-6 >4.0 pg/ml), anemia (<12 g/dl), and low serum albumin (<3.2 g/dl)) [2]. However, other diagnostic tools should not be discarded, such as decreased physical performance (total activity, handgrip strength, stairs climb, or 6-min walk distance) or biochemical tissue analysis (activation of proteolysis or apoptosis in skeletal muscle biopsies [5]).
Staging cachexia: the cachexia score
As already stated above, in addition to clear and objective diagnostic criteria, an essential requirement for both clinical trials and patient treatment is a staging system that allows for classification of the cancer patients according to the severity of the cachectic syndrome. Such a staging system would be beneficial not only for assessing the severity of the syndrome but also to decide the type of treatment. It was the aim of the present study to develop such a classification system. The result is the newborn cachexia score (CASCO) which, by means of a numerical scale, classifies cachexia into mild (0–25), moderate (26–50), severe (51–75), and terminal (76–100). It is therefore clear that the higher the score, the worst the syndrome (Table 1).
Table 1.
CASCO staging scale
BWC body weight loss and composition, IMD inflammation/metabolic disturbances/immunosupression, PHP physical performance, ANO anorexia, QOL quality of life
Components
The main components of CASCO are: body weight loss and composition, inflammation/metabolic disturbances/immunosupression, physical performance, anorexia, and quality of life.
Indeed, body weight loss and composition is an essential component of all definitions of cachexia. It is by far the most important requirement, in addition to the presence of an underlying disease such as cancer, COPD, chronic infection. However, and due to the fact that both loss of muscle and fat tissue coexist in the cachectic patient, it is also important to assess any changes that may occur in relation with lean body mass. From this point of view, a decrease in lean body mass in the cachectic patient represents impaired physical performance and therefore quality of life, weight changes may just give an idea of survival. Indeed, some authors claim that fat is associated with survival while skeletal muscle is associated with loss of quality of life [6]. In the CASCO, lean body mass modulates the importance of body weight loss (Table 2). Body weight loss and composition accounts for up 40% of the cachexia score (Table 2).
Table 2.
The CACHEXIA score (CASCO): a new tool for staging cachectic patients
| Symptom | Percent | Measurement | Total points | Parameter | Values |
|---|---|---|---|---|---|
| BWC | 40 | Body weight loss | 32 | <5% | |
| ≥5%, mild | |||||
| ≥10%, moderate | |||||
| ≥15%, severe | |||||
| ≥20%, terminal | |||||
| Lean body mass | 8 | No change in LBM | |||
| Loss of LBM >10% | |||||
| IMD | 20 | Inflammation | 8 | Plasma CRP | 5 mg/l ≤CRP ≤10 mg/l |
| 10 mg/l <CRP ≤20 mg/l | |||||
| CRP >20 mg/l | |||||
| Plasma IL6 | 4 pg/ml ≤IL6 ≤10 pg/ml | ||||
| 10 pg/ml <IL6 ≤30 pg/ml | |||||
| IL6 >30 pg/ml | |||||
| Metabolic disturbances | 8 | Plasma albumin | <3.2 g/dL | ||
| Plasma pre-albumin | <16 mg/dL | ||||
| Plasma lactate | >2.2 mM | ||||
| Plasma triglycerides | >200 mg/dL | ||||
| Anemia | Hb <12 g/dL | ||||
| Plasma urea | >50 mg/dL | ||||
| Oxidative stress: ROS plasma levels | >300 FORT U | ||||
| Glucose tolerance test or HOMA index | Altered | ||||
| Immunosupression | 4 | IL2 levels | >500 pg/ml | ||
| Peripheral lymphocytes: proliferation assay or skin hypersensitivity test | Positive | ||||
| PHP | 15 | 15 | Total activity | Physical performance, questionnaire, or monitoring | |
| Handgrip strength | |||||
| Stairs climb | |||||
| 6-min walk distance | |||||
| ANO | 15 | 15 | Simplified Nutrition Assessment Questionnaire | Yes | |
| QoL | 10 | 10 | Quality of life Questionnaire | Mild | |
| Moderate | |||||
| Severe |
BWC body weight loss and composition, IMD inflammation/metabolic disturbances/immunosupression, PHP physical performance, ANO anorexia, QoL quality of life
© 2010 Copyright by the authors; licensee University of Barcelona, Barcelona, Spain. From: Josep M. Argilés, Francisco J. López-Soriano, Míriam Toledo, Roberto Serpe and Sílvia Busquets
The second component of CASCO is inflammation/metabolic disturbances/immunosupression (IMD). Indeed, inflammation is a very important component of the cachectic response. Studies in pancreatic cancer patients [7] clearly demonstrate an inflammatory response characterized by increased levels of acute phase proteins (such as C-reactive protein (CRP)) and cytokines (such as IL-6). Other studies involving cachexia in pathological conditions other than cancer [8] also suggest the importance of the inflammatory response. The inflammatory component accounts for up to 40% of IMD in CASCO. In addition to inflammation, there are a number of metabolic disturbances that are present in most of the cachectic patients; such disturbances include glucose intolerance, anemia, and low levels of plasmatic albumin, among others. Metabolic disturbance component accounts for up to 40% of IMD in CASCO. Finally, immunosupression might be an early marker of cachexia [9]; therefore, assessment of the immune response could also be a good indicator for a cachexia staging system. The immunosupression component accounts for up to 20% of IMD in CASCO. IMD accounts for up 20% of the cachexia score (Table 2).
The third component relates to physical performance. Indeed, even if there is a relative small decrease in muscle mass due the cachectic syndrome, there may be a significant decrease in physical-related activities which are related to muscle performance [10, 11]. Therefore, assessing physical performance is an essential component of any cachectic staging system. Physical performance accounts for up 15% of the cachexia score (Table 2).
Anorexia constitutes the fourth parameter included in CASCO. Indeed, anorexia is an important component of cachexia of many types of diseases [12]. A decrease in food intake, by itself, promotes changes in quality of life and also conditions with many metabolic alterations. Anorexia accounts for up 15% of the cachexia score (Table 2).
Finally, the last component of CASCO is quality of life. Indeed, quality of life reflexes are not just changes in weight and physical performance but also in metabolic alterations [11, 13]. It is therefore essential to take it into consideration here. Quality of life accounts for up 10% of the cachexia score (Table 2).
It is clear that the five different factors mentioned clearly interact with one another and represent the most important set of variables that might indicate the severity of cachectic syndrome.
Methodology
The CASCO includes a comprehensive number of measurements. All of these can be carried out either with physical or biochemical tests together with the relevant questionnaires to be filled by the patients with or without sanitary assistance.
In addition to weight determination, lean body mass can be measured using either bioelectrical impedance analysis (BIA) [14, 15] or dual X-ray absorptiometry (DEXA) [15, 16], although the latter is preferred [17].
Concerning inflammation, both CRP and IL-6 are easily measured clinical parameters. The same applies for albumin, pre-albumin, lactate, triglycerides, hemoglobin, and urea. Decreases in plasma albumin have been related with the severity and the prognosis of different cachectic states [18]. Pre-albumin is a good indicator of nutritional status [18]. Elevations in plasma triglycerides are a common trend in catabolic conditions related with cachexia [19]. In the case of lactate, elevations in this marker are very frequent in cancer patients but also indicative of the acidosis present in other types of catabolic states [19]. Concerning hemoglobin, anemia is often a condition associated with cachexia, particularly in cancer [11]. Plasma urea, to some extent, reflects nitrogen catabolism [20] and is therefore included in the list. More time-consuming is the determination of glucose tolerance; however, this can be replaced by assessing the Homeostasis Model Assessment (HOMA index) [21], and finally, and to some extent, though not a normal clinical routine, is the determination of reactive oxygen species (ROS) plasma levels. Oxidative stress is clearly associated with cachexia, particularly in cancer [22, 23]. It is for this reason that we have included a simple method for the determination of plasma levels of ROS [24, 25]. In order to estimate immunosupression, IL-2 levels will be determined. This cytokine is known to be a clear activator of different T cells [26]. In addition to the cytokine measurement, a peripheral lymphocyte proliferation assay will be undertaken [27] since this is a very indicative measurement of immune response. Finally, skin hypersensitivity will also be determined with the relevant test [28]. These three measurements give a clear indication of the immunologic response of the patient. This is particularly important since a previously stated immunosuppression may appear before any weight loss takes place [9].
Concerning physical performance, standard measurements for total activity [29], handgrip strength [30], stairs climb [31], or 6-min walk distance [32, 33] will be undertaken (Tables 2 and 3). We have to take into consideration the fact that monitoring will only be possible after diagnosis; therefore, we have included a physical performance questionnaire which will be used at the moment of diagnosis in order to be able to use this parameter in the staging of the cachectic patient (Table 3, “Questionnaire”).
Table 3.
Physical performance
| Physical performance staging of a cachectic patient |
|---|
| Questionnaire (during the past week)a |
| Have you noticed any particular decrease in the physical activities (i.e., at work, at home, at leisure, etc.…) that you normally carry out during the day? |
| Have you had any problems doing strenuous activities, like carrying a heavy shopping bag or a suitcase? |
| Have you noticed any loss of handgrip force? |
| Did you have to put more effort on climbing stairs? |
| Have you felt tired after walking approximately half a kilometer? |
| Monitoringb |
| •Total physical activity |
| •Grip force |
| •Stair-climb |
| •6-min walk distance |
aAdapted from EORTC QLQ-C30 (version 3) ©Copyright 1995 EORTC Study Group on Quality of Life
bMonitoring will take place at the same moment as the questionnaire is filled, normally at the time of diagnose. The very first calculation of the CACHEXIA SCORE will use the values from the questionnaire. Subsequent calculations will use the monitored values of the items under “Monitoring”
Anorexia will be estimated using a standard questionnaire (Simplified Nutrition Assessment Questionnaire (SNAQ); Table 4).
Table 4.
Anorexia questionnaire
| Simplified Nutrition Assessment Questionnaire (SNAQ)a |
|---|
| My appetite is |
| a. Very poor |
| b. Poor |
| c. Average |
| d. Good |
| e. Very good |
| When I eat |
| a. I feel full after eating only a few mouthfuls |
| b. I feel full after eating about a third of a meal |
| c. I feel full after eating over half a meal |
| d. I feel full after eating most of the meal |
| e. I hardly ever feel full |
| Food tastes |
| a. Very bad |
| b. Bad |
| c. Average |
| d. Good |
| e. Very good |
| Normally I eat |
| a. Less than one meal a day |
| b. One meal a day |
| c. Two meals a day |
| d. Three meals a day |
| e. More than three meals a day |
aPoints are assigned for the patient’s answers as follows: a = 1, b = 2, c = 3, d = 4, e = 5. The sum of the scores for the individual items constitutes the SNAQ score
Concerning quality of life, the questionnaire is presented in Table 5. It has been adapted from EORTC QLQ-C30 [34], where questions related to physical performance or food intake have been withdrawn.
Table 5.
Quality of life questionnaire
| During the past week |
|---|
| Do you need to stay in bed or a chair during the day? |
| Do you need help with eating, dressing, washing yourself, or using the toilet? |
| Were you limited in doing either your work or other daily activities? |
| Were you limited in pursuing your hobbies or other leisure time activities? |
| Were you short of breath? |
| Have you had pain? |
| Did you need to rest? |
| Have you had trouble sleeping? |
| Have you felt weak? |
| Have you felt nauseated? |
| Have you vomited? |
| Have you been constipated? |
| Have you had diarrhea? |
| Did pain interfere with your daily activities? |
| Have you had difficulty in concentrating on things, like reading a newspaper or watching television? |
| Did you feel tense? |
| Did you worry? |
| Did you feel irritable? |
| Did you feel depressed? |
| Have you had difficulty remembering things? |
| Has your physical condition or medical treatment interfered with your family life? |
| Has your physical condition or medical treatment interfered with your social activities? |
| Has your physical condition or medical treatment caused you financial difficulties? |
| How would you rate your overall health during the past week? |
| How would you rate your overall quality of life during the past week? |
Adapted from EORTC QLQ-C30 (version 3) © Copyright 1995 EORTC Study Group on Quality of Life. First 23 questions: Not at all: 1; A little: 2; Quite a bit: 3; Very much: 4; Last two questions: Excellent: 1, Fine: 2, Poor: 3, Very poor: 4
Pre-cachexia
As we have previously mentioned in section 1, there may be patients that, although they have not yet lost any significant weight (less or equal to 5% in the last 12 months) and are subjected to an underlying disease, which is often associated with cachexia, may already have some of the peculiarities associated with cachexia such as inflammation or decreased physical performance. This condition has been named as pre-cachexia [4]. However, no consensus on how to classify the pre-cachectic patients has been reached in spite of many suggestions [4]. It is for this reason that the present publication also includes a tentative quantitative approach for the diagnosis of pre-cachexia (Table 6). Pre-cachexia would exist if the patient had at least 35 as the sum of the different parameters that specifically exclude body weight loss and composition; as we mentioned, it is essential to have no significant weight loss for pre-cachexia to exist (Table 6).
Table 6.
Pre-cachexia
| Quantitative approach |
|---|
| BWC = 0 |
| (IMD + PHP + QOL + ANO) >35 |
BWC body weight loss and composition, IMD inflammation/metabolic disturbances/immunosupression, PHP physical performance, ANO anorexia, QoL quality of life
Validation and conclusions
Future efforts will concentrate on the validation of such a staging system. Simple systems are generally preferable to allow bedside assessments. The issue of validation is difficult for such a score or staging system. There is a clear need for high-quality international data, representative of the disease populations. Cachexia researchers need to agree and to gather a large prospective data set. Obviously, interpretation of the data by appropriate statistical methods is crucial. Correlation with patient prognosis may be useful, but the relationship between score and outcome may be different in different underlying diseases. Also, the relationship between score and treatment response to cachexia interventions is a possible approach to validating such a score. Although this may be the preferable approach, it depends on availability of several treatment studies and their having assessed all relevant parameters of interest. It may be useful to generate a set of (simple) parameters that all trials should assess in order to allow assessment of such scores in the future and emphasize on the key importance of similar developments in the definition of related terms like sarcopenia, fatigue, and frailty. Although mainly intended and designed for cancer patients, CASCO could also be tentatively used for other wasting disorders such as chronic heart failure or chronic obstructive pulmonary disease, since many of both the biochemical and metabolic alterations are similar. Obviously, this would have to be validated.
In conclusion, a quantitative tentative (not yet validated) staging score for cancer cachectic patients is presented. It is already a tool for the (a) identification of pre-cachectic patients, and (b) classification and staging of the syndrome according to body weight loss and composition, inflammation/metabolic disturbances/immunosupression, physical performance, anorexia, and quality of life. When validated, the new CASCO might prove to be a useful tool for the treatment and nutritional recommendations of cachectic cancer patients in a similar way as other staging methodologies [35]. Future efforts will concentrate on a multicenter clinical validation of the score.
Practical questionnaire and CASCO quantitation procedure
The full CASCO calculation procedure and questionnaires can be found in the following address: http://www.fbg.ub.es/index.php?option=com_content&task=view&id=251&Itemid=
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic supplementary material (PDF 103 kb)
Acknowledgments
This work was supported by a grant from the Ministerio de Ciencia y Tecnología (SAF-02284-2008). Dr. Roberto Serpe was supported by grants CRP1_296 from the Regione Autonoma della Sardegna by PO Sardegna FSE 2007-2013 (L.R.7/2007) titled “Promotion of scientific and technological research in Sardinia”, Italy.
All authors of this manuscript complied with the guidelines of ethical authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle [36].
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Springer J, von Haehling S, Anker SD. The need for a standardized definition for cachexia in chronic illness. Nat Clin Pract Endocrinol Metab. 2006;2:416–7. doi: 10.1038/ncpendmet0247. [DOI] [PubMed] [Google Scholar]
- 2.Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–9. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Blum D, Omlin A, Fearon K, Baracos V, Radbruch L, Kaasa S, et al. Evolving classification systems for cancer cachexia: ready for clinical practice? Support Care Cancer. 2010;18:273–9. doi: 10.1007/s00520-009-0800-6. [DOI] [PubMed] [Google Scholar]
- 4.Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia–anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29:154–9. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Busquets S, Deans C, Figueras M, Moore-Carrasco R, Lopez-Soriano FJ, Fearon KC, et al. Apoptosis is present in skeletal muscle of cachectic gastro-intestinal cancer patients. Clin Nutr. 2007;26:614–8. doi: 10.1016/j.clnu.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Kuwae N, Wu DY, Shantouf RS, Fouque D, Anker SD, et al. Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am J Clin Nutr. 2006;83:202–10. doi: 10.1093/ajcn/83.2.202. [DOI] [PubMed] [Google Scholar]
- 7.Fearon KC, Barber MD, Falconer JS, McMillan DC, Ross JA, Preston T. Pancreatic cancer as a model: inflammatory mediators, acute-phase response, and cancer cachexia. World J Surg. 1999;23:584–8. doi: 10.1007/PL00012351. [DOI] [PubMed] [Google Scholar]
- 8.Delano MJ, Moldawer LL. The origins of cachexia in acute and chronic inflammatory diseases. Nutr Clin Pract. 2006;21:68–81. doi: 10.1177/011542650602100168. [DOI] [PubMed] [Google Scholar]
- 9.Faber J, Vos AP, Kegler D, Argiles J, Laviano A, Garssen J, et al. Impaired immune function: an early marker for cancer cachexia. Oncol Rep. 2009;22:1403–6. doi: 10.3892/or_00000581. [DOI] [PubMed] [Google Scholar]
- 10.Maddocks M, Byrne A, Johnson CD, Wilson RH, Fearon KC, Wilcock A. Physical activity level as an outcome measure for use in cancer cachexia trials: a feasibility study. Support Care Cancer. 2010;18:1539–44. doi: 10.1007/s00520-009-0776-2. [DOI] [PubMed] [Google Scholar]
- 11.Fouladiun M, Korner U, Gunnebo L, Sixt-Ammilon P, Bosaeus I, Lundholm K. Daily physical-rest activities in relation to nutritional state, metabolism, and quality of life in cancer patients with progressive cachexia. Clin Cancer Res. 2007;13:6379–85. doi: 10.1158/1078-0432.CCR-07-1147. [DOI] [PubMed] [Google Scholar]
- 12.Laviano A, Meguid MM, Inui A, Muscaritoli M, Rossi-Fanelli F. Therapy insight: cancer anorexia-cachexia syndrome–when all you can eat is yourself. Nat Clin Pract Oncol. 2005;2:158–65. doi: 10.1038/ncponc0112. [DOI] [PubMed] [Google Scholar]
- 13.Granda-Cameron C, DeMille D, Lynch MP, Huntzinger C, Alcorn T, Levicoff J, et al. An interdisciplinary approach to manage cancer cachexia. Clin J Oncol Nurs. 2010;14:72–80. doi: 10.1188/10.CJON.72-80. [DOI] [PubMed] [Google Scholar]
- 14.Simons JP, Schols AM, Westerterp KR, ten Velde GP, Wouters EF. The use of bioelectrical impedance analysis to predict total body water in patients with cancer cachexia. Am J Clin Nutr. 1995;61:741–5. doi: 10.1093/ajcn/61.4.741. [DOI] [PubMed] [Google Scholar]
- 15.Ellis KJ. Human body composition: in vivo methods. Physiol Rev. 2000;80:649–80. doi: 10.1152/physrev.2000.80.2.649. [DOI] [PubMed] [Google Scholar]
- 16.Plank LD. Dual-energy X-ray absorptiometry and body composition. Curr Opin Clin Nutr Metab Care. 2005;8:305–9. doi: 10.1097/01.mco.0000165010.31826.3d. [DOI] [PubMed] [Google Scholar]
- 17.Ellegard LH, Ahlen M, Korner U, Lundholm KG, Plank LD, Bosaeus IG. Bioelectric impedance spectroscopy underestimates fat-free mass compared to dual energy X-ray absorptiometry in incurable cancer patients. Eur J Clin Nutr. 2009;63:794–801. doi: 10.1038/ejcn.2008.35. [DOI] [PubMed] [Google Scholar]
- 18.Araujo JP, Lourenco P, Rocha-Goncalves F, Ferreira A, Bettencourt P. Nutritional markers and prognosis in cardiac cachexia. Int J Cardiol. 2009;146:359–363. doi: 10.1016/j.ijcard.2009.07.042. [DOI] [PubMed] [Google Scholar]
- 19.Argiles JM, Alvarez B, Lopez-Soriano FJ. The metabolic basis of cancer cachexia. Med Res Rev. 1997;17:477–98. doi: 10.1002/(SICI)1098-1128(199709)17:5<477::AID-MED3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 20.Pisters PW, Pearlstone DB. Protein and amino acid metabolism in cancer cachexia: investigative techniques and therapeutic interventions. Crit Rev Clin Lab Sci. 1993;30:223–72. doi: 10.3109/10408369309084669. [DOI] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 22.Mantovani G, Maccio A, Madeddu C, Massa E. Cancer-related cachexia and oxidative stress: beyond current therapeutic options. Expert Rev Anticancer Ther. 2003;3:381–92. doi: 10.1586/14737140.3.3.381. [DOI] [PubMed] [Google Scholar]
- 23.Barreiro E, de la Puente B, Busquets S, Lopez-Soriano FJ, Gea J, Argiles JM. Both oxidative and nitrosative stress are associated with muscle wasting in tumour-bearing rats. FEBS Lett. 2005;579:1646–52. doi: 10.1016/j.febslet.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Pavlatou MG, Papastamataki M, Apostolakou F, Papassotiriou I, Tentolouris N. FORT and FORD: two simple and rapid assays in the evaluation of oxidative stress in patients with type 2 diabetes mellitus. Metabolism. 2009;58:1657–62. doi: 10.1016/j.metabol.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 25.Mantovani G, Maccio A, Madeddu C, Mura L, Gramignano G, Lusso MR, et al. Antioxidant agents are effective in inducing lymphocyte progression through cell cycle in advanced cancer patients: assessment of the most important laboratory indexes of cachexia and oxidative stress. J Mol Med. 2003;81:664–73. doi: 10.1007/s00109-003-0476-1. [DOI] [PubMed] [Google Scholar]
- 26.Nakanishi K. Innate and acquired activation pathways in T cells. Nat Immunol. 2001;2:140–2. doi: 10.1038/84236. [DOI] [PubMed] [Google Scholar]
- 27.Mantovani G, Maccio A, Melis G, Mura L, Massa E, Mudu MC. Restoration of functional defects in peripheral blood mononuclear cells isolated from cancer patients by thiol antioxidants alpha-lipoic acid and N-acetyl cysteine. Int J Cancer. 2000;86:842–7. doi: 10.1002/(SICI)1097-0215(20000615)86:6<842::AID-IJC13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 28.Daly JM, Dudrick SJ, Copeland EM., 3rd Intravenous hyperalimentation. Effect on delayed cutaneous hypersensitivity in cancer patients. Ann Surg. 1980;192:587–92. doi: 10.1097/00000658-198011000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dahele M, Skipworth RJ, Wall L, Voss A, Preston T, Fearon KC. Objective physical activity and self-reported quality of life in patients receiving palliative chemotherapy. J Pain Symptom Manage. 2007;33:676–85. doi: 10.1016/j.jpainsymman.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 30.Luna-Heredia E, Martin-Pena G, Ruiz-Galiana J. Handgrip dynamometry in healthy adults. Clin Nutr. 2005;24:250–8. doi: 10.1016/j.clnu.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Brunelli A, Salati M. Preoperative evaluation of lung cancer: predicting the impact of surgery on physiology and quality of life. Curr Opin Pulm Med. 2008;14:275–81. doi: 10.1097/MCP.0b013e328300caac. [DOI] [PubMed] [Google Scholar]
- 32.Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–23. [PMC free article] [PubMed] [Google Scholar]
- 33.Wiedenmann B, Malfertheiner P, Friess H, Ritch P, Arseneau J, Mantovani G, et al. A multicenter, phase II study of infliximab plus gemcitabine in pancreatic cancer cachexia. J Support Oncol. 2008;6:18–25. [PubMed] [Google Scholar]
- 34.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 35.Ottery F. Patient-generated subjective global assessment. In: Polisena PMC, editor. The Clinical Guide to Oncology Nutrition. Chicago: The American Dietetic Association; 2000. pp. 11–23. [Google Scholar]
- 36.von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8. doi: 10.1007/s13539-010-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material (PDF 103 kb)