Abstract
Background
Cancer cachexia is a syndrome characterized by loss of skeletal muscle protein, depletion of lipid stores, anorexia, weakness, and perturbations of the hormonal homeostasis. Despite several therapeutic approaches described in the past, effective interventions countering cancer cachexia are still lacking.
Methods
The present work was aimed to verify the ability of eicosapentaenoic acid (EPA) to prevent the muscle depletion in Lewis lung carcinoma-bearing mice and to test the ability of endurance exercise training to increase the EPA effect.
Results
EPA alone did not prevent the muscle loss induced by tumor growth while the combination with exercise induced a partial rescue of muscle strength and mass. Moreover, the association of EPA and exercise reduced the dramatic PAX-7 accumulation and stimulated the increase of PCG-1 protein.
Conclusions
Overall, the present data suggest that exercise is an effective tool that should be added for combined therapeutic approaches against cancer cachexia.
Electronic supplementary material
The online version of this article (doi:10.1007/s13539-011-0028-4) contains supplementary material, which is available to authorized users.
Keywords: Experimental cancer cachexia, Training exercise, Muscle wasting, Eicosapentaenoic acid
Introduction
Cancer patients frequently develop a condition of general wasting known as cachexia. This is a multifactorial syndrome that complicates patients’ management, increases morbidity and mortality rates, reduces the tolerance to antineoplastic therapies, and results in poor quality of life [1]. Cachexia is characterized by muscle and fat wasting, anemia, anorexia, and perturbations of the hormonal homeostasis. The pathogenetic mechanisms underlying cachexia are complex and only partially identified, thereby effective therapeutic strategies are lacking.
Several therapeutic approaches have been proposed to counteract cachexia. Among these are nutritional interventions, frequently including omega-3 polyunsaturated fatty acids (PUFA) [2], antioxidants [3], branched-chain amino acids [4], agonists of the melanocortin receptor [5], ghrelin [6], and anti-myostatin agents [7]. The omega-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid have been shown to suppress the production of proinflammatory cytokines of several molecules deriving from the arachidonic acid cascade and of acute-phase reactants such as C-reactive protein (reviewed in [8, 9]). EPA administration to animals bearing the MAC16 tumor improves body weight loss and attenuates the catabolic effects of both lipid mobilizing factor and proteolysis-inducing factor (see [10]). In particular, in the MAC16 model system, EPA has been shown to inhibit the activity of the ubiquitin–proteasome-dependent proteolysis, thus reducing skeletal muscle wasting [11]. Finally, experimental studies suggest that omega-3 PUFAs may impair both tumor growth and metastatic spread, mainly by inducing apoptotic cell death, reducing pro-angiogenic factors, and inhibiting oncogene expression (reviewed in [10]). By contrast, EPA proved ineffective in preventing cachexia in rats bearing the AH-130 hepatoma [12].
Clinical trials performed in malnourished cancer patients have shown that EPA administration improves body weight and lean body mass, likely in force of its anti-inflammatory properties [9, 13]. These observations, however, have not been completely confirmed by a large multicenter study performed on pancreatic cancer patients [14]. Finally, EPA administration per se has been demonstrated to exert no statistically significant benefit in the treatment of cancer cachexia [15].
In addition to nutritional interventions, other therapeutic strategies could effectively contribute to attenuate cancer-related muscle wasting. In particular, exercise training has been proposed as a suitable tool, in view of recent observations, suggesting that decreased physical activity plays a role in the onset of muscle atrophy in cancer patients [16]. Indeed, exercise training stimulates the increase of muscle mass and strength and might improve cancer-associated skeletal muscle wasting by stimulating anabolic pathways as well as by down-regulating the activity of proinflammatory cytokines [17]. The molecular events that follow an acute bout of resistance exercise have been described by several studies (reviewed in [18]); by contrast, little is known about the effect induced by repeated exercise bouts.
The aim of the present study has been to investigate if EPA effectiveness in the prevention of cancer-related cachexia might be enhanced by the association with endurance exercise training. The study has been performed on a well-established model of cancer cachexia, namely mice bearing the Lewis lung carcinoma (LLC). The results obtained show that EPA administration to tumor hosts does not modify the wasting process, while the association with the exercise results in partial restoration of both muscle mass and strength.
Materials and methods
All materials were supplied by Sigma (St. Louis, MO, USA), unless differently specified.
Animals and experimental design
C57BL/6 mice weighing about 20 g (Interfauna, Barcelona, Spain) were maintained on a regular dark–light cycle (light from 08:00 to 20:00) with free access to food and water during the whole experimental period. They were cared for in compliance with the Policy on Humane Care and Use of Laboratory Animals elaborated by the National Institues of Health. The experimental protocol has been approved by the Bioethical Committee of the University of Barcelona. All animal manipulations were made in accordance with the European Community guidelines for the use of laboratory animals.
Animals were randomized and divided into two groups, namely controls (C, n = 15) and tumor bearers (LLC, n = 24). Tumor-bearing mice were inoculated intramuscularly with 5 × 105 LLC cells. Both controls and LLC where divided into three sub-groups (n = 5 for C, n = 8 for LLC): untreated, treated with EPA, and treated with EPA and submitted to endurance exercise. EPA-treated groups received daily intragastric administration of 0.5 g/kg EPA in corn oil starting from the fourth day of tumor growth (the same dose used by [19]) while untreated groups received vehicle alone. As for the exercise protocol, mice were exercised on a Panlab/Harvard-Apparatus treadmill (Barcelona, Spain). Briefly, mice were made to get used to the treadmill for 5 days before tumor injection (starting from 6 m/min for 15 min, increasing speed and time daily until reaching 60–70% of maximum oxygen consumption, corresponding to 14 m/min for 45 min). VO2 max (using a Panlab/Harvard-Apparatus Oxylet System, Barcelona, Spain) was measured after the 5 days adaptation period, and the exercise conditions were maintained constant for all the experimental period. Mice were exercised 5 days/week starting the day after tumor implantation. A schematic drawing of the experimental design including the time points of the different measures in the animals is presented in Fig. 1.
Fig. 1.
Schematic drawing of the experimental design including the time points of the different measurements performed in the mice. Group size is presented in parentheses
Animal weight and food intake were recorded daily. Tumor-bearing mice were sacrificed under anesthesia 14 days after tumor injection. Several muscle ant tissues were rapidly excised, weighed, frozen in isopentane cooled with liquid nitrogen, and stored at −80°C for further analysis.
Total physical activity
Total physical activity (IR ACTIMETER System and ACTITRAK software from PANLAB, Barcelona) was determined during the last 24 h before the sacrifice of the animals in control and tumor-bearing animals using activity sensors that translate individual changes in the infrared pattern caused by movements of the animals into arbitrary activity counts [20]. For the measurements, animals remained in their home cage. A frame containing an infrared beam system was placed on the outside of the cage; this minimized stress to the animals.
Grip force assessment
Skeletal muscular strength in mice was quantified by the grip-strength test [21, 22]. The grip-strength device (Panlab/Harvard Apparatus, Spain) comprised a triangular pull bar connected to an isometric force transducer (dynamometer). Basically, the grip-strength meter was positioned horizontally, and the mice are held by the tail and lowered towards the device. The animals are allowed to grasp the triangular pull bar and were then pulled backwards in the horizontal plane. The force applied to the bar just before it lost grip was recorded as the peak tension. At least three measurements were taken per mouse on both baseline and test days, and the results were averaged for analysis.
ELISA
γ-Interferon (IFN) serum levels were detected by a commercially available mouse ELISA kit, used according to the manufacturer instructions (Bender MedSystems, Vienna, Austria). Serum from each animal (50 μl) was assayed in duplicate. Quantitative calibration was obtained performing a standard curve with recombinant mouse γ-IFN.
Western blotting
Samples from gastrocnemius muscle (about 50 mg) were homogenized in 10 mM 4-(2-hydroxyethyl)-1 piperazineethanesulfonic acid (HEPES), pH 7.5, containing 10 mM MgCl2, 5 mM KCl, 0.1 mM ethylenediaminetetraacetic acid (EDTA), and 0.1% Triton X-100, with freshly added protease and phosphatase inhibitor cocktails, centrifuged at 3,000×g for 5 min at 4°C and the supernatant collected as cytosolic extract. The pellet was resuspended in 20 mM HEPES, pH 7.9, containing 1.5 mM MgCl2, 500 mM NaCl, 0.2 mM EDTA, and 25% glycerol, with freshly added protease and phosphatase inhibitor cocktails, incubated on ice for 30 min, centrifuged at 3,000×g for 5 min at 4°C, and the supernatant collected as nuclear extract. Protein concentration was assayed by the method of Lowry [23] using bovine serum albumine as working standard. Equal amounts of protein (30 μg) were heat-denaturated in sample-loading buffer (50 mM Tris–HCl, pH 6.8, 100 mM dithiothreitol, 2% Sodium Dodecyl Sulphate (SDS), 0.1% bromophenol blue, 10% glycerol), resolved by SDS-PolyAcrylamide Gel Electrophoresis and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). The filters were blocked with Tris-buffered saline containing 0.05% Tween and 5% non-fat dry milk and then incubated overnight with antibodies directed against: PGC-1 (Millipore, Billerica, MA, USA), atrogin-1 (ECM Biosciences, Versailles, KY, USA), and Pax7 (developed by Atsushi Kawakami, obtained from the Developmental Studies Hybridoma Bank, University of Iowa). Peroxidase-conjugated IgG (Bio-Rad, Hercules, CA, USA) were used as secondary antibodies. Membrane-bound immune complexes were detected by an enhanced chemiluminescence system (Santa Cruz Biotechnology, USA) on a photon-sensitive film (Hyperfilm ECL, GE Healthcare, Milano, Italy). Protein loading was normalized according to tubulin (Sigma, St. Louis, MO, USA) expression. Quantification of the bands was performed by densitometric analysis using specific software (TotalLab, NonLinear Dynamics, Newcastle upon Tyne, UK).
Proteasome enzymatic activity
Proteasome activity in the gastrocnemius was determined by cleavage of specific fluorogenic substrates. The tissue was homogenized in 20 mM Tris–HCl, pH 7.2, containing 0.1 mM EDTA, 1 mM 2-mercaptoethanol, 5 mM ATP, 20% glycerol, and 0.04% (v/v) Nonidet P-40. Muscle homogenates were then centrifuged at 13,000×g for 15 min at 4°C. The supernatant was collected and protein concentration determined as described above. Aliquots of 50 μg protein were then incubated for 60 min at 37°C in the presence of succinyl-Leu-Leu-Val-Tyr-7-AMC (LLVY). The incubation buffer for the evaluation of the enzymatic activity was 50 mM HEPES, pH 8.0, containing 5 mM ethylene glycol tetraacetic acid. Fluorescence was read with a spectrofluorometer (380 nm excitation, 460 nm emission; Perkin–Elmer, Norwalk, CT, USA). The activity was calculated by using free AMC as working standard.
Data analysis and presentation
All results were expressed as mean±SD. Significance of the differences was evaluated by analysis of variance followed by Tukey’s test.
Results
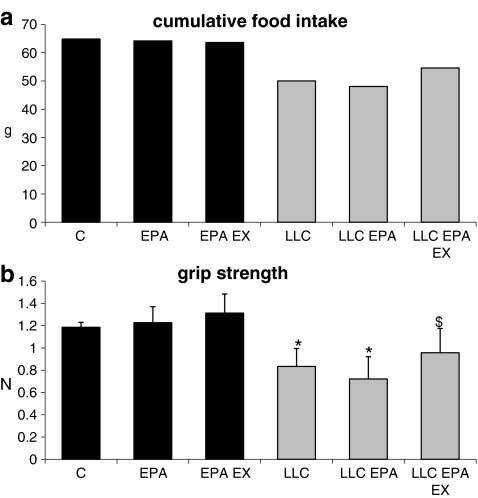
In comparison with control animals, mice bearing the LLC tumor markedly reduce their cumulative food intake; daily administration of EPA (0.5 g/kg; this dose effectively prevents cachexia in MAC16-bearing mice; see [19]) does not modify the decrease in food intake that is partially corrected by treatment with EPA and exercise (C = 64.8 g, LLC = 50.0 g, LLC EPA EX = 54.6 g; Fig. 2a).
Fig. 2.
Cumulative food intake (a) and voluntary grasping strength (b) in control (C) and LLC-bearing mice (LLC). Both groups were subdivided in untreated, EPA-treated (EPA), and EPA-treated plus exercised (EPA EX, see Section 2). Data (means±SD) expressed as grams (g) for food intake and Newtons (N) for strength. Significance of the differences: *p < 0.05 vs C; $ p < 0.05 vs LLC
In a voluntary grip-strength test, LLC-bearing mice develop a force 30% lower than control animals ( ,
,  ; Fig. 2b). EPA administration does not prevent force reduction in LLC hosts, whereas its combination with exercise results in a limited but significant recovery (
; Fig. 2b). EPA administration does not prevent force reduction in LLC hosts, whereas its combination with exercise results in a limited but significant recovery ( , p = 0.04 vs LLC). Muscle strength in control groups is not affected by EPA or EPA + exercise (Fig. 2b).
, p = 0.04 vs LLC). Muscle strength in control groups is not affected by EPA or EPA + exercise (Fig. 2b).
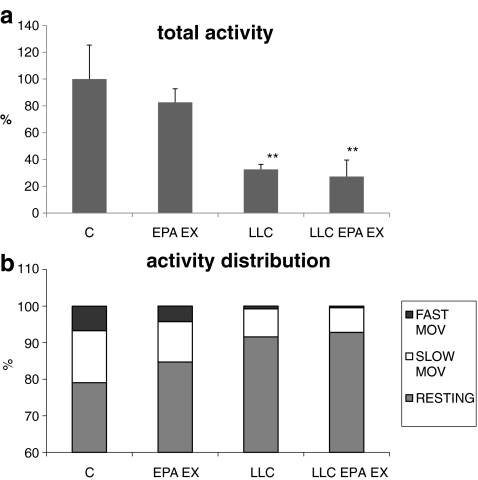
Analysis of the animal behavior, evaluated for 24 h in a continuous recording activity cage, shows a dramatic fall in the total activity of LLC-bearing mice (Fig. 3a); both fast and slow movements are reduced, the former more markedly than the latter (Fig. 3b). Despite the protection exerted against muscle weight loss, the combination of EPA and exercise reveals unable to prevent the decrease of both total and specific activity induced by LLC growth (Fig. 3a, b). Activity (total or specific) in control groups is qualitatively comparable to the corresponding LLC hosts (Fig. 3a, b).
Fig. 3.
a Spontaneous locomotor activity in control (C) and LLC-bearing mice (LLC) either untreated or EPA-treated and exercised (EPA EX). Data (means±SD) expressed as percentages of controls. Significance of the differences: **p < 0.01 vs C. b The total activity was subdivided in percentage of resting time, slow movements (between 2 and 5 cm/s) and fast movements (faster than 5 cm/s)
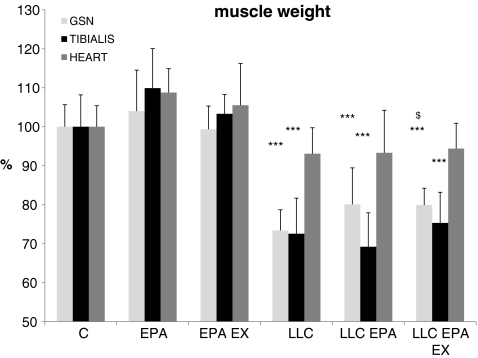
Tumor growth in LLC-bearing mice is associated with loss of skeletal muscle mass (Fig. 4). Consistently with the loss of muscle strength, 14 days after tumor injection, the weight of both gastrocnemius and tibialis muscles is about 70% of controls (gastrocnemius,  ,
,  ; tibialis:
; tibialis:  ,
,  initial body weight). By contrast, the heart is only slightly affected and loses about 7% of control weight (
initial body weight). By contrast, the heart is only slightly affected and loses about 7% of control weight ( ,
,  ). EPA treatment does not counteract muscle atrophy in LLC-bearing mice (Fig. 4). When EPA treatment is associated with training exercise, LLC-induced gastrocnemius depletion is partially prevented (
). EPA treatment does not counteract muscle atrophy in LLC-bearing mice (Fig. 4). When EPA treatment is associated with training exercise, LLC-induced gastrocnemius depletion is partially prevented ( , p = 0.02 vs LLC; Fig. 4).
, p = 0.02 vs LLC; Fig. 4).
Fig. 4.
Gastrocnemius (GSN), tibialis and heart weight in control (C), and LLC-bearing mice (LLC). Both groups were subdivided in untreated, EPA-treated (EPA), and EPA-treated plus exercised (EPA EX, see Section 2). Data (means±SD) expressed as percentages of controls. Significance of the differences: ***p < 0.001 vs C; $ p < 0.05 vs LLC
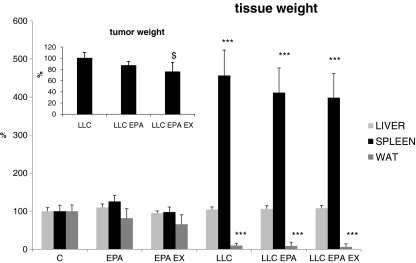
Among the other tissues analyzed, liver weight is unchanged in all experimental groups, while spleen mass increases about fourfold in all LLC-bearing animals, independently from the treatment (Fig. 5). The systemic inflammation, suggested by the spleen hypetrophy, is confirmed by the increased circulating levels of γ-IFN (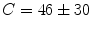 ,
,  ). Similar to the spleen weight, EPA administration, alone or in combination with the exercise, does not prevent the γ-IFN raise (
). Similar to the spleen weight, EPA administration, alone or in combination with the exercise, does not prevent the γ-IFN raise ( ,
,  ). Finally, white perirenal adipose tissue virtually disappears in both untreated and treated LLC hosts (Fig. 5).
). Finally, white perirenal adipose tissue virtually disappears in both untreated and treated LLC hosts (Fig. 5).
Fig. 5.
Liver, spleen, perirenal white adipose tissue (WAT) and tumor weight in control (C) and LLC-bearing mice (LLC). Both groups were subdivided in untreated, EPA-treated (EPA) and EPA-treated plus exercised (EPA EX, see Section 2). Data (means±SD) expressed as percentages of controls. Significance of the differences: ***p < 0.001 vs C; $ p < 0.05 vs LLC
Tumor mass remains comparable between untreated and EPA-treated mice (Fig. 5). However, when EPA is coupled to exercise, a small but statistically significant tumor reduction in size can be observed ( ,
,  , p = 0.013; Fig. 5).
, p = 0.013; Fig. 5).
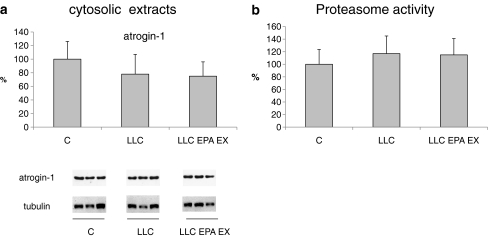
Muscle wasting in cancer cachexia mainly relies on a hypercatabolic response in which the ubiquitin–proteasome system seems to play a major role [24]. Atrogin-1 is a muscle-specific Ub-protein ligase (E3) critically involved in the enhancement of proteolysis [25], overexpressed in several conditions associated with muscle atrophy [26]. In apparent contrast with these reports, however, the present study shows that atrogin-1 protein expression in the gastrocnemius is comparable between untreated and treated LLC-bearing mice (Fig. 6a). This observation suggests that atrogin-1 expression might not the best marker of muscle proteolysis, as recently proposed [27], and/or that other catabolic systems likely contribute to muscle wasting in LLC hosts. The latter hypothesis is supported by the observation that proteasome chymotrypsin-like activity does not increase in the skeletal muscle of TB mice, at least at the time point considered in the present study (Fig. 6b).
Fig. 6.
a Atrogin-1 protein expression in the cytosolic fraction in the GSN of controls, LLC-bearing mice (LLC), and LLC-treated with EPA plus exercised (EPA EX, see Section 2). Densitometric quantifications were normalized according to tubulin levels. b Proteasome enzymatic activity in the above-mentioned experimental groups. Data (means±SD) expressed as percentages of controls
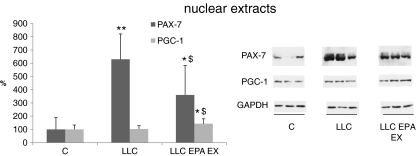
Protein hypercatabolism apart, muscle wasting could also be associated with alterations in the myogenic process. Impaired myogenesis has been recently suggested to contribute to the onset of muscle wasting in experimental cancer cachexia [28]. Pax7 protein levels, measured in the nuclear extracts, markedly increase in LLC-bearing mice (Fig. 7); such increase is prevented in part by the combination EPA + exercise.
Fig. 7.
PAX-7 and PGC-1 protein expression in the nuclear fraction in the GSN of controls, LLC-bearing mice (LLC) and LLC-treated with EPA plus exercised (EPA EX, see Section 2). Densitometric quantifications were normalized according to GAPDH levels. Data (means±SD) expressed as percentages of controls. Significance of the differences: *p < 0.05 vs C; **p < 0.01 vs C; $ p < 0.05 vs LLC
Previous observations have shown that the levels of the peroxisome proliferator-activated receptor γ (PPARγ) coactivator 1 (PGC-1), a factor that stimulates the switch from glycolytic to oxidative fibers, is reduced in different models of skeletal muscle atrophy, including cancer cachexia [29, 30]. In the present experimental setting, however, PGC-1 protein levels do not decrease in tumor-bearing mice with respect to controls, while they are increased by EPA administration coupled to exercise (Fig. 7).
Discussion
In the present study, EPA, a widely studied PUFA, was combined with exercise training in order to enhance its hypothetical effectiveness in the prevention of experimental cancer cachexia.
Several studies proposed PUFA supplementation as an effective tool against different pathologies, including sepsis, coronary artery disease, asthma, inflammatory bowel disease, and cancer (reviewed in [31]). The results obtained in the present study showed that EPA administration to LLC-bearing mice was completely ineffective in preventing tumor-induced muscle wasting. This is in contrast with a number of reports demonstrating a protective action of EPA in mice bearing the MAC16 tumor [19, 32–34]. The discrepancy between the present results on LLC-bearing mice and those reported in the MAC16 hosts could reside in the intrinsic differences that characterize these experimental models. In this regard, studies performed on rats bearing the AH-130 hepatoma [12] and clinical trials involving cachectic patients affected by gastrointestinal or lung cancer [15] reported the ineffectiveness of EPA supplementation. Finally, another fatty acid with antinflammatory properties, the c9t11 conjugated linoleic acid, did not improve muscle depletion in Colon26-bearing mice [35]. These considerations apart, however, the possibility that the dose of EPA adopted in the present study might be insufficient to elicit changes should be taken into account. Actually, there is no general consensus about the optimal EPA dosage needed to obtain pharmacological effects. As an example, 110 mg/day was required to revert insulin resistance in obese mice [36] while 0.49 mg/day were used in another study [37]; in both reports, EPA supplementation was effective, despite the very different range (200-folds). The doses above are 11-fold higher and 20-fold lower, respectively, than the one used in the present study (0.5 g/kg body weight, corresponding to 10 mg/day). Choosing EPA dosage, particular attention has been given to look for a therapeutic dose unable to interfere with tumor growth. In this regard, 0.5 g/kg was shown to prevent muscle wasting in MAC16-bearing mice [19] while 2.5 g/kg also resulted in tumor growth inhibition [32], rendering quite impossible to enucleate muscle-specific effects of the treatment.
In the clinical practice, exploitation of the potential benefits of PUFA administration is still largely limited by the poor knowledge of their mechanisms of action. In this regard, PUFA therapeutic effects are commonly accepted to rely on their antinflammatory actions, exerted by reducing the production of inflammatory cytokines, such as tumor necrosis factor alpha, interleukin-1, and interleukin-6 [31]. In the present study, spleen weight, an indirect indicator of the systemic inflammatory state, was increased by fourfold in LLC-bearing mice and not modified by EPA administration, suggesting that tumor-induced systemic inflammation was only marginally affected by the treatment. This hypothesis is further supported by the observation that circulating levels of γ-IFN, the mediator mainly involved in the pathogenesis of cachexia in LLC-bearing mice [38], increased in tumor hosts and were not modified by EPA. In this regard, a recent study proposed that suppression of immune response and proinflammatory cytokine production by PUFA may not be a general finding [39].
Summarizing, treatment with EPA alone does not appear suitable to prevent cancer cachexia, despite being a good candidate for the treatment of other experimental wasting conditions such as diabetes [40] and arthritis [41]. The different effectiveness of EPA in counteracting the various types of muscle atrophies may depend on the underlying pathogenetic mechanisms. As an example, the redox imbalance differently contributes to muscle depletion in diabetes and in cancer cachexia, as shown by the observation that antioxidant treatment markedly prevented muscle atrophy in the former while only partially in the latter [42].
Several reports suggested training exercise as a strategy to mitigate muscle wasting in chronic pathological conditions (reviewed in [17]). The underlying mechanisms accounting for these protective effects are still not entirely clear. Exercise training was proposed to stimulate mitochondrial biogenesis to increase muscle protein synthesis rate and to attenuate protein hypercatabolism [17]. Moreover, resistance training proved effective in restoring lean body mass and function in patients with rheumatoid arthritis-related cachexia, stimulating the enhancement of muscle IGF-1 levels [43].
Unfortunately, the usefulness of exercise in cancer patients received little attention in the past. The currently available data provide preliminary evidence that exercise training is a well-tolerated and safe complementary therapy that can mitigate several common treatment-related side effects, including breath shortness and pain [44]. A common feature of cancer patient is fatigue. In this regard, a large body of evidence supports the beneficial effect of exercise training in counteracting fatigue [45]. In the present work, we provide the first evidence that endurance training coupled to EPA administration partially prevented the loss of muscle mass and strength in tumor-bearing mice. The improvement of muscle depletion was associated with a small but significant decline in tumor burden in exercised, EPA-treated mice, while no differences were seen in mice treated with EPA alone. This observation is consistent with previous data showing that anaerobic resistance training in Waker-256-bearing rats strongly reduced tumor growth [46] while swimming exercise attenuated colon carcinogenesis in the rat [47]. On the contrary, however, another study reported that tumorigenesis increased in p53-deficient mice exercised with a treadmill protocol similar to the one used in the present study [48]. Exercise-induced suppression of tumor growth was hypothesized to depend on modulations of inflammation [49, 50]. However, both spleen hypertrophy and γ-IFN circulating levels in LLC-bearing mice were not affected by the combination of exercise and EPA, suggesting that, at least in the present experimental conditions, the systemic inflammatory response is not involved in the inhibition of LLC growth. On the whole, the effect of exercise training on tumor growth is still debated, and on the other side, the modulation of molecular pathways induced by the exercise plus EPA in the muscle suggests that the small reduction of tumor growth has a limited relevance in the anti-cachectic effects here reported.
EPA did not prevent muscle wasting when administered alone but was able to modulate both muscle mass and strength when coupled to exercise. From this point of view, the effectiveness of EPA + exercise could primarily result from exercise alone. Although this is a relevant point, such possibility was discarded in the present study, since previous results obtained on Colon26-bearing mice showed that the same exercise protocol adopted for the LLC hosts not only did not prevent, but even worsened tumor-induced muscle wasting (Electronic Supplementary Fig. S1). Similar results were obtained when Colon26-bearing mice were exposed to an 8-week exercise protocol, thus excluding that the lack of protection could depend on exercise duration and suggesting the adoption of a combined approach.
The association of EPA with exercise in LLC-bearing mice resulted in attenuation of tumor-induced anorexia, associated with unchanged voluntary (extra-exercise) activity that was close to untreated LLC bearers. In this regard, physical conditions in LLC hosts might be improved by exercise and EPA, leading tumor bearers to cope with the increased energy requirements by enhancing food intake rather than by further increasing the resting period, already significantly higher than that observed in treated, exercised controls. On the other side, food intake remained comparable between control groups (EPA-treated, exercised, and EPA untreated, sedentary). Such a discrepancy between trained, EPA-treated controls, and LLC hosts might reflect a different response to exercise. Indeed, voluntary activity was lower in trained than in sedentary controls, suggesting that healthy animals managed the enhanced energy requirement by simply recovering after exercise, without modifying food intake. On the contrary, in LLC hosts, resting time, already markedly higher than in controls, did not further increase after training. Also taking into consideration that cachexia can be associated with increased resting energy expenditure [10], modulation of food intake might be the mechanism by which trained animals manage the enhanced energy requirement.
Apart from reduced food intake that was shown to play a limited role in cancer-associated muscle depletion [26, 35], other tumor-induced metabolic alterations could be targeted by the exercise. Indeed, endurance exercise was shown to induce the restoration of lipid metabolism in tumor-bearing rats [51] while resistance exercise modulates both protein synthesis and degradation rates in normal or wasting conditions (reviewed by [52]). Finally, resistance training increased muscle mitochondrial biogenesis in patients affected by chronic kidney disease [53]. In the present work, endurance training in association with EPA induced the increase of PGC-1 protein levels in LLC-bearing mice, possibly stimulating mitochondrial biogenesis and a shift from fast- to slow-twitch oxidative fibers, less susceptible to atrophy.
Recent data showed that muscle wasting in Colon26-bearing mice was associated with reduced myogenin levels and increased expression of Pax7, an inhibitor of the myogenic program [54], suggesting the occurrence of impaired myogenesis [28]. The results obtained in the present work confirmed the enhancement of Pax7 expression also in the skeletal muscle of LLC-bearing mice; this might suggest an increased satellite cell proliferation or their impaired differentiation. Loss of myonuclei, previously reported in Colon26-bearing mice [55], could lead to enhancement of satellite cell proliferation; such increase, however, could be vanished by the inability to complete the myogenic program, contributing to the atrophic phenotype. The present work provides the first evidence that endurance exercise, even for the short period adopted for this study, in association with EPA, partially prevented muscle Pax7 increase in LLC hosts; this observation suggests that satellite cells might proceed into the myogenic program, thus partially explaining the protection exerted by such experimental protocol against LLC-induced muscle wasting.
In conclusion, the results shown in the present study demonstrate that the association between EPA and endurance exercise improved muscle wasting in LLC hosts, mainly by modulating some of the molecular pathways involved in the pathogenesis of muscle depletion, encouraging the inclusion of training exercise in the multi-disciplinary therapeutic approach that should be adopted to prevent cancer cachexia.
Electronic supplementary materials
Below is the link to the electronic supplementary material.
(DOC 34 kb)
Acknowledgment
All authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle [56].
Grant support
Fabio Penna received grant support from MFAG6211-Associazione Italiana per la Ricerca sul Cancro (AIRC), Milano. Paola Costelli received supprt from IG9153 AIRC, Milano; Fondazione San Paolo, Torino; Ministero per l’Università e la Ricerca, Roma (PRIN projects); University of Torino (ex-60% funds); and Regione Piemonte. Josep M. Argilés received support from Ministerio de Ciencia y Tecnología of Spain (SAF-02284-2008).
Disclosure of potential conflict of interest
The authors state they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 29:154–9. doi:10.1016/j.clnu.2009.12.004. [DOI] [PubMed]
- 2.Baracos VE, Mazurak VC, Ma DW. n-3 Polyunsaturated fatty acids throughout the cancer trajectory: influence on disease incidence, progression, response to therapy and cancer-associated cachexia. Nutr Res Rev. 2004;17:177–192. doi: 10.1079/NRR200488. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani G, Madeddu C. Cyclooxygenase-2 inhibitors and antioxidants in the treatment of cachexia. Curr Opin Support Palliat Care. 2008;2:275–281. doi: 10.1097/SPC.0b013e32830f47e4. [DOI] [PubMed] [Google Scholar]
- 4.May PE, Barber A, D’Olimpio JT, Hourihane A, Abumrad NN. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am J Surg. 2002;183:471–479. doi: 10.1016/S0002-9610(02)00823-1. [DOI] [PubMed] [Google Scholar]
- 5.Foster AC, Chen C. Melanocortin-4 receptor antagonists as potential therapeutics in the treatment of cachexia. Curr Top Med Chem. 2007;7:1131–1136. doi: 10.2174/156802607780906663. [DOI] [PubMed] [Google Scholar]
- 6.DeBoer MD. Emergence of ghrelin as a treatment for cachexia syndromes. Nutrition. 2008;24:806–814. doi: 10.1016/j.nut.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Benny Klimek ME, Aydogdu T, Link MJ, Pons M, Koniaris LG, Zimmers TA. Acute inhibition of myostatin-family proteins preserves skeletal muscle in mouse models of cancer cachexia. Biochem Biophys Res Commun. 391:1548–54. doi:10.1016/j.bbrc.2009.12.123. [DOI] [PubMed]
- 8.Ross PJ, Ashley S, Norton A, Priest K, Waters JS, Eisen T, et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer. 2004;90:1905–1911. doi: 10.1038/sj.bjc.6601781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laviano A, Meguid MM, Inui A, Muscaritoli M, Rossi-Fanelli F. Therapy insight: cancer anorexia-cachexia syndrome–when all you can eat is yourself. Nat Clin Pract Oncol. 2005;2:158–165. doi: 10.1038/ncponc0112. [DOI] [PubMed] [Google Scholar]
- 10.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 11.Khal J, Tisdale MJ. Downregulation of muscle protein degradation in sepsis by eicosapentaenoic acid (EPA) Biochem Biophys Res Commun. 2008;375:238–240. doi: 10.1016/j.bbrc.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Costelli P, Llovera M, Lopez-Soriano J, Carbo N, Tessitore L, Lopez-Soriano FJ, et al. Lack of effect of eicosapentaenoic acid in preventing cancer cachexia and inhibiting tumor growth. Cancer Lett. 1995;97:25–32. doi: 10.1016/0304-3835(95)03944-R. [DOI] [PubMed] [Google Scholar]
- 13.Barber MD, Fearon KC. Tolerance and incorporation of a high-dose eicosapentaenoic acid diester emulsion by patients with pancreatic cancer cachexia. Lipids. 2001;36:347–351. doi: 10.1007/s11745-001-0726-4. [DOI] [PubMed] [Google Scholar]
- 14.Fearon KC, Von Meyenfeldt MF, Moses AG, Van Geenen R, Roy A, Gouma DJ, et al. Effect of a protein and energy dense N-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut. 2003;52:1479–1486. doi: 10.1136/gut.52.10.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fearon KC, Barber MD, Moses AG, Ahmedzai SH, Taylor GS, Tisdale MJ, et al. Double-blind, placebo-controlled, randomized study of eicosapentaenoic acid diester in patients with cancer cachexia. J Clin Oncol. 2006;24:3401–3407. doi: 10.1200/JCO.2005.04.5724. [DOI] [PubMed] [Google Scholar]
- 16.Al-Majid S, Waters H. The biological mechanisms of cancer-related skeletal muscle wasting: the role of progressive resistance exercise. Biol Res Nurs. 2008;10:7–20. doi: 10.1177/1099800408317345. [DOI] [PubMed] [Google Scholar]
- 17.Zinna EM, Yarasheski KE. Exercise treatment to counteract protein wasting of chronic diseases. Curr Opin Clin Nutr Metab Care. 2003;6:87–93. doi: 10.1097/00075197-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Cameron-Smith D. Exercise and skeletal muscle gene expression. Clin Exp Pharmacol Physiol. 2002;29:209–213. doi: 10.1046/j.1440-1681.2002.03621.x. [DOI] [PubMed] [Google Scholar]
- 19.Russell ST, Tisdale MJ. Effect of eicosapentaenoic acid (EPA) on expression of a lipid mobilizing factor in adipose tissue in cancer cachexia. Prostaglandins Leukot Essent Fatty Acids. 2005;72:409–414. doi: 10.1016/j.plefa.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Toledo M, Busquets S, Sirisi S, Serpe R, Orpi M, Coutinho J et al. Cancer cachexia: Physical activity and muscle force in tumour-bearing rats. Oncol Rep. 25:189–93. [PubMed]
- 21.Zangarelli A, Chanseaume E, Morio B, Brugere C, Mosoni L, Rousset P, et al. Synergistic effects of caloric restriction with maintained protein intake on skeletal muscle performance in 21-month-old rats: a mitochondria-mediated pathway. FASEB J. 2006;20:2439–2450. doi: 10.1096/fj.05-4544com. [DOI] [PubMed] [Google Scholar]
- 22.Sinis N, Guntinas-Lichius O, Irintchev A, Skouras E, Kuerten S, Pavlov SP, et al. Manual stimulation of forearm muscles does not improve recovery of motor function after injury to a mixed peripheral nerve. Exp Brain Res. 2008;185:469–483. doi: 10.1007/s00221-007-1174-y. [DOI] [PubMed] [Google Scholar]
- 23.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 24.Acharyya S, Guttridge DC. Cancer cachexia signaling pathways continue to emerge yet much still points to the proteasome. Clin Cancer Res. 2007;13:1356–1361. doi: 10.1158/1078-0432.CCR-06-2307. [DOI] [PubMed] [Google Scholar]
- 25.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- 27.Attaix D, Baracos VE. MAFbx/Atrogin-1 expression is a poor index of muscle proteolysis. Curr Opin Clin Nutr Metab Care. 13:223–4. doi:10.1097/MCO.0b013e328338b9a6 [DOI] [PubMed]
- 28.Penna F, Costamagna D, Fanzani A, Bonelli G, Baccino FM, Costelli P. Muscle wasting and impaired myogenesis in tumor bearing mice are prevented by ERK inhibition. PLoS One. 5:e13604. doi:10.1371/journal.pone.0013604. [DOI] [PMC free article] [PubMed]
- 29.Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, et al. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA. 2006;103:16260–16265. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penna F, Bonetto A, Muscaritoli M, Costamagna D, Minero VG, Bonelli G et al. Muscle atrophy in experimental cancer cachexia: Is the IGF-1 signaling pathway involved? Int J Cancer. 127:1706–17. doi:10.1002/ijc.25146. [DOI] [PubMed]
- 31.Fetterman JW, Jr, Zdanowicz MM. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am J Health-Syst Pharm. 2009;66:1169–1179. doi: 10.2146/ajhp080411. [DOI] [PubMed] [Google Scholar]
- 32.Beck SA, Smith KL, Tisdale MJ. Anticachectic and antitumor effect of eicosapentaenoic acid and its effect on protein turnover. Cancer Res. 1991;51:6089–6093. [PubMed] [Google Scholar]
- 33.Smith HJ, Greenberg NA, Tisdale MJ. Effect of eicosapentaenoic acid, protein and amino acids on protein synthesis and degradation in skeletal muscle of cachectic mice. Br J Cancer. 2004;91:408–412. doi: 10.1038/sj.bjc.6601981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitehouse AS, Smith HJ, Drake JL, Tisdale MJ. Mechanism of attenuation of skeletal muscle protein catabolism in cancer cachexia by eicosapentaenoic acid. Cancer Res. 2001;61:3604–3609. [PubMed] [Google Scholar]
- 35.Tian M, Kliewer KL, Asp ML, Stout MB, Belury MA. c9t11-Conjugated linoleic acid-rich oil fails to attenuate wasting in colon-26 tumor-induced late-stage cancer cachexia in male CD2F1 mice. Mol Nutr Food Res. doi:10.1002/mnfr.201000176. [DOI] [PubMed]
- 36.Kalupahana NS, Claycombe K, Newman SJ, Stewart T, Siriwardhana N, Matthan N et al. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J Nutr. 140:1915–22. doi:10.3945/jn.110.125732. [DOI] [PubMed]
- 37.Bonnet N, Ferrari SL. Effects of long-term supplementation with omega-3 fatty acids on longitudinal changes in bone mass and microstructure in mice. J Nutr Biochem. doi:10.1016/j.jnutbio.2010.05.006. [DOI] [PubMed]
- 38.Matthys P, Heremans H, Opdenakker G, Billiau A. Anti-interferon-gamma antibody treatment, growth of Lewis lung tumours in mice and tumour-associated cachexia. Eur J Cancer. 1991;27:182–187. doi: 10.1016/0277-5379(91)90483-T. [DOI] [PubMed] [Google Scholar]
- 39.Rockett BD, Salameh M, Carraway K, Morrison K, Shaikh SR. n-3 PUFA improves fatty acid composition, prevents palmitate-induced apoptosis, and differentially modifies B cell cytokine secretion in vitro and ex vivo. J Lipid Res. 51:1284–97. doi:10.1194/jlr.M000851. [DOI] [PMC free article] [PubMed]
- 40.Figueras M, Olivan M, Busquets S, Lopez-Soriano FJ, Argiles JM. Effects of eicosapentaenoic acid (EPA) treatment on insulin sensitivity in an animal model of diabetes: improvement of the inflammatory status. Obesity (Silver Spring). doi:10.1038/oby.2010.194. [DOI] [PubMed]
- 41.Castillero E, Martin AI, Lopez-Menduina M, Villanua MA, Lopez-Calderon A. Eicosapentaenoic acid attenuates arthritis-induced muscle wasting acting on atrogin-1 and on myogenic regulatory factors. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1322–R1331. doi: 10.1152/ajpregu.00388.2009. [DOI] [PubMed] [Google Scholar]
- 42.Mastrocola R, Reffo P, Penna F, Tomasinelli CE, Boccuzzi G, Baccino FM, et al. Muscle wasting in diabetic and in tumor-bearing rats: role of oxidative stress. Free Radic Biol Med. 2008;44:584–593. doi: 10.1016/j.freeradbiomed.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 43.Lemmey AB, Marcora SM, Chester K, Wilson S, Casanova F, Maddison PJ. Effects of high-intensity resistance training in patients with rheumatoid arthritis: a randomized controlled trial. Arthritis Rheum. 2009;61:1726–1734. doi: 10.1002/art.24891. [DOI] [PubMed] [Google Scholar]
- 44.Jones LW, Peppercom J, Scott JM, Battaglini C. Exercise therapy in the management of solid tumors. Curr Treat Options Oncol. 11:45–58. doi:10.1007/s11864-010-0121-5. [DOI] [PMC free article] [PubMed]
- 45.Barsevick AM, Newhall T, Brown S. Management of cancer-related fatigue. Clin J Oncol Nurs. 2008;12:21–25. doi: 10.1188/08.CJON.S2.21-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Lima C, Alves LE, Iagher F, Machado AF, Bonatto SJ, Kuczera D, et al. Anaerobic exercise reduces tumor growth, cancer cachexia and increases macrophage and lymphocyte response in Walker 256 tumor-bearing rats. Eur J Appl Physiol. 2008;104:957–964. doi: 10.1007/s00421-008-0849-9. [DOI] [PubMed] [Google Scholar]
- 47.Demarzo MM, Martins LV, Fernandes CR, Herrero FA, Perez SE, Turatti A, et al. Exercise reduces inflammation and cell proliferation in rat colon carcinogenesis. Med Sci Sports Exerc. 2008;40:618–621. doi: 10.1249/MSS.0b013e318163274d. [DOI] [PubMed] [Google Scholar]
- 48.Colbert LH, Westerlind KC, Perkins SN, Haines DC, Berrigan D, Donehower LA, et al. Exercise effects on tumorigenesis in a p53-deficient mouse model of breast cancer. Med Sci Sports Exerc. 2009;41:1597–1605. doi: 10.1249/MSS.0b013e31819f1f05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lira FS, Rosa JC, Zanchi NE, Yamashita AS, Lopes RD, Lopes AC, et al. Regulation of inflammation in the adipose tissue in cancer cachexia: effect of exercise. Cell Biochem Funct. 2009;27:71–75. doi: 10.1002/cbf.1540. [DOI] [PubMed] [Google Scholar]
- 50.Batista ML, Jr., Rosa JC, Lopes RD, Lira FS, Martins E, Jr., Yamashita AS et al. Exercise training changes IL-10/TNF-alpha ratio in the skeletal muscle of post-MI rats. Cytokine. 49:102–8. doi:10.1016/j.cyto.2009.10.007. [DOI] [PubMed]
- 51.Lira FS, Tavares FL, Yamashita AS, Koyama CH, Alves MJ, Caperuto EC, et al. Effect of endurance training upon lipid metabolism in the liver of cachectic tumour-bearing rats. Cell Biochem Funct. 2008;26:701–708. doi: 10.1002/cbf.1495. [DOI] [PubMed] [Google Scholar]
- 52.Little JP, Phillips SM. Resistance exercise and nutrition to counteract muscle wasting. Appl Physiol Nutr Metab. 2009;34:817–828. doi: 10.1139/H09-093. [DOI] [PubMed] [Google Scholar]
- 53.Balakrishnan VS, Rao M, Menon V, Gordon PL, Pilichowska M, Castaneda F et al. Resistance training increases muscle mitochondrial biogenesis in patients with chronic kidney disease. Clin J Am Soc Nephrol. 5:996–1002. doi:10.2215/CJN.09141209. [DOI] [PMC free article] [PubMed]
- 54.Olguin HC, Olwin BB. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev Biol. 2004;275:375–388. doi: 10.1016/j.ydbio.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berardi E, Aulino P, Murfuni I, Toschi A, Padula F, Scicchitano BM, et al. Skeletal muscle is enriched in hematopoietic stem cells and not inflammatory cells in cachectic mice. Neurol Res. 2008;30:160–169. doi: 10.1179/174313208X281046. [DOI] [PubMed] [Google Scholar]
- 56.von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8. doi: 10.1007/s13539-010-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 34 kb)