Abstract
Nitrogen supply is pivotal for the maintenance of life. Amino acids can be utilized to synthesize both glucose and lipids. The opposite, i.e., production of amino acids from either one of them, is not possible in the absence of other amino acids as donors of nitrogen. The quality of amino acid content in protein has been re-evaluated recently, and the relevance of essential amino acids has been repeatedly underlined. Essential amino acid requirements in different mammals are not identical, and ratios among them should be taken into account when projecting an efficient formulation. Recent research has demonstrated that genes respond to different qualities and quantities of nutritional supply, and increased provision of essential amino acids increases lifespan in animal experiments through mitochondriogenesis and maintenance of elevated rates of synthesis of anti-oxidant molecules. Moreover, genetic expression of key controllers of synthesis, like mTOR, may be particularly important for understanding skeletal muscle maintenance. Losses of muscle mass and impaired immune function are related to reduced protein supply, and there is increasing evidence that regular essential amino acid intake as part of an oral diet is effective in reversing muscle catabolism, promoting muscle anabolism, and restoring immunological function. Therefore, the use of amino acids as supplements to diet would be expanding in the near future. Is this safe? Few data are available on amino acid toxicity, and only one essential amino acid may be considered to have clinically relevant toxicity: methionine, because it is transformed into a toxic intermediate, homocysteine, when cysteine synthesis is required by metabolic needs. Matching of stoichiometric ratios between methionine and cysteine may solve the problem of supplying sufficient amounts of sulfur to the body. Arginine and glutamine are two non-essential amino acids than can become “conditionally essential” because of elevated needs during pathological conditions, and metabolism may not be able to maintain their concentrations at sufficient levels to match metabolic requirements. Chronic exogenous arginine supplementation has not proven to exert positive clinical effects in different trials, and sequential articulation of the knowledge of introduction of arginine-driven transcriptional, translational, and epigenetic adaptations may give us a key for interpreting those puzzling results.
Keywords: Amino acids, Nutrition, Toxicogenomic, Muscle, Definition of life
Introduction
In 1944, Schrödinger proposed that life could be based on the laws of physics [1]. Many attempts to define life have been proposed since then. All attempts, however, received major criticism, but at least, there is general agreement on three characteristics typical for all living systems: self-reproduction (permitting preservation of information), mutation (permitting evolution), and metabolism (permitting selection of a system for a certain function) [2].
From the rough point of view of the researcher on the role of amino acids (AA) in human metabolism, life is the huge energetic cost of maintaining concentrated amounts of atoms, mostly carbon, oxygen, hydrogen, and nitrogen. These atoms are enclosed in the limited environment that we identify as cells, tissues, organs, and organisms. Life sciences are the study of the complex organization that allows maintenance of continuous production of this energy. In mammals, energy is derived mostly from breaking down carbon–carbon bonds present in some nutrients. Such nutrients need to be introduced in adequately large amounts, and thus are identified as macro-nutrients (from ancient greek—macro = big, large). Organic molecules, particularly AAs, were present on earth long before life developed [3], and life eventually became possible by the adaptation of activities and functions according to nutritional opportunities available in the environment. In contrast to other organisms such as plants, mammals do not possess the genetic capability to utilize inorganic nitrogen for the maintenance of life. Thus, we depend on constant nitrogen supply for growth, development, and survival [4].
Skeletal muscles as an amino acids reservoir
Therefore, conditions that are accompanied by a dysbalance between supply and requirements ultimately yield depletion of the main AA reservoir in the body, skeletal muscle [5, 6].
Sarcopenia, wasting, and cachexia are the clinical demonstration that AAs are important substrates to the first priority of life—production of energy. Adequate nutrition, on the other hand, could provide sufficient amounts of carbohydrates and lipids to spare excessive degradation of AAs for energy metabolism, and providing enough essential amino acids (EAA) to promote and maintain synthesis [7]. In this context, the relevance of the concept that only EAAs promote and maintain muscle synthesis [8] cannot be stressed enough with regards to the fullness of its implications [9]. EAA availability controls the balance between synthesis and degradation of human muscle, EAA availability is the limiting factor for maintaining synthesis of new proteins. The ratio among degradation and synthesis is fundamental for maintaining efficient protein-based activities and functions, and changing the dimensions of AA supply deeply alters this balance [10].
The role of essential amino acids in muscle metabolism
Concentrations of AAs in human cells are regulated by the expression of AA transporters, which are controlled and upregulated by increasing EAA availability [11]. Availability of all the EAAs in adequate stoichiometric ratios is not the only limiting factor for protein synthesis, but EAAs are also signaling molecules and gene expression modulators. Thus, prolonged supplementation of EAAs increases basal and post-insulin stimulation of key signals of the insulin/AKT/mTOR pathway, and is prompted to re-establish responsiveness to post insulin IRS1 degradation in muscles of aging rats [12]. Another feature accompanying aging, loss of mitochondria, is reversed by EAA supplementation through activation of Sirt-1 dependent mitochondrial-biogenesis [13]. This feature is paralleled by improved oxygen supply by restoration of expression of endothelial nitric oxide synthase in key tissues such as the kidneys [14]. As a consequence of these effects, chronic EAA supplementation increased lifespan when compared to ad libitum normal diet or calorie restriction [13].
In humans, different studies have shown that EAAs are efficient in promoting protein synthesis independent of age [15] and in reducing muscular catabolism even in prolonged bed rest in the elderly [16]. Dietary EAA supplementation was also successful in other pathological conditions characterized by the presence of a sarcopenia-wasting-cachexia syndrome, like in chronic obstructive pulmonary disease, in which supplementation of EAAs improved protein synthesis, physical strength, and arterial pO2 [17].
The knot of calculating nitrogen needs by urea synthesis
It is important to discuss potential mistakes that may originate from calculating nitrogen needs independent of EAAs requirements [7, 18]. Calculation of nitrogen balance provides the gold standard for the evaluation of matching nitrogen demand and supply [19]. It is widely accepted to calculate nitrogen balance by using urea synthesis and analyzing the relationship between oral protein intake and urea excretion in the urine. In that context, nitrogen (N) balance (B) is estimated according to the formula:
Protein intake/6.5 expresses the protein nitrogen content in grams, and 4 is the conventional sum of nitrogen losses due to incomplete nitrogen digestion. Such losses include fecal losses and daily nitrogen losses through other sources (i.e., hair, nails, skin desquamation, sweat, etc.). Positive balance means nitrogen retention, indicating that protein intake has been sufficient to provide more nitrogen than is lost. Negative balance means that protein intake does not match the needs, and catabolism of muscle proteins may become prevalent. Therefore, evaluation of nitrogen balance is mainly based on dynamics of urea synthesis. But, although nitrogen balance remains a pivotal clinical tool, a lot of knowledge has accumulated over the past decades about the genetic control of arginase expression, the enzyme that synthesizes urea, although dynamics of urea synthesis have not been critically revised. Arginase exists in two isoenzymes (or isoforms), whose expression is controlled by different genes located on different chromosomes. Arginase I is a cytosolic enzyme and comprises about 98% of the high arginase activity present in liver, but it accounts for only 50% of arginase activity in the kidneys and other organs. In these organs, the remaining arginase activity is represented by the mitochondrial isozyme arginase, arginase II. Both isoforms are quite ubiquitous, although expressed in different ratios in different cell types [20]. Arginase I is also present in red blood cells and in plasma, but the role of red blood cell arginases in degrading arginine and thus regulating availability of arginine for nitric oxide (NO) synthesis has never been investigated in much detail. What is really important is that genetic expression and the level of activity of arginases is induced by arginine concentrations, and thus the rate of exogenous arginine supply determines arginase expression and the rate of urea synthesis and arginine plasma levels [21]. These facts imply that the same nutritional nitrogen supply may still yield different arginase expression, dependent on the amounts of arginine provided by food. Consequently, a more or less prominent activity on arginine catabolism and urea production are induced according to arginine content.
This is a quite disturbing point, because Allison identified the reference protein ovalbumin in 1956, suitable to provide humans with adequate amounts of EAAs, and all enteral or parenteral nutritional formulations have ever since used stoichiometric ratios of AAs similar to those contained in the reference protein. In fact, Allison was very foresighted, and he also underlined the fact that ovalbumin had a content of non-essential AAs (like arginine) that are rather high, but probably necessary mostly to spare EAAs for essential organs by oviparous. Indeed, non-essential AAs could be used for less important structures such as feathers [22].
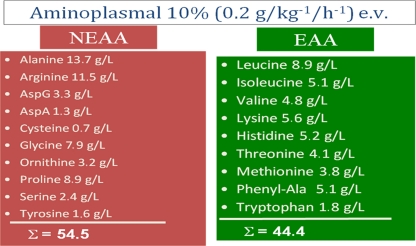
Moreover, EAAs are not necessary all in the same amount. As an example, the daily requirements for leucine and those for tryptophan are not the same. Therefore, it could be calculated that only five EAAs match 70% of total nitrogen EAA requirements: leucine, isoleucine, valine, histidine, and lysine [23]. In different studies dealing with protein or AA effects, a formulation of AA like that presented in Fig. 1 was used to perform protocols evaluating possible diabetogenic effects of amino acids [24]. This formulation mimicked ovalbumin AA content. If we calculate the number of molecules contained in the molecular weight of any AA provided by that formulation (i.e., the stoichiometric ratio) for just three non-essential AAs like arginine, alanine and glutamine as well as the number of molecules of those five EAAs that cover 70% of human needs, we have the dramatic sensation that we are providing enormous numbers of molecules that are not suitable for promoting protein syntheses [8]. However, all of them directly or indirectly (e.g., by being used for energy) contribute first to arginine then to urea synthesis [25]. Arginine is also implicated in the development of insulin resistance by nitrogen introduction [26, 27].
Fig. 1.
Typical formulation of amino acids used in different clinical studies, formulation inspired to ovalbumin amino acids content. Proportions based on 100 mg, L-forms. This formulation contains both essential (EAA) and non-essential amino acids (NEAA). There is prevalence of non-essential amino acids on essential ones. But if the weight would be transformed in number of molecules, the content in just two non-essential amino acids, alanine, and arginine (alanine, 230 mmol; arginine, 66 mmol; sum, 296 mmol) is enormously superior to those of the five essential amino acids that are required to cover 70% of human nitrogen needs (leucine, 56 mmol; isoleucine, 39 mmol; valine, 50 mmol; histidine, 33.5 mmol; lysine, 38 mmol; sum, 219.5 mmol). Alanine and arginine have significant interferences with glucose oxidative metabolism
In patients with chronic kidney disease, EAA supplementation proved to be more effective than ovalbumin-similar formulations in promoting synthesis of visceral proteins without altering urea production [28]. Similar findings were observed in diabetic patients affected by chronic heart failure [29, 30], and in intensive care unit patients after neurosurgery [31]. Therefore, we should always ask ourselves, if increases in urea excretion are a sign of an ameliorated nitrogen balance, or if such increases simply mirror an inadequate EAA availability as part of excessive non-essential AA supply, i.e., the failure of supply to match requirements and needs.
In my opinion, dynamical evaluation of changes in synthesis by repeated monitoring of plasma concentrations of visceral proteins (albumin, transferrin, prealbumin, or rethinol-binding protein) would be the best clinical parameter for general practitioners to evaluate their patients. A prerequisite is that the liver has a sufficiently large working cell mass. All patients with albumin plasma levels below normal should be considered at risk of protein malnutrition independent of any other medical condition. Such finding should prompt further clinical investigation. Albumin has a long half-life (about 3 weeks), but other proteins like transferrin (half-life about 1 week) or prealbumin (better identified as trans-thyretin, or TTR—half-life of about 48 h) or rethinol-binding protein (RBP—half-life of about 12 h) can be used to monitor more accurately and at shorter intervals success or failure of treatment options. In any case, any patient with plasma albumin levels of 3.2 g/L should be considered severely malnourished and possibly cachectic and treated aggressively. Indeed, low plasma albumin levels are predictors of poor prognosis in different pathological conditions [32, 33]. The only limit of the use of those parameters in evaluating malnutrition is that the integrity of liver function is conditioning for syntheses.
Toxicity of amino acids
There are very few papers dealing with upper limits of safe AA supply or toxicity of AAs in healthy humans [34, 35] Most, if not all, deal with alterations connected with single AAs [36, 37]. A PubMed search under “amino acids safe upper level of intake” yielded just 12 papers. In my opinion, methionine is the only EAA that can be considered toxic. Experimental studies dating back to 1971 identified methionine as the “most toxic” AA. Indeed, when methionine was added to diet in a ratio of 5%, it produced the largest depression of growth in rats fed 10% casein-rich diets, and when compared to any other AA it was particularly efficient in depressing growth as well as protein, DNA and RNA content in liver of animals receiving the same amount of any of the 18 AAs examined. Interestingly, the L-form was even more toxic than the D-form. Moreover, increasing the percentage of casein fed with diet blunted the toxicity of any other AA. On the contrary, methionine supplementation toxicity was most inefficiently reduced even by increasing intake of proteins up to 50% of total food intake [38]. Most of methionine toxicity is dependent on the intermediate metabolite formed when cysteine and/or cystine have to be synthesized from methionine to match metabolic requirements. This metabolic intermediate, homocysteine (Hcys), may have different toxic effects. A most interesting one is that Hcys seems to control increased expression of β-hydroxy-β-methylglutaryl coenzyme A (HMG-CoA) reductase in arteries walls [39]. Indeed, in conditions associated with chronic hyperhomocysteinemia, atheromatous lesions of carotid arteries are peculiarly frequent [40]. But other possible negative effects have been ascribed to increased oxidative damage [41] and impairment of nitric oxide (NO) production [42]. Increased homocysteinization of proteins in cells may lead to increased atherosclerosis either by eliciting both inflammation and cell death and/or auto-immunoresponse due to anti-N-Hcys-protein antibodies [43].
Sulfur-containing AAs are indispensable for life. Among these, methionine is the only one considered essential. In order to prevent excess Hcys synthesis, two to three molecules of cysteine for any molecule of methionine supplied would be the best way to match bodily requirements and safety aspects [44]. Arginine is one of the non-essential AAs that can become “conditionally essential”, i.e., not synthesized in adequate amounts during highly demanding pathological conditions. It is the indispensable substrate for NO production, but the VINTAGE study showed that exogenous chronic supply was potentially deleterious in patients after myocardial infarction [45]. However, acute administration elicits increased NO production even if endothelial intracellular arginine concentrations are deemed to be largely sufficient to totally saturate NO synthases, a phenomenon known as “the arginine paradox” [46].
Chronic arginine supplementation proved to increase mortality in patients after myocardial infarction; thus, we must answer in the affirmative, but its supplementation is still recommended in different pathological conditions [47]. Should arginine be considered toxic? Although literature about the arginine paradox is immense, very few papers have discussed the possible reasons for its toxicity in specific pathological situations [21]. I believe that this is a classic example of how important toxicogenomic studies may become. Indeed, exogenous arginine supplementation elicits a late (48 h) and progressive increased expression of arginase, the enzyme producing urea and competing with NO synthases for arginine. Thus, the more arginine is supplied, the more is destroyed. In patients with compromised recycling back to arginine of citrulline formed by NO production, as well as in those presenting with insulin resistance and excess beta-oxydation, acute supplementation would elicit improved acute NO production. Chronic supplementation, on the other hand, would progressively impair peripheral NO production. Similar considerations may be valid for glutamine, an AA also “conditionally essential” in sepsis, but exogenous administration has not always proven safe [48]. Also, glutamine toxicogenomics should be evaluated, because exogenous glutamine suppresses glutamino-synthase expression [49]. Therefore, it should be taken into account that patients may become totally dependent on exogenous glutamine supply for maintenance of sufficiently elevated glutamine plasma concentrations in order to maintain leucocyte and immune function [50]. At physiological food concentrations, the largest part of oral glutamine intake is disposed of by enterocytes during absorption and by splanchnic bed at first passage, and mostly used for energy [51]. Thus, we are challenging the hypothesis that a progressive discontinuation of glutamine supplementation should be planned, and correct queuing could be done most safely if EAA would be supplied on a daily basis. Supplementation by EAA should be performed until genetic expression of glutamine synthetic pathway would be restored and adequate glutamine synthesis from EAA and intermediates of TCA would be achieved [52].
Concluding remarks
EAAs are indispensable for the maintenance of life under normal and pathological conditions. Genetic expression modifications induced by increasing supply of EAA suggest beneficial effects of chronically modifying the ratio with non-essential AAs.
Therefore, supplementing diet with EAA is an efficient method to increase efficiency of nitrogen supply and maintaining integrity of the largest reservoir of amino acids, skeletal muscles, while optimizing urea synthesis. The suggestion to the skilled clinician could be to take into account the chance to use a 1–1.5 g/10 kg−1 day−1 supplementation with EAA in any patient with plasma albumin lower than 3.5 g/L−1, because those are the amounts used with success in clinical published studies. Patients not responding to such supplementation within 2–3 weeks or with worsening albumin values, or those presenting with albumin values lower than 3.2 g/L−1 should be referred to the clinical nutritionist's intensive care. For general clinical and prognostic purposes, we have no better method for understanding the sufficient supply of EAAs other than monitoring synthesis of visceral proteins.
Acknowledgments
The author of this manuscript certifies that he complies with the Principles of Ethical Publishing in the Journal of Cachexia, Sarcopenia, and Muscle [53]. The author would like to thank Dr. Stephan von Haehling and Ms. Kathrin Weiss for fruitful discussions and useful suggestions.
Conflict of interest
The author serves as a consultant with fees for Professional Dietetics s.r.l, Milano, Italy.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Schrödinger E. What is life? Cambridge: Cambridge University Press; 1944. [Google Scholar]
- 2.Jortner J. Conditions for the emergence of life on the early Earth: summary and reflections. Phil Trans Biol Sci. 2006;361:1877–1891. doi: 10.1098/rstb.2006.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bar-Nun A, Bar-Nun N, Bauer SH, Sagan C. Shock synthesis of amino acids in simulated primitive environments. Science. 1970;168:470–472. doi: 10.1126/science.168.3930.470. [DOI] [PubMed] [Google Scholar]
- 4.Halac E., Jr Studies of protein reserves. The relation between protein intake and resistance to protein deprivation. Am J Clin Nutr. 1962;11:574–576. doi: 10.1093/ajcn/11.6.574. [DOI] [PubMed] [Google Scholar]
- 5.Forbes EB, Voris L, Bratzler JW, Wainio W. The utilization of energy-producing nutrients and protein as affected by the plane of protein intake. J Nutr. 1938;15:285. [Google Scholar]
- 6.Timmerman K, Volpi E. Amino acid metabolism and regulatory effects in aging. Curr Opin Clin Nutr Metab Care. 2008;11:45–49. doi: 10.1097/MCO.0b013e3282f2a592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Layman DK. Dietary Guidelines should reflect new understandings about adult protein needs. Nutr Metab. 2009;6:12. doi: 10.1186/1743-7075-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–258. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129–133. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinbock HL, Tarver H. Plasma protein.V. The effect of the protein content of the diet on turnover. J Biol Chem. 1954;209:127–132. [PubMed] [Google Scholar]
- 11.Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen B. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E1011–E1018. doi: 10.1152/ajpendo.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasini E, Flati V, Paiardi S, Rizzoni D, Porteri E, Aquilani R, et al. Intracellular molecular effects of insulin resistance in patients with metabolic syndrome. Cardiovasc Diabetol. 2010;9:46. doi: 10.1186/1475-2840-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metabol. 2010;12:362–372. doi: 10.1016/j.cmet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Corsetti G, Stacchiotti A, D' Antona G, Nisoli E, Dioguardi FS, Rezzani R. Supplementation with essential amino acids in middle age maintains the health of rat kidney. Int J Immunopathol Pharmacol. 2010;23:523–533. doi: 10.1177/039463201002300214. [DOI] [PubMed] [Google Scholar]
- 15.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 16.Ferrando AA, Paddon-Jones D, Hays NP, Kortebein P, Ronsen O, Williams RH, et al. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr. 2010;29:18–23. doi: 10.1016/j.clnu.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Dal Negro RW, Aquilani R, Bertacco S, Boschi F, Micheletto C, Tognella S. Comprehensive effects of supplemented essential amino acids in patients with severe COPD and sarcopenia. Monaldi Arch Chest Dis. 2010;73:25–33. doi: 10.4081/monaldi.2010.310. [DOI] [PubMed] [Google Scholar]
- 18.Holt LE., Jr Some problems in dietary amino acid requirements. Am J Clin Nutr. 1968;21:367–375. doi: 10.1093/ajcn/21.5.367. [DOI] [PubMed] [Google Scholar]
- 19.Xi P, Jiang Z, Zheng C, Lin Y, Wu G. Regulation of protein metabolism by glutamine: implications for nutrition and health. Front Biosci. 2011;16:578–597. doi: 10.2741/3707. [DOI] [PubMed] [Google Scholar]
- 20.Groody WW, Argyle C, Kern RM. Dizikes, Spector EB, Strickland AD, Klein D, Cedrebaum SD. Differential expression of the two human arginase genes in hyperargininemia. Enzymatic, pathologic and molecular analysis. J Clin Invest. 1989;83:602–609. doi: 10.1172/JCI113923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dioguardi FS. To give or not to give? Lessons from arginine paradox. J Nutrigen Nutrigenom. 2011. doi:10.1159/000327777. [DOI] [PubMed]
- 22.Allison JB. Optimal nutrition correlated with nitrogen retention. Am J Clin Nutr. 1956;4:662–672. doi: 10.1093/ajcn/4.6.662. [DOI] [PubMed] [Google Scholar]
- 23.Young VR, Bier DM. Amino acid requirements in the adult human: how well do we know them? J Nutr. 1987;117:1484–1487. doi: 10.1093/jn/117.8.1484. [DOI] [PubMed] [Google Scholar]
- 24.Krebs M, Krssak M, Bernroider E, et al. Mechanism of amino acid–induced skeletal muscle insulin resistance in humans. Diabetes. 2002;51:599–605. doi: 10.2337/diabetes.51.3.599. [DOI] [PubMed] [Google Scholar]
- 25.Dioguardi FS. The alanine-arginine connection: a key piece in the puzzle of protein renal toxicity, low-protein diets, and the risk of malnutrition in patients with chronic kidney disease. Nutr Ther & Metab. 2009.27, 1, pp.
- 26.Dioguardi FS. Wasting and the substrate-to-energy controlled pathway: a role for insulin resistance and amino acids. Am J Cardiol. 2004;93:6A–12A. doi: 10.1016/j.amjcard.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 27.De Castro Barbosa T, Lourenço Poyares L, Fabres Machado U, Nunes MT. Chronic oral administration of arginine induces GH gene expression and insulin resistance. Life Sci. 2006;79:1444–1449. doi: 10.1016/j.lfs.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Bolasco P, Caria S, Cupisti A, Secci R, Dioguardi FS. A novel amino acids oral supplementation in hemodialysis patients: a pilot study. Ren Fail. 2011;33:1–5. doi: 10.3109/0886022X.2010.536289. [DOI] [PubMed] [Google Scholar]
- 29.Solerte SB, Gazzaruso C, Bonacasa R, Rondanelli M, Zamboni M, Basso C, et al. Nutritional supplements with oral amino acid mixtures increases whole-body lean mass and insulin sensitivity in elderly subjects with sarcopenia. Am J Cardiol. 2008;101:69E–77E. doi: 10.1016/j.amjcard.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Solerte SB, Fioravanti M, Locatelli E, Bonacasa R, Zamboni M, Basso C, et al. Improvement of blood glucose control and insulin sensitivity during a long-term (60 weeks) randomized study with amino acid dietary supplements in elderly subjects with type 2 diabetes mellitus. Am J Cardiol. 2008;101:82E–88E. doi: 10.1016/j.amjcard.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Laviano A, Aghilone F, Colagiovanni D, Fiandra F, Giambarresi R, Tordiglione P, et al. Metabolic and clinical effects of the supplementation of a functional mixture of amino acids in critically ill patients: a pilot study. Neurocritical Care. 2011;14:44–49. doi: 10.1007/s12028-010-9461-z. [DOI] [PubMed] [Google Scholar]
- 32.Aquilani R, Zuccarelli GC, Dioguardi FS, Baiardi P, Frustaglia A, Rutili C, et al. Effects of oral amino acid supplementation on long-term-care-acquired infections in elderly patients. Arch Gerontol Geriatr. 2010. doi:10.1016/j.archger.2010.09.005. [DOI] [PubMed]
- 33.Famakin B, Weiss P, Hertzberg V, McClellan W, Presley R, Krompf K, et al. Hypoalbuminemia predicts acute stroke mortality: Paul Coverdell Georgia Stroke Registry. J Stroke Cerebrovasc Dis. 2010;19:17–22. doi: 10.1016/j.jstrokecerebrovasdis.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Jn H, Shao A. Expanded approach to tolerable upper intake guidelines for nutrients and bioactive substances. J Nutr. 2008;38:1992S–1995S. doi: 10.1093/jn/138.10.1992S. [DOI] [PubMed] [Google Scholar]
- 35.Renwick AG. Establishing the upper end of the range of adequate and safe intakes for amino acids: a toxicologist's viewpoint. J Nutr. 2004;134:1617S–1624S. doi: 10.1093/jn/134.6.1617S. [DOI] [PubMed] [Google Scholar]
- 36.Benevenga NJ, Steele RD. Adverse effects of excessive consumption of amino acids. Annu Rev Nutr. 1984;4:157–181. doi: 10.1146/annurev.nu.04.070184.001105. [DOI] [PubMed] [Google Scholar]
- 37.Shao A, Hathcock JN. Risk assessment for the amino acids taurine, l-glutamine and l-arginine. Regul Toxicol Pharmacol. 2008;50:376–399. doi: 10.1016/j.yrtph.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Muramatsu K, Odagiri H, Morishita S, Takeuchi H. Effect of excess levels of individual amino acids on growth of rats fed casein diets. J Nutr. 1971;101:1117–1126. doi: 10.1093/jn/101.9.1117. [DOI] [PubMed] [Google Scholar]
- 39.Li H, Lewis A, Brodsky S, Rieger R, Iden C, Goligorsky MS. Homocysteine induces 3-hydroxy-3-methyl-glutaryl coenzyme A (HMGCoA) reductase in vascular endothelial cells. A mechanism for development of atherosclerosis? Circulation. 2002;105:1037–1043. doi: 10.1161/hc0902.104713. [DOI] [PubMed] [Google Scholar]
- 40.Selhub J, Jacques PF, Bostom AG, D'Agostino RB, Wilson PW, Belanger AJ, et al. Association between plasma Homocysteine concentrations and extracranial carotid-artery stenosis. N Engl J Med. 1995;332:286–291. doi: 10.1056/NEJM199502023320502. [DOI] [PubMed] [Google Scholar]
- 41.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338:1042–1050. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 42.Morris SM. Arginine: beyond protein. Am J Clin Nutr. 2006;83:508s–512s. doi: 10.1093/ajcn/83.2.508S. [DOI] [PubMed] [Google Scholar]
- 43.Jakubowski H. Pathophysiological consequences of homocysteine excess. J Nutr. 2006;136:1741S–1749S. doi: 10.1093/jn/136.6.1741S. [DOI] [PubMed] [Google Scholar]
- 44.Di Buono M, Wykes LJ, Ball RO, Pencharz PB. Dietary cysteine reduces the methionine requirement in men. Am J Clin Nutr. 2001;74:761–766. doi: 10.1093/ajcn/74.6.761. [DOI] [PubMed] [Google Scholar]
- 45.Schulman SP, Becker LC, Kass DA, Champion HC, Terrin ML, Forman S, et al. L-Arginine therapy in acute myocardial infarction. The vascular interaction with age in myocardial infarction (VINTAGE MI) randomized trial. JAMA. 2006;295:58–64. doi: 10.1001/jama.295.1.58. [DOI] [PubMed] [Google Scholar]
- 46.Kurz S, Harrison DG. Insulin and the arginine paradox. J Clin Invest. 1997;99:369–370. doi: 10.1172/JCI119166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luiking YC, Poeze M, Dejong CH, Ramsay G, Deutz NE. Sepsis: an arginine deficiency state? Crit Care Med. 2004;32:2135–2145. doi: 10.1097/01.CCM.0000142939.81045.A0. [DOI] [PubMed] [Google Scholar]
- 48.Bracco D. Glutamine: a double edged sword in the intensive care unit? Crit Care Med. 2005;33:2692–2694. doi: 10.1097/01.CCM.0000186750.06199.0F. [DOI] [PubMed] [Google Scholar]
- 49.Huang Y-F, Wang Y, Watford M. Glutamine directly downregulates glutamine synthetase protein levels in mouse C2C12 skeletal muscle myotubes. J Nutr. 2007;137:1357–1362. doi: 10.1093/jn/137.6.1357. [DOI] [PubMed] [Google Scholar]
- 50.Cetinbas F, Yelken B, Gulbas Z. Role of glutamine administration on cellular immunity after total parenteral nutrition enriched with glutamine in patients with systemic inflammatory response syndrome. J Crit Care. 2010;25:661.e1–661.e6. doi: 10.1016/j.jcrc.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 51.Van der Schoor SRD, Schierbecek H, Bet PM, Veremulen MMM, Lafeberer HN, van Goudoever JB, et al. Majority of dietary glutamine is utilized in first pass in preterm infants. Pediatr Res. 2010;67:194–199. doi: 10.1203/PDR.0b013e3181c34609. [DOI] [PubMed] [Google Scholar]
- 52.Shambaugh GE. Urea bisosynthesis I. The urea cycle and relationships to the citric acid cycle. Am J Clin Nutr. 1977;30:2083–2087. doi: 10.1093/ajcn/30.12.2083. [DOI] [PubMed] [Google Scholar]
- 53.von Haehling S, Morley JE, Coats AJ, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8. doi: 10.1007/s13539-010-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]