Abstract
Background
Nutritional status, weight loss and cachexia have important prognostic implications in patients with chronic obstructive pulmonary disease (COPD). Body mass index (BMI) has been implicated in COPD risk assessment, but information is mostly limited to composite scores or to patients with stable disease. We aimed to analyse the association between BMI and mortality in acute exacerbation of COPD.
Methods
This retrospective survey included 968 patients hospitalized due to acute exacerbation of COPD at the University Clinic Golnik from February 2002 to June 2007. Vital status was ascertained with Central Population Registry, and database was censored on November 1, 2008.
Results
Median BMI was 25.08 kg/m2 (interquartile range, 21.55–29.05 kg/m2) and 210 patients (22%) had BMI < 21 kg/m2. During median follow-up of 3.26 years (1.79–4.76 years), 430 patients (44%) died. Lowest mortality was found for BMI 25.09–29.05 kg/m2. When divided per BMI decile, mortality was lowest for BMI 25.09–26.56 kg/m2 (33%). In univariate analysis, BMI per quartile and BMI per unit increase were predictive for all-cause mortality. In an adjusted model, BMI per 1 kg/m2 unit increase was associated with 5% less chance of death (hazard ratio 0.95, 95% confidence interval 0.93–0.97).
Conclusions
Low BMI < 21 kg/m2 is frequent in patients hospitalized due to acute exacerbation of COPD. Higher BMI was independently predictive of better long-term survival. A better outcome in obese patients compared to normal weight is in contrast to primary prevention data but concurs with observations of an obesity paradox in other cardiovascular diseases.
Keywords: Chronic obstructive pulmonary disease, Body mass index, Cachexia, Survival
Introduction
Nutritional status, weight loss and cachexia have important prognostic implications in patients with chronic disease [1, 2]. In contrast to common public beliefs and guidelines for primary preventive medicine, high body mass index (BMI) confers protective effects for patients with established chronic diseases such as chronic obstructive pulmonary disease (COPD), heart failure (HF) and chronic kidney disease [3–5]. Similar observations have been reported for other chronic and acute disease conditions which summarized to term reverse epidemiology [6].
Along the natural course of COPD, poor nutritional status, weight loss and development of cachexia frequently occur [7] and contribute to excessive morbidity and mortality burden [8]. In clinical practice, nutritional status is most frequently estimated by BMI calculation and low BMI has been found as an independent predictor of increased mortality [3, 7, 8]. Most of the initial studies, however, were conducted in limited samples or in clinically stable ambulatory patients, and, therefore, may not reflect the full picture of COPD patients [8–11]. For hospitalized patients, less information is available and reports are divergent in their findings. Most of the studies had relatively short follow-up period, and data about association between BMI and long-term mortality after hospitalization for acute exacerbation of COPD is particularly scarce [12–14].
The aim of the present study was therefore to assess the association between BMI and long-term mortality in COPD patients after acute deterioration necessitating hospital care.
Methods
Study design
This study used data from a survey which revised all consecutive discharges from University Clinic Golnik from February 2002 to June 2007 [15]. Patients discharged with COPD (J44.0–J44.9 ICD 10 code) as a primary diagnosis were screened for the analysis. The study protocol was approved by the National Ethics Committee of Slovenia.
Patients and data collection
Initial review of medical records identified 1,391 potentially eligible patients. After verification of COPD as primary admission cause by an independent review of medical documentation and availability of required data such as complete pulmonary function testing and information about body height and body weight to allow for BMI calculation, we included 968 patients. Patients’ demographic characteristics, smoking status, length of hospital stay, concomitant disease, discharge medication and long-term oxygen therapy (LTOT) were retrieved from index hospitalization medical records.
Study endpoints and statistical analysis
The primary outcome of this a priori analysis was all-cause mortality, which was assessed through the Central Population Registry, and the database was censored on November 1, 2008. Secondary endpoints included BMI distribution and relative risk of death across BMI deciles.
Continuous variables are presented as mean values ± standard deviation or as median (interquartile range—IQR). Categorical variables are presented as absolute numbers (%). Differences between the patient subgroups (e.g. per BMI quartiles) were evaluated using the Student’s t test, chi-squared test, Mann–Whitney U test and analysis of variance as appropriate. Kaplan–Meier survival curves were used to present occurrence of primary endpoints during follow-up. The difference across BMI quartiles was compared using the log-rank test. The relationship between all-cause mortality and BMI per 1 kg/m2 increase (model 1) or BMI quartiles (model 2) was evaluated with Cox models of proportional hazards. To identify independent predictors of mortality, age, gender, Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage, HF, cancer and asthma were forced into the multivariate model. We report hazard ratios (HR) and corresponding 95% confidence intervals (CI). SPSS 16.0 software (SPSS Inc., 2007, Chicago, IL, USA) was used for statistical calculations. For all tests, a p value of <0.05 was considered statistically significant.
Results
A total of 968 patients were included in this study, median BMI was 25.08 kg/m2 (IQR 21.55–29.05 kg/m2); 210 patients (22%) had BMI > 21 kg/m2, 294 patients (30%) were overweight (BMI 25–30 kg/m2) and 194 patients (20%) were obese (BMI > 30 kg/m2). Table 1 summarizes patient characteristics over BMI quartiles. For most parameters, there were no differences between patient subgroups. A significant increase was observed for prevalence of concomitant asthma and HF and forced expiratory volume in 1 s (FEV1). By contrast, GOLD stage decreased over BMI quartiles.
Table 1.
Patient characteristics per body mass index quartiles
| All patients (N = 968) | Body mass index < 21.55 kg/m2 (N = 242) | Body mass index 21.55–25.08 kg/m2 (N = 242) | Body mass index 25.09–29.05 kg/m2 (N = 242) | Body mass index > 29.05 kg/m2 (N = 242) | p | |
|---|---|---|---|---|---|---|
| Age | 70 ± 9 | 69 ± 9 | 70 ± 9 | 70 ± 9 | 69 ± 9 | 0.082 |
| Men | 700 (72%) | 167 (69%) | 190 (78%) | 174 (72%) | 169 (70%) | 0.100 |
| Length of stay | 12 ± 8 days | 12 ± 8 | 13 ± 10 | 11 ± 9 | 12 ± 7 | 0.351 |
| Mortality | 430 (44%) | 132 (54%) | 123 (51%) | 82 (34%) | 93 (38%) | <0.001 |
| Current smoker | 233 (24%) | 74 (31%) | 60 (25%) | 40 (17%) | 59 (24%) | 0.229 |
| GOLD | 2.8 ± 0.9 | 3.0 ± 0.9 | 2.8 ± 0.9 | 2.7 ± 0.8 | 2.6 ± 0.8 | <0.001 |
| FEV1 | 1,304 ± 577 ml | 1,145 ± 554 ml | 1,311 ± 620 | 1,344 ± 555 | 1,418 ± 545 | <0.001 |
| VC | 2,885 ± 910 ml | 2,843 ± 897 ml | 2,998 ± 948 | 2,889 ± 946 | 2,810 ± 840 | 0.116 |
| Asthma | 178 (18%) | 31 (13%) | 39 (16%) | 49 (20%) | 59 (24%) | 0.006 |
| Heart failure | 263 (27%) | 29 (12%) | 54 (22%) | 70 (29%) | 110 (45%) | <0.001 |
| Cancer | 108 (11%) | 32 (13%) | 30 (12%) | 24 (10%) | 22 (9%) | 0.428 |
| SABA | 882 (91%) | 218 (90%) | 225 (93%) | 223 (92%) | 216 (89%) | 0.458 |
| LABA | 656 (68%) | 159 (66%) | 161 (66%) | 167 (69%) | 169 (70%) | 0.693 |
| ICS | 627 (65%) | 145 (60%) | 162 (67%) | 153 (63%) | 167 (69%) | 0.175 |
| Tiotropium | 262 (27%) | 73 (30%) | 62 (25%) | 71 (29%) | 56 (23%) | 0.255 |
| Teophylline | 497 (51%) | 132 (54%) | 132 (54%) | 116 (48%) | 117 (48%) | 0.294 |
| LTOT | 194 (20%) | 54 (22%) | 49 (20%) | 38 (16%) | 53 (22%) | 0.257 |
FEV 1 forced expiratory volume in 1 s, VC vital capacity, SABAs short-acting β2-agonists, LABAs long-acting β2-agonists, ICSs inhaled corticosteroids, LTOT long-term oxygen treatment, GOLD Global Initiative for Obstructive Lung Disease
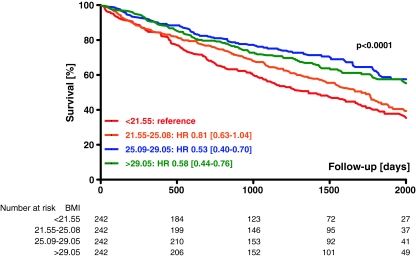
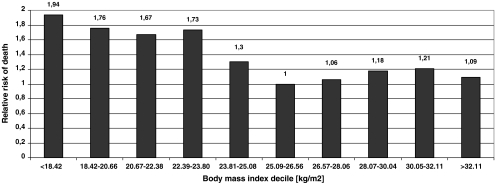
During follow-up (median 3.26 years; range 1.79 to 4.76 years), 430 patients (44%) died. Mortality rates at year 1, 2, and 3 after discharge were 12% (95% confidence interval [CI] 10-15%), 25% (21-26%), and 32% (29-35%), respectively. Lowest mortality was found for patients in third BMI quartile (25.09–29.05 kg/m2) and increased to lowest BMI quartile—Table 1 and Fig. 1. When patients were divided per BMI decile, mortality was lowest for those with BMI of 25.09–26.56 kg/m2 (33%). A linear increase was observed for patients below optimal BMI decile (a relative risk of 1.94 for patients with BMI < 18.42 kg/m2), whereas risk plateaued with increasing BMI—Fig. 2.
Fig. 1.
Kaplan–Meier survival curves per body mass index quartiles and univariate analysis of mortality. HR hazard ratio, CI confidence interval
Fig. 2.
Relative risk for all-cause mortality per body mass index deciles (body mass index 25.09–26.56 kg/m2 as reference)
BMI was associated with all-cause mortality which was independent of other parameters (Table 2). In univariate survival analysis, BMI per quartile and BMI per unit increase were predictive for all-cause mortality. The associations remained significant after adjustment for other parameters that are known to predict survival in patients with COPD. In an adjusted model, BMI per 1 kg/m2 increase was associated with 5% less chance of death (95% CI 0.93–0.97). Similarly, patients in two highest BMI quartiles had 48% and 46% less chance of death (95% CI 0.41–0.72) when compared to patients in lowest BMI quartile.
Table 2.
Cox proportional hazard models for all-cause mortality
| Univariate | Multivariate | ||
|---|---|---|---|
| Model 1 | Model 2 | ||
| Body mass index [per 1 kg/m2] | 0.95 [0.94–0.97] | 0.95 [0.93–0.97] | NA |
| Body mass index | |||
| <21.55 kg/m2 | Reference | NA | reference |
| 21.55–25.08 kg/m2 | 0.81 [0.63–1.04] | NA | 0.82 [0.64–1.06] |
| 25.09–29.05 kg/m2 | 0.53 [0.40–0.70] | NA | 0.52 [0.39–0.69] |
| >29.05 kg/m2 | 0.58 [0.44–0.75] | NA | 0.54 [0.41–0.72] |
| Age [per 1 year] | 1.04 [1.03–1.05] | 1.03 [1.02–1.04] | 1.03 [1.02–1.04] |
| Gender [male vs female] | 0.90 [0.73–1.12] | 0.87 [0.70–1.09] | 0.87 [0.70–1.09] |
| GOLD stage I | Reference | Reference | Reference |
| GOLD stage II | 1.29 [0.75–2.20] | 1.13 [0.66–1.95] | 1.12 [0.65–1.93] |
| GOLD stage III | 1.41 [0.83–2.40] | 1.04 [0.60–1.79] | 1.06 [0.62–1.83] |
| GOLD stage IV | 2.82 [1.66–4.80] | 2.07 [1.20–3.56] | 2.11 [1.23–3.63] |
| Heart failure | 1.60 [1.31–1.95] | 1.72 [1.39–2.14] | 1.65 [1.33–2.05] |
| Cancer | 1.80 [1.37–2.36] | 1.60 [1.21–2.11] | 1.54 [1.17–2.03] |
| Asthma | 0.61 [0.47–0.80] | 0.75 [0.57–0.98] | 0.75 [0.57–0.98] |
Data are presented as hazard ratios and 95% confidence intervals
GOLD Global Initiative for Obstructive Lung Disease, NA not applicable
Discussion
This study found that 22% of patients hospitalized due to acute exacerbation have BMI < 21 kg/m2. We were able to demonstrate that BMI, either per unit increase or per quartile, is an independent predictor of long-term mortality. Importantly, higher BMI was associated with better survival. Optimal BMI with lowest risk of death was in the overweight category (BMI of 25.09–26.56 kg/m2).
In patients with COPD, poor nutritional status as depicted with low BMI is a frequent finding [16–18]. Our results are confirming previous studies [8, 12] and extend current knowledge as we evaluated patients with acute admission due to COPD. Low BMI could also be suggestive for whole body wasting and occurrence of cachexia [19, 20]. This generally is a non-reversible condition, and no effective remedy is available to date [21, 22].
Acute exacerbation of COPD is a common and serious situation where hospital admissions are frequent [9]. Low BMI, along with some other predictors, precipitates need for hospital care [23]. In contrast to other chronic diseases, only few strategies are in place to keep COPD patients away from hospitals and assessment of self-rated health could drive preventive measures to prevent unnecessary hospitalizations [24, 25]. This could be particularly relevant in the context of body size because risk for disability and poor/fair self-rated health was found to be lowest in overweight or even obese [26].
Our study has investigated body size in an unselected population hospitalized due to acute exacerbation of COPD. Overweight and obesity are firmly established risk factors for cardiovascular and other diseases, and increased disease severity of the disease may have been expected in this COPD population. Importantly, most of the patient characteristics that are available in daily clinical practice were not different across the BMI spectrum. Also no differences were found for COPD specific treatments. The prognosis was predicted by BMI independently of other established mortality predictors with better survival observed in those patients who were overweight and obese. Implications of our results are relevant for clinical practice because BMI is an easily accessible parameter, even in acutely deteriorated patients. For COPD, several scoring systems to predict prognosis were developed. The BODE score includes body-mass index (B), the degree of airflow obstruction (O) and dyspnea (D), and exercise capacity (E). The BODE composite score [23] is allegedly the most frequently used in everyday practice but it is primarily limited to patients with clinically stable disease as seen in outpatient setting. Due to its availability and clinical importance, the BMI has also been included in other scoring systems in patients with COPD [27]. Overall, the low BMI is included in the scoring systems to predict outcome in COPD patients as managed by primary and secondary care physicians [23, 27]. Our study confirms increased risk in patients with low BMI. But more importantly, we show that overweight and moderate obesity are not associated with increased mortality. Further, during acute exacerbation, however, some of the score components (e.g. forced expiratory volume in 1 second, dyspnoea score) may be particularly vulnerable to short-term changes which limit the applicability of risk assessment during patient deterioration. Our results demonstrated that BMI, even on top of other recognized risk stratificators that are reliably accessible during course of transient clinical deterioration, is predictive of all-cause mortality in patients admitted due to COPD exacerbation. Although retrospective in design, our study has relevant advantages over previous reports. We have included a large sample of patients with reliable diagnosis of COPD, and we have followed them for long-term outcome. Importantly, we have included only patients already experiencing worsening which necessitated hospital admission. Such an event is a relevant point to both patient and caring physician in a patient’s course of disease to predict future disease progression and to re-consider therapeutic strategies [25, 28]. In an era of complex patient assessment, future studies need to look into composite scores including cardiovascular therapy and treatment as well as laboratory parameters beyond COPD [29]. This could identify novel therapeutic strategies either to prevent patient deterioration or to improve rehabilitation and outcome once clinical condition has worsened [30–32]. It is important to stress that management strategies have to shift beyond the symptom relief interventions [33, 34] and to assess the global patient situation beyond COPD, where concomitant disease may play a decisive role [2, 35]. A tailored approach accounting for patient complexity and disease severity, preferably in an outpatient setting [36], could prove as particularly advantageous.
Limitations
This study has to be interpreted in view of certain limitations. Through selected methodology, we were unable to control for potential effects of parameters beyond the included variables thus there exists a risk of residual confounding by additional variables and co-morbidities beyond the study design. It is important to notice that our findings are based upon index hospitalization information which was not controlled for any changes through the follow-up period. This limitation, however, is driven per study design and is shared with many previous publications [12–14].
Clinical implications
BMI is an easily accessible parameter which carries important information for patient assessment and risk stratification. This is particularly important in the presence of chronic disease like COPD. In patients experiencing acute exacerbation of COPD, assessment of functional parameters like dyspnoea, exercise capacity and pulmonary function may be of limited value for long-term risk assessment which could importantly impact risk stratification. Our results, along with some of previous studies [3, 7–15], suggest that BMI calculation carries a potential to identify patient at risk of poor prognosis. This enables the clinicians to identify patients at risk and to plan future therapeutic efforts to prevent body wasting and to improve prognosis for patients with COPD [37, 38].
Acknowledgement
The authors of this manuscript certify that they complied with the Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle [39].
Conflicts of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle. 2010;1:1–5. doi: 10.1007/s13539-010-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doehner W, von Haehling S, Anker SD, Lainscak M. Neurohormonal activation and inflammation in chronic cardiopulmonary disease: a brief systematic review. Wien Klin Wochenschr. 2009;121:293–296. doi: 10.1007/s00508-009-1194-7. [DOI] [PubMed] [Google Scholar]
- 3.Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1791–1797. doi: 10.1164/ajrccm.157.6.9705017. [DOI] [PubMed] [Google Scholar]
- 4.Davos CH, Doehner W, Rauchhaus M, Cicoira M, Francis DP, Coats AJ, et al. Body mass and survival in patients with chronic heart failure without cachexia: the importance of obesity. J Card Fail. 2003;9:29–35. doi: 10.1054/jcaf.2003.4. [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81:543–554. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Horwich TB, Oreopoulos A, Kovesdy CP, Younessi H, Anker SD, et al. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care. 2007;10:433–442. doi: 10.1097/MCO.0b013e3281a30594. [DOI] [PubMed] [Google Scholar]
- 7.Wagner PD. Possible mechanisms underlying the development of cachexia in COPD. Eur Respir J. 2008;31:492–501. doi: 10.1183/09031936.00074807. [DOI] [PubMed] [Google Scholar]
- 8.Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1856–1861. doi: 10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- 9.Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest. 2003;124:459–467. doi: 10.1378/chest.124.2.459. [DOI] [PubMed] [Google Scholar]
- 10.Connors AF, Jr, Dawson NV, Thomas C, Harrell FE, Jr, Desbiens N, Fulkerson WJ, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments) Am J Respir Crit Care Med. 1996;154:959–967. doi: 10.1164/ajrccm.154.4.8887592. [DOI] [PubMed] [Google Scholar]
- 11.Gunen H, Hacievliyagil SS, Kosar F, Mutlu LC, Gulbas G, Pehlivan E, et al. Factors affecting survival of hospitalised patients with COPD. Eur Respir J. 2005;26:234–241. doi: 10.1183/09031936.05.00024804. [DOI] [PubMed] [Google Scholar]
- 12.Hallin R, Gudmundsson G, Suppli Ulrik C, Nieminen MM, Gislason T, Lindberg E, et al. Nutritional status and long-term mortality in hospitalised patients with chronic obstructive pulmonary disease (COPD) Respir Med. 2007;101:954–1960. doi: 10.1016/j.rmed.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Tsimogianni AM, Papiris SA, Stathopoulos GT, Manali ED, Roussos C, Kotanidou A. Predictors of outcome after exacerbation of chronic obstructive pulmonary disease. J Gen Intern Med. 2009;24:1043–1048. doi: 10.1007/s11606-009-1061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranieri P, Bianchetti A, Margiotta A, Virgillo A, Clini EM, Trabucchi M. Predictors of 6-month mortality in elderly patients with mild chronic obstructive pulmonary disease discharged from a medical ward after acute nonacidotic exacerbation. J Am Geriatr Soc. 2008;56:909–913. doi: 10.1111/j.1532-5415.2008.01683.x. [DOI] [PubMed] [Google Scholar]
- 15.Sarc I, Jeric T, Ziherl K, et al. Adherence to treatment guidelines and long-term survival in hospitalized patients with chronic obstructive pulmonary disease. J Eval Clin Pract. 2011 doi: 10.1111/j.1365-2753.2010.01617.x. [DOI] [PubMed] [Google Scholar]
- 16.Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82:53–59. doi: 10.1093/ajcn.82.1.53. [DOI] [PubMed] [Google Scholar]
- 17.Lenk K, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2010;1:9–21. doi: 10.1007/s13539-010-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segurola H, Pérez-Portabella C, Ferrer J, Hermosilla E, Planas M, Burgos R. Muscle wasting in patients with chronic obstructive pulmonary disease (COPD) J Cachexia Sarcopenia Muscle. 2010;1:47. [Google Scholar]
- 19.Lainscak M, Filippatos GS, Gheorghiade M, Fonarow GC, Anker SD. Cachexia: common, deadly, with an urgent need for precise definition and new therapies. Am J Cardiol. 2008;101:8E–10E. doi: 10.1016/j.amjcard.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 20.Lainscak M, Majc Hodoscek L, Düngen HD, Rauchhaus M, Doehner W, Anker SD, et al. The burden of chronic obstructive pulmonary disease in patients hospitalized with heart failure. Wien Klin Wochenschr. 2009;121:309–313. doi: 10.1007/s00508-009-1185-8. [DOI] [PubMed] [Google Scholar]
- 21.Lainscak M, Andreas S, Scanlon PD, Somers VK, Anker SD. Ghrelin and neurohumoral antagonists in the treatment of cachexia associated with cardiopulmonary disease. Intern Med. 2006;45:837. doi: 10.2169/internalmedicine.45.1867. [DOI] [PubMed] [Google Scholar]
- 22.Koehler F, Doehner W, Hoernig S, Witt C, Anker SD, John M. Anorexia in chronic obstructive pulmonary disease—association to cachexia and hormonal derangement. Int J Cardiol. 2007;119:83–89. doi: 10.1016/j.ijcard.2006.07.088. [DOI] [PubMed] [Google Scholar]
- 23.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 24.Farkas J, Kosnik M, Zaletel-Kragelj L, Flezar M, Suskovic S, Lainscak M. Distribution of self-rated health and association with clinical parameters in patients with chronic obstructive pulmonary disease. Wien Klin Wochenschr. 2009;121:297–302. doi: 10.1007/s00508-009-1170-2. [DOI] [PubMed] [Google Scholar]
- 25.Farkas J, Kosnik M, Flezar M, Suskovic S, Lainscak M. Self-rated health predicts acute exacerbations and hospitalisations in patients with COPD. Chest. 2010;138:323–330. doi: 10.1378/chest.09-2459. [DOI] [PubMed] [Google Scholar]
- 26.Imai K, Gregg EW, Chen YJ, Zhang P, de Rekeneire N, Williamson DF. The association of BMI with functional status and self-rated health in US adults. Obesity. 2008;16:402–408. doi: 10.1038/oby.2007.70. [DOI] [PubMed] [Google Scholar]
- 27.Schembri S, Anderson W, Morant S, Winter J, Thompson P, Pettitt D, et al. A predictive model of hospitalisation and death from chronic obstructive pulmonary disease. Respir Med. 2009;103:1461–1467. doi: 10.1016/j.rmed.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 28.Steer J, Gibson GJ, Bourke SC. Predicting outcomes following hospitalization for acute exacerbations of COPD. QJM. 2010;103:817–829. doi: 10.1093/qjmed/hcq126. [DOI] [PubMed] [Google Scholar]
- 29.Stolz D, Christ-Crain M, Morgenthaler NG, Leuppi J, Miedinger D, Bingisser R, et al. Copeptin, C-reactive protein, and procalcitonin as prognostic biomarkers in acute exacerbation of COPD. Chest. 2007;131:1058–1067. doi: 10.1378/chest.06-2336. [DOI] [PubMed] [Google Scholar]
- 30.van Hees HWH, Linkels M, Pigmans CJC, Dekhuijzen PNR, Heunks LMA. Akt activation is not reduced in the diaphragm of emphysematous hamsters. J Cachexia Sarcopenia Muscle. 2010;1:65. [Google Scholar]
- 31.Kamiide Y, Sugiyama M, Tamaki C, Matsuo T, Takagi H, Furuya M. Effect of SUN11031 (a synthetic human ghrelin) on pulmonary and systemic abnormalities in the cigarette smoke-induced chronic obstructive pulmonary disease (COPD) model. J Cachexia Sarcopenia Muscle. 2010;1:67. [Google Scholar]
- 32.Gertner JM, Oo C. Performance improvement in chronic obstructive pulmonary disease (COPD) cachexia with SUN11031 (a synthetic human ghrelin) in a placebo-controlled trial. J Cachexia Sarcopenia Muscle. 2010;1:100. [Google Scholar]
- 33.Rabe KF, Hurd S, Anzueto A, et al. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 34.Calverley PM, Anderson JA, Celli B, et al. TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 35.Dransfield MT, Rowe SM, Johnson JE, et al. Use of beta blockers and the risk of death in hospitalised patients with acute exacerbations of COPD. Thorax. 2008;63:301–305. doi: 10.1136/thx.2007.081893. [DOI] [PubMed] [Google Scholar]
- 36.Sridhar M, Taylor R, Dawson S, Roberts NJ, Partridge MR. A nurse led intermediate care package in patients who have been hospitalised with an acute exacerbation of chronic obstructive pulmonary disease. Thorax. 2008;63:194–200. doi: 10.1136/thx.2007.077578. [DOI] [PubMed] [Google Scholar]
- 37.van Wetering CR, Hoogendoorn M, Broekhuizen R, et al. Efficacy and costs of nutritional rehabilitation in muscle-wasted patients with chronic obstructive pulmonary disease in a community-based setting: a prespecified subgroup analysis of the INTERCOM trial. J Am Med Dir Assoc. 2010;11:179–187. doi: 10.1016/j.jamda.2009.12.083. [DOI] [PubMed] [Google Scholar]
- 38.Lan CC, Yang MC, Lee CH, et al. Pulmonary rehabilitation improves exercise capacity and quality of life in underweight patients with chronic obstructive pulmonary disease. Respirology. 2011;16:276–283. doi: 10.1111/j.1440-1843.2010.01895.x. [DOI] [PubMed] [Google Scholar]
- 39.von Haehling S, Morley JE, Coats AJ, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8. doi: 10.1007/s13539-010-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]