Abstract
Chronic administration of the atypical antipsychotic drug, clozapine, to rodents has been shown to increase the concentration of apolipoprotein D (apoD) in several area of the brain, suggesting that apoD could be involved in the therapeutic effects of antipsychotic drugs and/or the pathology of psychotic illnesses. Here, we measured a significant decrease in the concentration of apoD in serum samples from schizophrenic patients. In contrast, apoD levels were significantly increased (92–287%) in dorsolateral prefrontal cortex (Brodmann's area 9) of schizophrenic and bipolar subjects. Elevated levels of apoD expression were also observed in the caudate of schizophrenic and bipolar subjects (68–89%). No differences in apoD immunoreactivity were detected in occipital cortex (Brodmann's area 18) in either group, or in the hippocampus, substantia nigra, or cerebellum of the schizophrenic group. The low serum concentrations of apoD observed in these patients supports recent hypotheses involving systemic insufficiencies in lipid metabolism/signaling in schizophrenia. Elevation of apoD expression selectively within central nervous system regions implicated in the pathology of these neuropsychiatric disorders suggests a focal compensatory response that neuroleptic drug regimens may augment.
Psychiatric disturbances, such as schizophrenia and bipolar disorder, bear similarities in several epidemiologic aspects, including genetic susceptibility, lifetime risk, age of onset, and course of illness (1). Although each disturbance has distinct clinical features, their treatment regimens often overlap considerably in terms of antipsychotic pharmacotherapy, which is used to treat psychiatric symptoms in both disorders. The efficacy of such medications in these disorders has been well established in that antipsychotic drugs reduce symptomatology and prevent relapse in a large percentage of patients. Therefore, molecular and biochemical changes resulting from administration of antipsychotic drugs may be associated with the pathology of psychoses.
Clozapine, a widely used atypical antipsychotic drug, has been shown to be effective and relatively well tolerated in acute and long-term treatment of patients with bipolar disorder, schizophrenia, and schizoaffective disorder, especially those who have not responded to conventional pharmacotherapies (2). Previously, by using TOGA (Total Gene Expression Analysis) to identify clozapine-induced changes in gene expression in mouse central nervous system (CNS), we detected a gradual accumulation in the expression of apolipoprotein D (apoD) mRNA and protein in response to chronic clozapine administration (3). Increases in apoD expression were detected in white matter regions, including corpus callosum, internal capsule, and optic tract, and gray matter regions, including the striatum and globis pallidus (3). These results implicate apoD in the mechanisms of action of clozapine and could suggest that apoD is associated with biochemical pathways underlying psychiatric disorders. ApoD was initially identified as a constituent of plasma high-density lipoproteins (4); however, it shares little homology with the other plasma lipoproteins. Rather, it is a member of the lipocalin superfamily of proteins that function in the transport of small hydrophobic molecules (5). In addition to abundant expression in human serum, apoD is also widely expressed in numerous tissues, including liver, kidney, intestine, spleen, and brain (5). The physiological role for apoD within the brain is not known. However, it has been shown to bind several hydrophobic ligands, including steroid hormones and heme-containing molecules (6, 7), suggesting a role in extracellular lipid transport in the brain. ApoD has also been shown to bind arachidonic acid (AA) (8), implicating it in pathways associated with membrane phospholipid signal transduction and metabolism.
Here we have measured apoD levels in serum samples of schizophrenic subjects and from brain tissue obtained postmortem from schizophrenic and bipolar subjects and subjects with no history of psychiatric illness (controls) using both Western blot and ELISA analyses.
Materials and Methods
Tissue Collection.
Following approval from the North-Western Health Care Human Ethics Committee of the Victorian Institute of Forensic Medicine, tissue samples were obtained from the left brain hemisphere. In all cases, the cadavers were refrigerated within 5 h of being found and the tissue was rapidly frozen to −70°C within 30 min of autopsy and stored until used. The mean postmortem interval and pH for the tissue from each group was not significantly different (Tables 1 and 2). The cohorts consisted of 20 subjects with a diagnosis of schizophrenia and eight subjects with bipolar disorder (Tables 1 and 2). All bipolar subjects were noted as displaying psychotic behavior at time of death. Tissue was also collected from 19 subjects (controls) with no known history of psychiatric illness (Table 1). All psychiatric diagnoses of the subjects from whom tissue was collected were made by using Diagnostic and Statistical Manual version IV criteria (9) by a senior psychiatrist and psychologist after an extensive case history review. All schizophrenic patients, and six of the eight bipolar subjects, had a history of treatment with typical neuroleptic drugs, except two who were reported to have been treated with clozapine and another that had been neuroleptic-free for over 1 year (Table 1). The sex distribution and mean age of the control and schizophrenic groups in this study were not significantly different (Table 1). The control group used in the bipolar study was a subset of the control subjects used in the schizophrenic study; however, the mean age of the bipolar group was significantly higher (Table 2). Importantly, the tissue samples from these control subjects were reanalyzed in separate experiments with the bipolar subjects.
Table 1.
Demographic data for the schizophrenic and control subjects
| Schizophrenia
|
Control
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. | Sex | Age, y | Tissue pH | PMI, h | DOI | Drug dose, mg* | Patient no. | Sex | Age, y | Tissue pH | PMI, h |
| 1 | M | 23 | 6.40 | 42 | 6 | 1750 | 1 | M | 23 | 6.13 | 36 |
| 2 | M | 38 | 5.52 | 40 | N/A | 160 | 2 | M | 30 | 6.46 | 24 |
| 3 | F | 27 | 5.85 | 41 | 10 | N/A | 3 | F | 21 | 6.03 | 58 |
| 4 | M | 55 | 6.10 | 25 | 33 | 40 | 4 | M | 50 | 6.43 | 69 |
| 5 | F | 36 | 6.28 | 45 | 4 | 160 | 5 | F | 32 | 6.16 | 56 |
| 6 | M | 22 | 6.29 | 49 | 20 | 2920 | 6 | M | 22 | 6.58 | 51 |
| 7 | M | 36 | 6.04 | 38 | 12 | 200 | 7 | M | 38 | 6.42 | N/A |
| 8 | M | 44 | 6.28 | 32 | 23 | 600 | 8 | M | 43 | 6.25 | 45 |
| 9 | M | 48 | 6.62 | 30 | 24 | 1250 | 9 | M | 25 | 6.15 | 35 |
| 10 | M | 42 | 6.26 | 47 | 22 | N/A | 10 | M | 42 | 6.32 | 26 |
| 11 | M | 25 | 6.38 | 49 | 2 | N/A | 11 | M | 42 | 6.32 | 26 |
| 12 | M | 22 | 6.07 | 37 | 3 | 450 | 12 | M | 25 | 6.48 | 50 |
| 13 | F | 35 | 6.26 | 15 | 7 | 300 | 13 | M | 26 | 6.42 | 24 |
| 14 | M | 41 | 6.20 | 31 | 11 | 500 | 14 | M | 30 | 5.86 | 27 |
| 15 | M | 45 | 6.48 | 68 | 12 | 300 | 15 | M | 38 | 6.19 | 44 |
| 16 | M | 38 | 6.02 | 50 | 4 | 500 | 16 | F | 33 | 6.41 | 42 |
| 17 | F | 31 | 6.27 | 27 | 13 | 875 | 17 | M | 42 | 6.61 | 43 |
| 18 | F | 38 | 6.43 | 20 | 17 | N/A | 18 | M | 43 | 6.43 | 51 |
| 19 | M | 22 | 6.17 | 37 | 3 | 200 | 19 | F | 38 | 6.26 | 52 |
| 20 | M | 26 | 6.39 | 52 | 2 | 500 | |||||
| Mean ± SEM | 34.7 ± 2.2 | 6.21 ± 0.05 | 38.7 ± 2.9 | Mean ± SEM | 33.2 ± 2.2 | 6.32 ± 0.04 | 41.5 ± 4.5 | ||||
PMI, post-mortem interval; DOI, duration of illness; N/A, not available.
Drug doses are given as chlorpromazine equivalents.
Table 2.
Demographic data for the bipolar and control subjects
| Bipolar
|
Control
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. | Sex | Age, y | Tissue pH | PMI, h | DOI | Neuroleptic drugs | Patient no. | Sex | Age, y | Tissue pH | PMI, h |
| 1 | F | 74 | 6.26 | 45 | 12 | Fluphenazine | 1 | F | 38 | 6.26 | 52 |
| 2 | F | 58 | 5.68 | 41 | 40 | none | 2 | F | 33 | 6.41 | 42 |
| 3 | M | 59 | 6.46 | 34 | 24 | none | 3 | M | 38 | 6.19 | 44 |
| 4 | M | 38 | 6.42 | 24 | 10 | Chlorpromazine | 4 | M | 30 | 6.42 | N/A |
| 5 | M | 66 | 6.41 | 17 | 3 | Fluphenazine | 5 | M | 26 | 6.42 | 24 |
| 6 | F | 55 | 6.46 | 52 | 14 | Trifluorperazine | 6 | M | 43 | 6.25 | 45 |
| 7 | F | 60 | 6.08 | 50 | 23 | Flupenthixol | 7 | F | 32 | 6.16 | 56 |
| 8 | M | 61 | 6.44 | 58 | 35 | Melleril | 8 | M | 42 | 6.61 | 43 |
| Mean ± SEM | 58.8 ± 3.6* | 6.27 ± 0.09 | 40.1 ± 5.0 | Mean ± SEM | 35.3 ± 2.1 | 6.34 ± 0.05 | 43.7 ± 3.8 | ||||
, P < 0.0001.
Dissection of the cortical regions was carried out with reference to a Brodmann map. All tissue was excised from the cortex only and therefore excluded the underlying white matter. The hippocampus was dissected with reference to the system of Amaral and Insausti (10). The substantia nigra was identified by pigmention and then carefully dissected to exclude the subthalamic nuclei. No attempts were made to differentiate between the subdivisions of the substantia nigra.
Serum Collection.
ApoD concentrations were determined in the serum from consenting neuroleptic-free patients, patients receiving typical neuroleptic drugs, and patients enrolled in the clozapine monitoring system at the Mental Health Research Institute. Patients were deemed neuroleptic-free if they had not received neuroleptic drugs orally for 1 month or by depot injection for 3 months before blood collection. The schizophrenic patients consisted of 24 males and 8 females. The control group consisted of 13 male and 17 female volunteers made up of staff members of the Mental Health Research Institute with no previous history of psychiatric illness. There was no significant difference in the mean age of the schizophrenic subjects versus the control subjects (schizophrenic subjects: mean age = 35 ± 10; control subjects: mean age = 30 ± 7), nor a significant difference in apoD concentrations between male (286 ± 10.3) and female subjects (271 ± 16.0).
Membrane Preparation.
Membrane homogenates were prepared from various regions of normal human brain, including Brodmann's area 9 (BA9), BA10, CA1, CA3, dentate gyrus, subiculum, parahippocampal gyrus, caudate, and substantia nigra, by homogenization in Tris buffer (20 mM Tris⋅HCl/0.2 mM EGTA/0.1 mM EDTA, pH 7.4) including 3× “complete” protein inhibitor tablets (Roche Molecular Biochemicals). Membrane homogenates were also prepared in the same manner for BA9, BA18, caudate, hippocampus, substantia nigra, and cerebellum from control and schizophrenic subjects, and BA9, BA18, and caudate from bipolar subjects.
Protein Separation and Western Blot Analysis.
Aliquots of the membrane homogenates (50 μg of total protein per lane) were subjected to SDS/PAGE using a 12% acrylamide gel. The gels were transferred to nitrocellulose membranes, blocked with 5% milk in T-TBS (Tris-buffered saline/0.1% Tween-20, pH 7.5), and then probed with a monoclonal antibody directed against human apoD (1:500 dilution; NovoCastra, Newcastle, U.K.). Enhanced chemiluminescence (ECL from Amersham Pharmacia) was used to detect immunoreactivity and blots were visualized by exposure to autoradiography film.
ELISA.
ApoD was quantified in membrane homogenates prepared above and serum using a modified ELISA. Two monoclonal antibodies to apoD from Signet Laboratories (Dedham, MA) were used in a sandwich assay: a coating antibody, which recognized a major apoD immunoreactive band of ≈31 kDa in serum and two minor bands of ≈22 and 46 kDa, and an HRP-conjugated apoD antibody. Microtitre high-capacity binding plates (Costar) were coated with 50 μl of a 4.7 μg/ml apoD antibody for 1–2 h at room temperature. The wells were washed four times with T-TBS, blocked with 5% BSA in T-TBS for 1 h at room temperature and then washed again four times with T-TBS. An aliquot (50 μl) of the various tissue homogenates (50 μg of total protein) or serum samples (1:750 dilution) was added and incubated for 1 h at room temperature. The wells were washed four times with T-TBS, and then 50 μl of a second, HRP-conjugated, apoD antibody was added to each well and incubated 1 h at room temperature. After extensive washing with T-TBS, 50 μl of TMB substrate system (Sigma) was added to allow color formation. The reaction was quenched with 0.2 N HCl (50 μl) and absorbance was read at 450 nM. Purified apoD (kindly provided by D. A. Haagensen, Mercy Healthcare, Sacramento, CA) was used as a standard in all assays.
Statistical Analysis.
The ELISA data were subjected to two-way ANOVA to discern significant differences among brain regions from the same cohort of subjects, and then student's t test (two-tailed) was used to determine exact P values. A Pearson Product Moment correlation analysis of experimental data and demographic [age and duration of illness (DOI)], treatment related (final recorded drug dose), and tissue-related data (pH) were carried out by using an assumed straight-line curve fit. All statistical analyses (student's t test, two-way ANOVA, and linear regression analysis) were carried out by using prism computer software.
Results
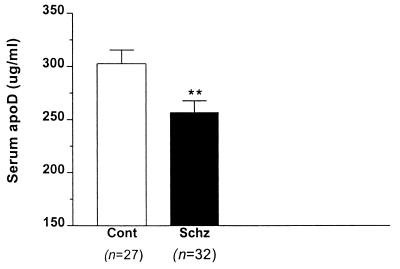
Our previous studies in rodents demonstrated an increase in apoD expression in response to clozapine, suggesting that apoD itself, or some aspect of lipid metabolism for which apoD serves as a reporter, may be associated with the mechanisms of clozapine action. Therefore, we hypothesized that apoD levels might be low in patients with schizophrenia and that elevated levels of apoD, such as those caused by clozapine, would be beneficial for patients. By using ELISA with two different antibodies to human apoD, we quantified apoD concentrations in serum samples from normal subjects and patients with schizophrenia. A significant decrease in the concentration of apoD was observed in schizophrenic patients relative to normal subjects (256 μg/ml ± 11 vs. 303 μg/ml ± 12; P = 0.0084) (Fig. 1). We detected no significant differences in apoD levels among those patients receiving typical neuroleptic drugs, clozapine, or those that were deemed neuroleptic-free, nor did we find any correlation between apoD levels and age in these subjects (r2 = 0.0071).
Figure 1.
Serum apoD levels in control subjects and schizophrenic patients. ApoD concentrations were measured with ELISA, using purified apoD as a standard. The number of subjects in each group is indicated in parentheses. Asterisks denote a significant decrease, P = 0.0083.
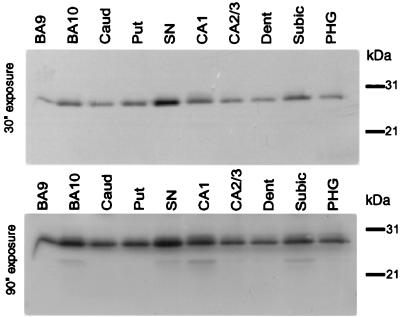
We next examined apoD distribution in the brain of a normal individual. Western immunoblotting of various brain regions, including prefrontal cortex (BA9 and BA10), components of the hippocampal formation (CA1, CA3, dentate gyrus, subiculum, parahippocampal gyrus) and basal ganglia (caudate and substantia nigra) with an apoD antibody revealed an immunoreactive band of ≈29 kDa in all regions examined (Fig. 2). An additional, less prominent band of ≈22 kDa was observed in a few brain regions, primarily BA10, substantia nigra, CA1, and subiculum. Molecular variation of apoD has been reported in human plasma and regenerating rat sciatic nerve (4, 11, 12) and likely reflects different glycosylation states of the protein.
Figure 2.
Western blot analysis showing distribution of apoD isoforms in human brain. Western blots containing 50 μg of total protein per lane were probed with a monoclonal antibody directed against human apoD. Enhanced chemiluminescence (ECL) was used to detect immunoreactivity and blots were visualized by exposure to autoradiography film. (A) 30 s exposure; (B) 90 s exposure. Caud, caudate; Put, putamen; Dent, dentate gyrus; Subic, subiculum; SN, substantia nigra; PHG, parahippocampal gyrus.
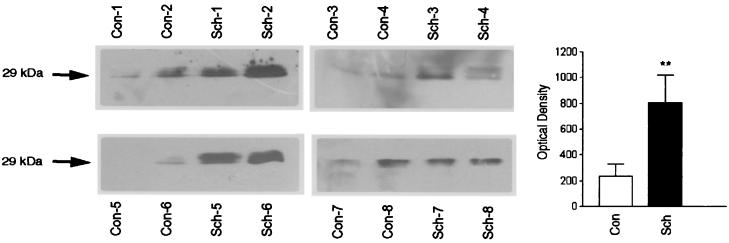
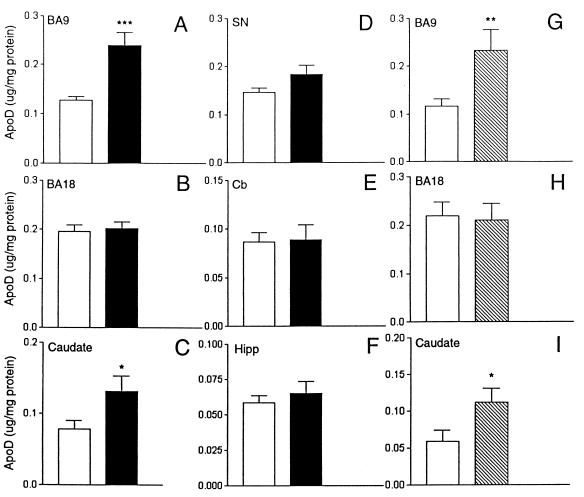
We then focused our analysis of apoD expression to the dorsolateral prefrontal cortex, BA9, a region previously implicated in the pathophysiology of schizophrenia (for a review, see ref. 13). ApoD immunoreactivity was measured in BA9 extracts prepared from eight control and eight schizophrenic subjects by using Western blot analysis followed by quantification by densitometric analysis. ApoD levels were significantly increased in schizophrenic subjects [802.5 ± 217 optical density (OD) units; P = 0.0232] compared with age- and sex-matched controls (207 ± 85.0 OD units) (Fig. 3). In addition, we used an ELISA to quantify apoD levels in BA9 regions from the same and additional subjects. A significant increase was detected in the BA9 from schizophrenic patients (0.244 ± 0.027 μg/mg protein; n = 18; P = 0.0002) vs. controls (0.127 ± 0.008 μg/mg protein; n = 19) (Fig. 4A and Table 3). Elevated levels of apoD levels were also observed in the caudate of schizophrenic subjects (0.132 ± 0.021 μg/mg protein; n = 18) vs. control (0.078 ± 0.011 μg/mg protein; n = 18; P = 0.045) (Fig. 4C and Table 3). Similar measurements were performed on membrane preparations from the occipital cortex (BA18), substantia nigra, cerebellum, and hippocampus and no differences in apoD levels were detected between control and schizophrenic subjects (Fig. 4 B and D–F and Table 3).
Figure 3.
Western blot analysis demonstrating apoD expression in dorsolateral prefrontal cortex of control (Con) and schizophrenic (Sch) subjects. Western blots containing 50 μg of total protein per lane were probed with a monoclonal antibody directed against human apoD. Eight schizophrenic subjects (Sch-1–Sch-8) are shown with their age- and sex-matched controls (Con-1–Con-8). Only the regions of the blots corresponding to the 29-kDa apoD immunoreactive species are shown.
Figure 4.
ApoD levels in dorsolateral prefrontal cortex (BA9; A and G), occipital cortex (BA18; B and H), caudate (C and I), substantia nigra (SN; D), cerebellum (Cb; E), and hippocampus (Hipp; F) of control (open bars), schizophrenic (solid bars), and bipolar (stippled bars) subjects. ApoD concentrations were measured by ELISA using purified apoD as a standard. Significant differences are indicated by asterisks as determined by student's t test (two-tailed). ***, P = 0.0002; **, P = 0.02; *, P = 0.04.
Table 3.
ApoD protein levels in various brain regions from normal, schizophrenic, and bipolar subjects
| Brain region | ApoD, μg/mg protein | |
|---|---|---|
| Control | Schizophrenic | |
| DLPFC | 0.127 ± 0.008 (n = 19) | 0.244 ± 0.027 (n = 20)*** |
| OC | 0.196 ± 0.013 (n = 18) | 0.201 ± 0.014 (n = 19) |
| Caudate | 0.078 ± 0.011 (n = 18) | 0.132 ± 0.021 (n = 20)* |
| SN | 0.146 ± 0.008 (n = 17) | 0.183 ± 0.019 (n = 17) |
| Hipp | 0.059 ± 0.005 (n = 17) | 0.069 ± 0.008 (n = 14) |
| Cb | 0.086 ± 0.009 (n = 18) | 0.088 ± 0.015 (n = 18) |
| Control | Bipolar | |
| DLPFC | 0.115 ± 0.015 (n = 8) | 0.233 ± 0.043 (n = 8)** |
| OC | 0.220 ± 0.028 (n = 9) | 0.205 ± 0.034 (n = 8) |
| Caudate | 0.059 ± 0.015 (n = 8) | 0.112 ± 0.018 (n = 8)* |
ApoD concentrations were measured by ELISA using purified apoD as a standard. DLPFC, dorsolateral prefrontal cortex; OC, occipital cortex; SN, substantia nigra; Hipp, hippocampus; Cb, cerebellum. Values are mean concentration ± SEM. Significant differences are indicated by asterisks (student's t test; two-tailed). ***, P = 0.0002; **, P = 0.02; *, P = 0.04.
To test for disease specificity, we measured apoD expression levels in prefrontal and occipital cortices and caudate from patients diagnosed with bipolar disease and from a subset of the control subjects. By using ELISA, an increase in apoD concentration, similar in magnitude to that observed in schizophrenic patients, was detected in the BA9 region of the bipolar patients (bipolar, 0.233 ± 0.043 μg/mg protein; n = 8; P = 0.0424 vs. control, 0.115 ± 0.015 μg/mg protein; n = 8) (Fig. 4G and Table 3). The apoD levels in the occipital cortex were not significantly different between control and bipolar subjects (0.220 ± 0.028 μg/mg protein; n = 9, vs. 0.205 ± 0.034 μg/mg protein; n = 8) (Fig. 4H and Table 3). However, a significant increase in apoD expression was observed in the caudate of bipolar subjects (0.112 ± 0.018 μg/mg protein; n = 8; P = 0.0218) vs. control subjects (0.059 ± 0.015 μg/mg protein; n = 8) (Fig. 4I and Table 3). In no cases were there any significant correlations between levels of apoD with age, final recorded drug dose, postmortem interval, or brain pH.
Discussion
In this study, we have detected a decrease in the serum levels of apoD in schizophrenic patients, which we suggest reflects a systemic deficiency in lipid pathways associated with apoD. This is in agreement with numerous studies that have reported alterations in total membrane phospholipid content, fatty acid content, and cholesteryl esters in membranes from erythrocytes, red blood cells, and fibroblasts and in frontal cortex of schizophrenic patients (for a review, see ref. 14). Decreased concentrations of essential fatty acids, especially AA, in schizophrenic patients have been replicated in several studies. For example, low levels of AA-enriched phospholipids have been observed in cultured fibroblasts in chronic and first-episode schizophrenic patients (15, 16). Studies have also demonstrated a marked depletion of AA in red blood cells and an abnormal incorporation/esterification of AA into platelet membranes of patients with schizophrenia (17–19). These alterations in fatty acid concentrations are consistent with the increased levels of PLA2 activity detected in the serum and cortex of schizophrenic patients (20, 21). It has also been suggested that a defect in the transport of dietary fatty acids is associated with the pathophysiology of schizophrenia (22). In light of these studies and the reported ability of apoD to bind AA, the decreased serum apoD levels observed in this study might result from preexisting AA and/or phospholipid deficiencies.
Our measurements in the CNS revealed a significant 1.9- to 3.9-fold increase in apoD expression in the dorsolateral prefrontal cortex (DLPFC; BA9) in schizophrenic subjects versus sex- and age-matched controls. Numerous experimental and clinical studies have provided evidence of pathophysiological changes in the prefrontal cortex of patients with schizophrenia. Studies using neuroimaging techniques have demonstrated decreased blood flow activation and metabolism in prefrontal cortex of schizophrenic patients, especially during behavioral tasks (23–27). Neuropsychological and neurophysiological observations of schizophrenic patients have also revealed impairments in cognitive tasks and working memory skills, behavioral processes that require intact prefrontal functioning (24, 28). We did not observe differences in apoD expression in the occipital cortex (BA18), substantia nigra, cerebellum, or hippocampus, indicating regional specificity for apoD expression induction. The increases in apoD levels observed in the dorsolateral prefrontal cortex (DLPFC) and caudate of bipolar subjects indicate that increased apoD accumulation is not specific to schizophrenia. However, components of bipolar disorder have also been associated with abnormal functioning of the prefrontal cortex (29–31). The increases observed in the caudate are also consistent with studies implicating basal ganglia structures in the pathophysiology of psychiatric disorders (32). We did not observe a correlation between apoD levels and age in the serum (r2 = 0.0071) or BA9 (r2 = 0.119) samples. These findings are consistent with Terrisse and coworkers (33), who did not find a correlation between cerebrospinal fluid (CSF) apoD levels and age, but in contrast to Kalman and coworkers (34), who reported an increase in apoD immunoreactivity in cortical astrocytes in aged vs. young human subjects.
As samples from never-medicated patients are difficult to acquire, most of the schizophrenic and bipolar subjects in this study had been treated with typical neuroleptic drugs (haloperidol, fluphenazine, thioridazine, or chlorpromazine) before death. The elevated apoD CNS levels detected in this study are, therefore, seemingly consistent with our previous studies, which demonstrated apoD levels were elevated in the rodent brain after clozapine administration (3). However, several arguments suggest that these changes are not simply due to drug treatment before death. First, we did not observe a correlation between apoD levels and antipsychotic drug dose (chlorpromazine equivalents) in these subjects (r2 = 0.045); nor did we observe a correlation between apoD levels and duration of illness (DOI; r2 = 0.164). DOI may be considered an indication of how long subjects have been exposed to neuroleptic drugs. Secondly, our previous studies in rodents did not detect significant increases in apoD expression in response to the typical neuroleptic, haloperidol (3), and most of the subjects in this study were treated with typical neuroleptic drugs (six specifically treated with haloperidol). Finally, there has recently been a large body of literature describing apoD induction under various other neuropathological conditions. For example, apoD levels have been shown to be elevated in brains of patients with other neurological disorders (such as Alzheimer's disease, cerebrovascular disease, motoneuron diseases, and meningoencephalitis), and presumably these patients had not been exposed to neuroleptic drugs (33). Thus, on balance, currently available data would argue against the changes in apoD reported in this study simply being an effect of antipsychotic drugs.
The functional role for apoD in the CNS and in human neurological disease remains unclear. Several studies in rodents have implicated apoD in neuronal degeneration after CNS injury. For example, increased apoD immunoreactivity and mRNA levels have been observed in glial cells and neurons of the hippocampus and/or cortex after kainic acid lesioning and traumatic brain injury, two experimental insults that result in massive excitotoxic damage (35–37). In addition, apoD mRNA and protein levels were found to be elevated in the cerebellum of a mouse strain considered to be a model of Niemann–Pick disease, a human condition that is characterized by abnormal lysosomal cholesterol storage and chronic progressive neurodegeneration (38, 39). Studies have also supported a neuroprotective role for apoD in the nervous system. A 42% increase in apoD mRNA levels and up to 16-fold increases in protein expression have been observed in the hippocampus after entorhinal cortex lesioning (40), which results in reactive synaptogenesis and compensatory glial functions. And after peripheral nerve injury, apoD was found to be up-regulated in sites undergoing regeneration (12). Given its hypothesized role as a lipid-binding protein, apoD may be involved in the binding of steroids or fatty acids released upon CNS insult, or the transport of lipid molecules necessary for cellular regeneration, and therefore may function in CNS maintenance and tissue repair.
It has also been hypothesized that apoD is a marker for neuropathology associated with human neurological disease. Previous studies have demonstrated increases in apoD immunoreactivity in the cerebrospinal fluid (CSF) (300%) and in the hippocampus (60–350%) of Alzheimer's patients, and in the CSF of patients with other neurological diseases, including cerebrovascular disease, motoneuron diseases and, meningoencephalitis (33). Separate studies have observed apoD increases in scattered cortical astrocytes in subjects with Alzheimer's dementia relative to aged controls, but did not detect quantitative differences in cortical gray matter regions (34), as we have observed in the present study in the prefrontal cortex of schizophrenics and bipolar subjects. The lack of apoD induction observed in the hippocampus of schizophrenic subjects is in contrast to that seen in Alzheimer's subjects, indicating regional specificity for the changes in apoD expression.
Because apoD expression is elevated under apparently diverse conditions, it is possible that apoD expression represents a nonspecific response to stress or pathological insult. However, given the distinct sites of apoD up-regulation observed after CNS insult in the rodent studies and the regional specificity of apoD induction observed in human disease and mouse models (Niemann–Pick), rather, it is possible that apoD is a region-specific marker for a pathological process. Our previous findings that clozapine induced apoD accumulation in rodent brains had suggested the simple hypothesis that increases apoD may be beneficial to patients with neuropsychiatric disorders. The present findings suggest that apoD accumulation might be a natural response to regional neuropathology, and that one reason clozapine is an effective antipsychotic drug is its ability to augment increases in apoD already present in the brain.
Changes in apoD expression in the CNS could have potentially diverse consequences that could account for the wide variety of molecules found at abnormal levels in patients with schizophrenia and other psychiatric disorders. ApoD has been shown to specifically bind the fatty acid AA, which, together with docosahexaenoic acid, makes up >90% of the polyunsaturated fatty acid content in the CNS (41), and is also thought to play a role in the transport of cholesterol, which makes up 25% of gray and white matter (42). Both of these are major components of the lipid bilayer of cellular membranes. Variation in composition and hydrocarbon chain saturation state determine membrane order and fluidity, and these properties affect the binding and function of extrinsic membrane proteins and second messenger signaling. Hence, changes in the levels of apoD can potentially affect membrane phospholipid composition by increasing or decreasing transport and uptake of these membrane constituents. Phospholipids play a critical role in almost every function of the cell membrane and its metabolic products are crucial for cellular functions and cell-to-cell communication. In the prefrontal cortex, a site of increased apoD expression, numerous reports have demonstrated increases and/or decreases in neurotransmitter receptors, ion channels, and membrane-bound proteins in subjects with schizophrenia (for a review, see ref. 32). Alterations in membrane phospholipids and consequential effects on neural cell membranes would also have profound effect on brain development and maturation. Considerable evidence indicates that dysfunction during neurodevelopment contributes to pathogenesis of schizophrenia (43, 44), and specific proteins associated with development processes, reelin, and GAP-43 have been found at abnormal levels in schizophrenic and bipolar subjects (45–47). By means of binding to AA, apoD could also affect developmental processes. AA acts as a second messenger in several neurotransmitter systems, including the action of basic fibroblast growth factors that are critical for normal brain development. Synaptic organization would also dependent on the integrity of the membrane structure. Recent studies have demonstrated increases in various presynaptic proteins (48) and synapsin and synaptophysin, two synaptic vesicle-associated proteins (49, 50), in cerebral cortex of schizophrenic subjects.
In summary, we have shown that apoD levels are low in the serum of schizophrenic subjects, but elevated in the dorsolateral prefrontal cortex (DLPFC) and caudate of schizophrenic and bipolar subjects. Although the specific functions of apoD in the CNS and in psychiatric illnesses remain unclear, we suggest that apoD may be a compensatory region-specific marker for a neuropathological process that is initiated because of systemic lipid metabolism insufficiencies.
Acknowledgments
We thank Robyn Bradbury for assistance in tissue preparations, Andrew McLaren for preparation of serum samples, and Nicholas Keks, Christine Hill, and David Copolov for case-history review and subsequent diagnosis of the schizophrenic and bipolar subjects. We also thank Floyd Bloom for helpful comments and suggestions on the manuscript. This work was supported in part by National Institutes of Health Grant GM32355 and Digital Gene Technologies.
Abbreviations
- apoD
apolipoprotein D
- BA
Brodmann's area
- AA
arachidonic acid
- CNS
central nervous system
References
- 1.Berrettini W H. Biol Psychol. 2000;48:531–538. doi: 10.1016/s0006-3223(00)00883-0. [DOI] [PubMed] [Google Scholar]
- 2.Ciapparelli A, Dell'Osso L, Pini S, Chiavacci M C, Fenzi M, Cassano G B. J Clin Psychiatry. 2000;61:329–334. doi: 10.4088/jcp.v61n0502. [DOI] [PubMed] [Google Scholar]
- 3.Thomas E A, Danielson P E, Nelson P A, Pribyl T M, Hilbush B S, Hasel K W, Sutcliffe J G. J Neurochem. 2001;76:789–797. doi: 10.1046/j.1471-4159.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- 4.McConathy W J, Alaupovic P. FEBS Lett. 1973;37:178–182. doi: 10.1016/0014-5793(73)80453-3. [DOI] [PubMed] [Google Scholar]
- 5.Drayna D, Fielding C, McLean J, Baer B, Castro G, Chen E, Comstock L, Henzel W, Kohr W, Rhee L. J Biol Chem. 1986;261:16535–16539. [PubMed] [Google Scholar]
- 6.Dilley W G, Haagensen D E, Cox C E, Wells S A. Breast Cancer Res Treat. 1990;16:253–260. doi: 10.1007/BF01806333. [DOI] [PubMed] [Google Scholar]
- 7.Lea O A. Steroids. 1988;52:337–338. doi: 10.1016/0039-128x(88)90135-3. [DOI] [PubMed] [Google Scholar]
- 8.Morais-Cabral J H, Atkins G L, Sanchez L M, Lopez-Boado Y S, Lopez-Otin C, Sawyer L. FEBS Lett. 1995;366:53–56. doi: 10.1016/0014-5793(95)00484-q. [DOI] [PubMed] [Google Scholar]
- 9.Association A P. Diagnostic and Statistical Manual of Mental Disorders. Washington, D.C.: Am. Psychiatr. Assoc.; 1994. [Google Scholar]
- 10.Amaral D G, Insausti R. Hippocampal Formation. New York: Academic; 1990. [Google Scholar]
- 11.Kamboh M I, Albers J J, Majumder P P, Ferrel R E. Am J Hum Genet. 1989;45:147–154. [PMC free article] [PubMed] [Google Scholar]
- 12.Boyles J K, Notterpek L M, Anderson L J. J Biol Chem. 1990;265:17805–17815. [PubMed] [Google Scholar]
- 13.Goldman-Rakic P S, Seleman L D. Schizophr Bull. 1997;23:437–458. doi: 10.1093/schbul/23.3.437. [DOI] [PubMed] [Google Scholar]
- 14.Horrobin D F, Bennet C N. Prostaglandins Leukotrienes Essent Fatty Acids. 1999;60:141–167. doi: 10.1054/plef.1999.0027. [DOI] [PubMed] [Google Scholar]
- 15.Mahadik S P, Mukherjee S, Correnti E E, Kelkar H S, Wakade C G, Costa R M, Scheffer R. Schizophr Res. 1994;13:239–247. doi: 10.1016/0920-9964(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 16.Mahadik S P, Mukherjee S, Horrobin D F, Jenkins K, Correnti E E, Scheffer R. Psychiatry Res. 1996;63:133–142. doi: 10.1016/0165-1781(96)02899-5. [DOI] [PubMed] [Google Scholar]
- 17.Peet M, Laugharne J D, Mellor J, Ramchand C N. Prostaglandins Leukotrienes Essent Fatty Acids. 1996;55:71–75. doi: 10.1016/s0952-3278(96)90148-9. [DOI] [PubMed] [Google Scholar]
- 18.Demisch L, Heinz K, Gerbaldo H, Kirsten R. Prostaglandins Leukotrienes Essent Fatty Acids. 1992;46:47–52. doi: 10.1016/0952-3278(92)90058-q. [DOI] [PubMed] [Google Scholar]
- 19.Yao J K, van Kammen D P, Gurklis J A. Psychiatry Res. 1996;60:11–21. doi: 10.1016/0165-1781(95)02832-3. [DOI] [PubMed] [Google Scholar]
- 20.Ross B M, Turenne S, Moszczynska A, Warsh J J, Kish S J. Brain Res. 1999;821:407–413. doi: 10.1016/s0006-8993(99)01123-3. [DOI] [PubMed] [Google Scholar]
- 21.Gattaz W F, Kollisch M, Thuren T, Virtanen J A, Kinnunen P K J. Biol Psychiatry. 1987;22:421–426. doi: 10.1016/0006-3223(87)90164-8. [DOI] [PubMed] [Google Scholar]
- 22.Glen A I, Glen E M, Horrobin D F, Vaddadi K S, Spellman M, Morse-Fisher N, Ellis K, Skinner F S. Schizophr Res. 1994;12:53–61. doi: 10.1016/0920-9964(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 23.Franzen G, Ingvar D H. J Neurol Neurosurg Psychiatry. 1975;38:1027–1032. doi: 10.1136/jnnp.38.10.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinberger D R, Berman K F, Zec R F. Arch Gen Psychiatry. 1986;43:114–134. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 25.Liddle P F, Friston K J, Frith C D, Hirsch S R, Jones T, Frackowiak R S. Br J Psychiatry. 1992;160:179–186. doi: 10.1192/bjp.160.2.179. [DOI] [PubMed] [Google Scholar]
- 26.Ragland J D, Gur R C, Glahn D C, Censits D M, Smith R J, Lazarev M G, Alavi A, Gur R E. Neuropsychology. 1998;12:399–413. doi: 10.1037//0894-4105.12.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter C S, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen J D. Am J Psychiatry. 1998;155:1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- 28.Liddle P F, Morris D L. Br J Psychiatry. 1991;158:340–345. doi: 10.1192/bjp.158.3.340. [DOI] [PubMed] [Google Scholar]
- 29.Blumberg H P, Stern E, Ricketts S, Martinez D, de Asis J, White T, Epstein J, Isenberg N, McBride P A, Kemperman I, et al. Am J Psychiatry. 1999;156:1986–1988. doi: 10.1176/ajp.156.12.1986. [DOI] [PubMed] [Google Scholar]
- 30.Knable M B. Schizophr Res. 1999;39:149–152. doi: 10.1016/s0920-9964(99)00114-0. [DOI] [PubMed] [Google Scholar]
- 31.Drevets W C, Ongur D, Price J L. Mol Psychiatry. 1998;3:220–226. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- 32.Harrison P J. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 33.Terrisse L, Poirier J, Bertrand P, Merched A, Visvikis S, Siest G, Milne R, Rassart E. J Neurochem. 1998;71:1643–1650. doi: 10.1046/j.1471-4159.1998.71041643.x. [DOI] [PubMed] [Google Scholar]
- 34.Kalman J, McConathy W, Araoz C, Kasa P, Lacko A. Neurol Res. 2000;22:330–336. doi: 10.1080/01616412.2000.11740678. [DOI] [PubMed] [Google Scholar]
- 35.Franz G, Reindl M, Patel S C, Beer R, Unterrichter I, Berger T, Schmutzhard E, Poewe W, Kampfl A. J Neurochem. 1999;73:1615–1625. doi: 10.1046/j.1471-4159.1999.0731615.x. [DOI] [PubMed] [Google Scholar]
- 36.Ong W Y, He Y, Suresh S, Patel S C. Neuroscience. 1997;79:359–367. doi: 10.1016/s0306-4522(96)00608-2. [DOI] [PubMed] [Google Scholar]
- 37.Montpied P, de Bock F, Lerner-Natoli M, Bockaert J, Rondouin G. Epilepsy Res. 1999;35:135–146. doi: 10.1016/s0920-1211(99)00003-0. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida K, Cleaveland E S, Nagle J W, French S, Yaswen L, Ohshima T, Brady R O, Pentchev P G, Kulkarni A B. DNA Cell Biol. 1996;15:873–882. doi: 10.1089/dna.1996.15.873. [DOI] [PubMed] [Google Scholar]
- 39.Suresh S, Yan Z, Patel R C, Patel Y C, Patel S C. J Neurochem. 1998;70:242–251. doi: 10.1046/j.1471-4159.1998.70010242.x. [DOI] [PubMed] [Google Scholar]
- 40.Terrisse L, Sequin D, Bertrand P, Poirier J, Milne R, Rassart E. Brain Res Mol Brain Res. 1999;70:26–35. doi: 10.1016/s0169-328x(99)00123-0. [DOI] [PubMed] [Google Scholar]
- 41.O'Brien J S, Sampson E L. J Lipid Res. 1965;6:537–544. [PubMed] [Google Scholar]
- 42.Snipes G J, Suter U. In: Subcellular Biochemistry. Bittman R, editor. Vol. 28. New York: Plenum; 1997. pp. 173–204. [DOI] [PubMed] [Google Scholar]
- 43.Bloom F E. Arch Gen Psychiatry. 1993;50:224–227. doi: 10.1001/archpsyc.1993.01820150074008. [DOI] [PubMed] [Google Scholar]
- 44.Weinberger D R. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 45.Guidotti A, Auta J, Davis J M, Gerevini V D, Dwivedi Y, Grayson D R, Impagnatiello F, Pandey G, Pesold C, Sharma R, et al. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 46.Impagnatiello F, Guidotti A R, Pesold C, Dwivedi Y, Caruncho H, Pisu M G, Uzunov D P, Smalheiser N R, Davis J M, Pandey G N, et al. Proc Natl Acad Sci USA. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perrone-Bizzozero N I, Sower A C, Bird E D, Benowitz L I, Ivins K J, Neve R L. Proc Natl Acad Sci USA. 1996;93:14182–14187. doi: 10.1073/pnas.93.24.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gabriel S M, Haroutunian V, Powchik P, Honer W G, Davidson M, Davies P, Davis K L. Arch Gen Psychiatry. 1997;54:559–566. doi: 10.1001/archpsyc.1997.01830180077010. [DOI] [PubMed] [Google Scholar]
- 49.Browning M D, Dudek E M, Rapier J L, Leonard S, Freedman R. Biol Psychiatry. 1993;34:529–535. doi: 10.1016/0006-3223(93)90195-j. [DOI] [PubMed] [Google Scholar]
- 50.Honer W G, Falkai P, Chen C, Arango V, Mann J J, Dwork A J. Neuroscience. 1999;91:1247–1255. doi: 10.1016/s0306-4522(98)00679-4. [DOI] [PubMed] [Google Scholar]