Abstract
Introduction
The aim of this study is to evaluate computed tomography perfusion (CTP) during admission baseline period (days 0–3) in aneurysmal subarachnoid hemorrhage (A-SAH) for development of vasospasm.
Methods
Retrospective analysis was performed on A-SAH patients from Dec 2004 to Feb 2007 with CTP on days 0–3. Cerebral blood flow (CBF), cerebral blood volume (CBV), and mean transit time (MTT) maps were analyzed for qualitative perfusion deficits. Quantitative analysis was performed using region-of-interest placement to obtain mean CTP values. Development of vasospasm was determined by a multistage hierarchical reference standard incorporating both imaging and clinical criteria. Student's t test and threshold analysis were performed.
Results
Seventy-five patients were included, 37% (28/75) were classified as vasospasm. Mean CTP values in vaso-spasm compared to no vasospasm groups were: CBF 31.90 ml/100 g/min vs. 39.88 ml/100 g/min (P<0.05), MTT 7.12 s vs. 5.03 s (P<0.01), and CBV 1.86 ml/100 g vs. 2.02 ml/100 g (P=0.058). Fifteen patients had qualitative perfusion deficits with 73% (11/15) developed vasospasm. Optimal threshold for CBF is 24–25 mL/100 g/min with 91% specificity and 50% sensitivity, MTT is 5.5 s with 70% specificity and 61% sensitivity and CBV is 1.7 mL/ 100 g with 89% specificity and 36% sensitivity.
Conclusion
These initial results support our hypothesis that A-SAH patients who develop vasospasm may demonstrate early alterations in cerebral perfusion, with statistically significant CBF reduction and MTT prolongation. Overall, CTP has high specificity for development of vasospasm. Future clinical implications include using CTP during the baseline period for early identification of A-SAH patients at high risk for vasospasm to prompt robust preventative measures and treatment.
Keywords: Brain, CT perfusion, Subarachnoid hemorrhage, Vasospasm
Introduction
Delayed cerebral vasospasm remains the most serious cause of morbidity and mortality in patients with aneurysmal subarachnoid hemorrhage (A-SAH). Vasospasm affects greater than half this population and typically develops 4 to 9 days following aneurysm rupture [1, 2]. Patients with vasospasm are at high risk for poor clinical outcomes with long-term disability and death. Therefore, clinical management is focused on early diagnosis to initiate treatment of vasospasm and preventative measures for its complications of permanent neurologic deficits, stroke and death.
Clinical exam, transcranial Doppler ultrasound (TCD) and digital subtraction angiography (DSA) are used in the determination of vasospasm. Recently, computed tomography (CT) angiography (CTA) and CT perfusion (CTP) have also been added at some institutions. Perfusion methods, such as CTP, are recognized for providing important information on the hemodynamic status of the brain. Several studies have evaluated perfusion imaging techniques to detect vasospasm during its typical time-point of occurrence. There is considerable evidence to suggest that alterations in perfusion, manifested as reduced blood flow and prolonged transit time, during days 4–9 after A-SAH are related to vasospasm and delayed cerebral ischemia (DCI) [3-8].
To our knowledge, there is very limited data reported in the literature regarding the use of CTP during the admission baseline period, defined as days 0–3 following A-SAH. However, assessing patients' risks for developing vasospasm at admission would be the ideal time-point. Theoretically, patients can be identified early as high risk for developing vasospasm in order to begin robust preventative measures and treatment regimens. Early and effective treatment of vasospasm may improve the clinical outcome in A-SAH patients by preventing complications of permanent neurologic deficits, infarction and death. Our hypothesis is that A-SAH patients who develop vasospasm demonstrate early alterations in cerebral perfusion on baseline CTP. The purpose of this study is to qualitatively and quantitatively evaluate CTP during the baseline period in A-SAH for the development of vasospasm.
Materials and methods
Study design
A retrospective analysis was performed on consecutive patients admitted to our institution with A-SAH from Dec 2004 to Feb 2007 who underwent an admission baseline CTP on days 0–3 following aneurysmal rupture. Day 0 is defined as the day of the initial hemorrhagic event. Institutional review board approval was obtained. A-SAH was determined by a combination of noncontrast head CT, CTA, DSA, and/or cerebrospinal fluid analysis. At our institution, CTP is performed during the baseline period according to the neurological intensive care unit protocol to assess baseline cerebral perfusion and to compare with later perfusion exams for interval change to detect vasospasm. Patients with symptoms for vasospasm and elevated TCD velocities underwent DSA with intention to perform endovascular treatment. Therapeutic decisions were based on the patient's clinical status, TCD velocities and DSA. Clinical and demographic data was obtained from chart review. Clinical outcomes were determined by a neurologic deficit on clinical exam and/or delayed cerebral ischemia on follow-up CT or MRI.
The reference standard to determine if vasospasm developed during the hospital course is a validated multi-stage hierarchical design [9], summarized in Fig. 1. Reichman et al. thoroughly describe the implementation of this reference standard along with its advantages and limitations [10]. At the primary level, DSA is used as the reference standard to determine vasospasm. Angiographic criteria for vasospasm were determined by the arterial luminal narrowing compared with the normal parent vessel diameter and comparison with the initial DSA performed on presentation. Mild angiographic vasospasm was considered at less than 50% arterial narrowing, moderate vasospasm as 50–75% arterial narrowing, and severe vasospasm as greater than 75% arterial narrowing. Assessment of arterial narrowing on DSA was based on qualitative interpretation by two observers, an Interventional Neuroradiologist who performed the exam (with either 10 or 25 years experience) and a Neuroradiologist blinded to all patient information (22 years experience). For disagreements, a third Neuroradiologist (10 years experience) independently reviewed the exam in a blinded fashion. The intracranial segments of the internal carotid arteries, vertebral artery, basilar artery and the major vessels that comprise the circle-of-Willis (anterior, middle, and posterior cerebral arteries), including its first, second, and third order segments were analyzed.
Fig. 1.
Flow diagram for the reference standard
In those patients who did not undergo DSA for evaluation of vasospasm, excluding the initial DSA at presentation for diagnosis of the ruptured aneurysm, vasospasm was determined on the secondary or tertiary level using clinical and imaging criteria with consideration of the effects of vasospasm treatment. “Symptomatic” vasospasm or DCI has been used as a reference standard for vasospasm since it has been shown to be more clinically relevant to patient outcomes [11-15]. At the secondary level, the following criteria were used:
Clinical criteria: Permanent delayed neurologic deficit on clinical exam, distinct from the deficit at baseline produced by the initial A-SAH event, which was not attributable to any other cause.
Imaging criteria: DCI on follow-up CT and/or MR imaging. DCI was defined as ischemia/infarction that occurred during the vasospasm period, after day 4 that had not been present on the initial CT within 3 days after onset. This criterion effectively excluded primary brain damage from aneurysm rupture and complications of surgical intervention which mostly occur during the initial 3 days [12, 13].
Patients with either of the above criteria were assigned a vasospasm diagnosis at the secondary level. Patients without the above criteria and those who did not receive therapeutic intervention for vasospasm, such as hypervolemic–hyperdynamic–hemodilution (HHH) therapy, were assigned a no vasospasm diagnosis. However, patients without the above criteria who did receive HHH therapy proceeded to the tertiary level for consideration of the effects of treatment.
At the tertiary level, vasospasm diagnosis was based on the assessment of response-to-treatment. Patients who demonstrated an improvement in symptoms and/or clinical exam following HHH therapy as determined by medical record review were considered responders to appropriate treatment and were assigned a vasospasm diagnosis. However, patients who did not improve following treatment or had another cause for their new symptoms (i.e., re-hemorrhage from aneurysm, hydrocephalus, infection, metabolic disturbances, seizure, etc...) were classified as no vasospasm.
Imaging protocols and data processing
CTP was performed on a 16-slice multi-detector scanner according to standard protocol utilizing cine 4i scanning mode with 45 s acquisition using 80 kVp and 190 mA, consisting of 45 gantry rotations at the rate of one rotation per second. A scanning volume of 2 cm was used with its inferior extent selected at the level of the basal ganglia, above the orbits, to minimize radiation exposure to the lenses. A total of 45 ml of low osmolar (300 mg/ml) or isoosmolar (320 mg/ml) nonionic iodinated contrast was administered intravenously at 5 ml/s using a power injector with a 5-s delay. In general, patients with a creatinine clearance of less than 40 ml/min received iso-osmolar nonionic iodinated contrast while all others received low osmolar contrast.
Data analysis
CTP post-processing into cerebral blood flow (CBF), cerebral blood volume (CBV), and mean transit time (MTT) maps were performed on a General Electric Advantage Workstation using CT Perfusion software version 3.0 (General Electric Medical Systems, Milwaukee, WI). This software employs a deconvolution method, which is considered most accurate for low contrast injection rates [16]. Standardized selection of the arterial input function as the A2 segment [17] and venous output as the superior sagittal sinus were used in all patients. CBF, CBV, and MTT maps were then evaluated qualitatively by two blinded observers (attending Neuroradiologist and Neuroradiology fellow) to determine the presence of qualitative perfusion deficits, defined as areas of CBF reduction and MTT prolongation. These deficits were considered separate from the perfusion abnormalities related to the primary hemorrhagic event and/or surgical intervention. After reviewing the images independently, consensus judgment was determined.
Quantitative analysis was conducted using a standardized method of contiguous regions-of-interest (ROI) placement measuring 157 mm2 sampling the cortex and basal ganglia. Each CTP slice had a total of 22 ROIs in the following territories: two ROIs in anterior cerebral artery, 16 ROIs in middle cerebral artery, two ROIs in posterior cerebral artery, and two ROIs in the basal ganglia. Prior studies have shown that these standardized methods used in post-processing and quantitative analysis of CTP are reproducible amongst different observers [18, 19].
Statistical analysis
For patients with qualitative focal perfusion deficits, ROIs within the affected region were isolated and arithmetic means were generated for CBF, CBV, and MTT. In patients with globally decreased perfusion, all of the ROIs were included in the arithmetic means. Similarly, for patients without perfusion deficits, the global arithmetic means were used. Quantitative CTP data for all patients were evaluated for statistically significant differences between the vasospasm and no vasospasm groups utilizing a two-tailed, unpaired Student's t test. Statistical significance was accepted at P<0.05. Threshold analysis was performed using the maximum Youden's index method to obtain the optimal cut-off value for each CTP parameter. The sensitivity and specificity were determined for the threshold values of CBF, CBV, and MTT.
Subgroup analysis was also performed with the same methods above including only patients diagnosed at the primary level using DSA as a single reference standard.
Results
Study population
Overall, 78 patients were identified for inclusion in this study. Three patients were excluded due to uninterpretable CTP studies resulting from excess patient motion. A total of 75 patients were included in the data analysis. Clinical and imaging data are presented in Table 1. The median day baseline CTP was performed was on day 2 for both the vasospasm and no vasospasm groups. The differences observed in the type of aneurysm treatment, and the Hunt Hess (HH) grade were not statistically significant (P>0.05). As expected, the no vasospasm group had more patients with lower HH grades and fewer patients with hydrocephalus or extraventricular drain (EVD) placement compared with the vasospasm group.
Table 1.
Clinical and imaging characteristics
| Characteristic | All (n=75) | Vasospasm (n=28) | No vasospasm (n=47) |
|---|---|---|---|
| Median age (years) | 48 | 48 | 49 |
| Range | 28–73 | 28–73 | 28–73 |
| Median day of baseline CTP | 2 | 2 | 2 |
| Range | 0–3 | 0–3 | 0–3 |
| Female gender (n (%)) | 52 (69) | 21 (75) | 31 (66) |
| Ruptured aneurysm location (n (%)) | |||
| Anterior | 54 (72) | 21 (75) | 33 (70) |
| ACOM | 23 (31) | 8 (29) | 15 (32) |
| ACA | 5 (7) | 2 (7) | 3 (6) |
| ICA | 13 (17) | 5 (18) | 8 (17) |
| MCA | 11 (15) | 5 (18) | 6 (13) |
| Pericallosal | 2 (3) | 1 (4) | 1 (2) |
| Posterior | 21 (28) | 7 (25) | 14 (30) |
| PCOM | 15 (20) | 5 (18) | 10 (21) |
| PCA | 1 (1) | 0 | 1 (2) |
| Basilar | 1 (1) | 1 (4) | 0 |
| PICA | 3 (4) | 1 (4) | 2 (4) |
| SCA | 1 (1) | 0 | 1 (2) |
| Median aneurysm size (mm) | 5 | 5 | 5 |
| Range | 2–25 | 2–25 | 2–13 |
| Aneurysm treatment (n (%)) | |||
| Clip | 41 (55) | 16 (57) | 25 (53) |
| Coil | 33 (44) | 12 (43) | 21 (45) |
| Coil and clip | 1 (1) | 0 | 1 (2) |
| Hunt Hess grade | |||
| Median | 2 | 2.5 | 2 |
| Range | 1–5 | 1–5 | 1–4 |
| Low grade (grades 1–2) | 45 (60) | 14 (50) | 31 (66) |
| High grade (grades 3–5) | 30 (40) | 14 (50) | 16 (34) |
| Noncontrast CT findings | |||
| Hydrocephalus | 19 (25) | 7 (25) | 12 (26) |
| EVD | 28 (37) | 13 (46) | 15 (32) |
| Hydrocephalus and EVD | 9 (12) | 4 (14) | 5 (11) |
| No hydrocephalus or EVD | 19 (25) | 4 (14) | 15 (32) |
According to the reference standard, 37% (28/75) patients were classified as vasospasm and 63% (47/75) as no vasospasm. Vasospasm was determined at the primary level for 51% (38/75) patients, secondary level for 32% (24/75), and tertiary level for 17% (13/75). Ninety-three percent (26/28) of patients in the vasospasm group were diagnosed at the primary level using DSA. Day 8 was the median time-point DSA was performed. The additional two patients in the vasospasm group were determined on the tertiary level, indicating a response-to-treatment. In both of these cases, the patients developed motor deficits (e.g., pronator drifts) on days 6 and 9 that improved after HHH therapy on days 8 and 10, respectively. The range of development of vasospasm occurred from days 2–14 in these patients. Clinical outcomes of the vasospasm group include 54% (15/28) patients with no permanent neurological deficit, 39% (11/28) with permanent neurological deficits, and 7% (2/28) died during hospitalization.
Sixty-three percent (47/75) patients were classified as no vasospasm. Twenty-six (12/47) percent of patients in the no vasospasm group were determined at the primary level, 50% (24/47) at the secondary level, and 24% (11/47) at the tertiary level. Clinical outcomes of the no vasospasm group included 91% (43/47) patients with no permanent neurological deficit, 9% (4/47) patients with permanent neurological deficits referable to causes other than vasospasm (e.g., post-surgical complications), and 0% died during hospitalization.
In the subgroup analysis (n=38) using DSA as a single reference standard, 68% (26/38) patients were classified as vasospasm and 32% (12/38) as no vasospasm. Using DSA as the reference standard increases the incidence of vasospasm to two thirds of patients in the subgroup.
Quantitative analysis
The mean quantitative values and 95% confidence intervals of CBF, CBV, and MTT for the vasospasm and no vasospasm groups are summarized in Table 2. Similarly, the results of the subgroup analysis (n=38) are summarized in Table 3. Threshold analysis reveals the optimal cut-off for CBF is 24–25 mL/100 g/min with a 91% specificity and 50% sensitivity. The optimal cut-off for MTT is 5.5 s with 70% specificity and 61% sensitivity and CBV is 1.7 mL/100 g with 89% specificity and 36% sensitivity. Similar threshold values were seen in the subgroup with the optimal cut-off for CBF is 24–26 mL/100 g/min with 100% specificity and 54% sensitivity, MTT is 6.0–6.5 s with 75% specificity and 54% sensitivity and CBV is 1.9 mL/100 g with 92% specificity and 54% sensitivity.
Table 2.
Mean CT perfusion values for all patients
| CTP value | Vasospasm (n=28) | No vasospasm (n=47) | P value |
|---|---|---|---|
| All patients n=75 | |||
| CBF (ml/100 g/min) | 31.90 (95% CI: 25.78, 38.01) | 39.88 (95% CI: 36.84, 42.93) | 0.021 |
| CBV (ml/100 g) | 1.86 (95% CI: 1.71,2.01) | 2.02 (95% CI: 1.94, 2.10) | 0.058 |
| MTT (s) | 7.12 (95% CI: 5.73, 8.50) | 5.03 (95% CI: 4.45, 5.61) | 0.0073 |
Table 3.
Mean CT perfusion values in the subgroup using DSA as reference standard
| CTP value | Vasospasm (n=26) | No vasospasm (n=12) | P value |
|---|---|---|---|
| DSA patients n=38 | |||
| CBF (ml/100 g/min) | 31.00 (95% CI: 24.54, 37.46) | 39.77 (95% CI: 33.13, 46.41) | 0.052 |
| CBV (ml/100 g) | 1.82 (95% CI: 1.68, 1.97) | 2.09 (95% CI: 1.96, 2.22) | 0.0062 |
| MTT (s) | 7.20 (95% CI: 5.73, 8.67) | 5.16 (95% CI: 4.08, 6.24) | 0.024 |
Qualitative analysis
Twenty percent (15/75) of patients had qualitative perfusion deficits, manifested as areas of CBF reduction and MTT prolongation. Of these patients with perfusion deficits, 40% (6/15) were located in the MCA territory, 33% (5/15) in the MCA and ACA territories, 20% (3/15) in the MCA and PCA territories, and 7% (1/15) in the ACA territory. Examples are provided in Figs. 2 and 3. Seventy-three percent (11/15) of these patients were classified as vasospasm. A 2×2 table was generated and the test characteristics of CTP for a qualitative perfusion deficit are 92% specificity, 39% sensitivity, 73% positive predictive value and 72% negative predictive value.
Fig. 2.
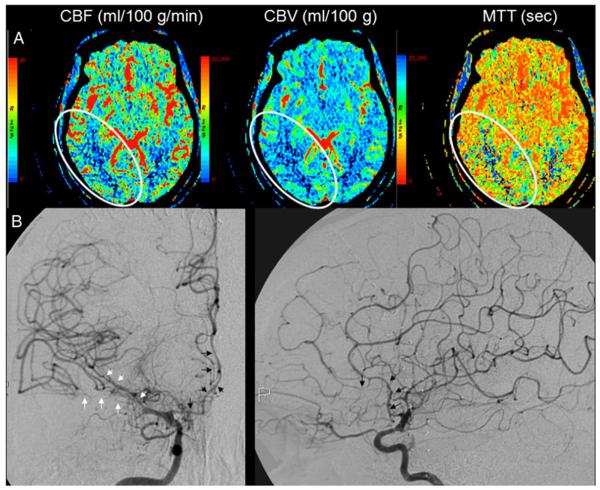
A 39-year-old with A-SAH from a right posterior communicating artery aneurysm: a CTP on day 0 demonstrates matched perfusion deficit on CBF, CBV, and MTT in the posterior right middle and posterior cerebral artery distributions (delineated by the white oval outline); b DSA performed on day 8 demonstrates mild-moderate stenosis of M1 segment and severe stenosis of M2 segments of the right middle cerebral artery (white arrows). Also noted is moderate–severe stenosis of the right anterior cerebral artery (black arrows)
Fig. 3.
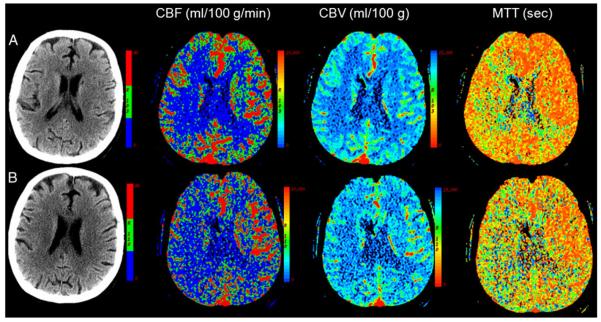
A 47-year-old with A-SAH from a right middle cerebral artery aneurysm: a CTP on day 2 demonstrates a perfusion deficit predominantly involving right middle cerebral artery distribution; b CTP on day 8 demonstrates marked progression in decreased perfusion to right cerebral hemisphere and posterior left cerebral hemisphere; there is no infarction in this region, re-hemorrhage or hydrocephalus on non-contrast CT; DSA (not shown) performed on the same day confirmed angiographic vasospasm
Discussion
Since the advent of CT, there has been increasing interest in developing CTP as a means of evaluating cerebral perfusion in A-SAH patients given its many advantages that include its widespread availability, non-invasive technique, and relatively low cost. CTP has specific advantages in the setting of critically ill patients with its rapid acquisition time, ability to continuously monitor patient's status and few patient contra-indications. The risks of CTP are principally related to the administration of intravenous contrast (e.g., contrast reaction, nephrotoxicity) and the effects of radiation exposure. However, when CTP is performed using standard guidelines and techniques, then the possible benefits of early diagnosis and treatment of vasospasm outweigh these potential risks for A-SAH patients. Furthermore, since CT is already the most often employed modality for following evolution of SAH and for development of delayed cerebral ischemia, CTP can be performed in combination with routine follow-up non contrast CT without delaying patient care.
A review of the current literature reveals evidence to suggest that perfusion abnormalities detected on CTP during days 4–14 following A-SAH are related to vasospasm and delayed cerebral ischemia. Aralasmak et al. studied the correlation between the degree of vasospasm and the presence of a perfusion abnormality in its corresponding territory [20]. A perfusion abnormality was noted in 83% patients with severe vasospasm compared with 26% of mild-moderate vasospasm and 15% of no vasospasm patients. The severe vasospasm group in this study supports defining a significant luminal narrowing for cerebral vessels as≥50% as these patients have a high likelihood of a perfusion abnormality in the territory of the vasospastic vessel. The study performed by Dankbaar et al. further demonstrates the relationship between the degree of vasospasm and the quantitative perfusion abnormality reporting that the flow territory of the vessel with the most severe vasospasm corresponded with the least perfused region, representing an inverse relationship [21]. This study also revealed that patients with severe vasospasm more often experienced DCI than patients without vasospasm. However, almost half of the patients with severe vasospasm in this study did not experience DCI, suggesting that severe vasospasm alone is not sufficient to cause DCI. Specifically, the relationship of the perfusion parameters on perfusion-weighted MRI in patients with angiographically visible cerebral vasospasm was studied by Hattingen et al. [22]. A simultaneous decrease in the relative CBF (rCBF) and CBV (rCBV) was found in patients with vasospasm with a strongly associated positive correlation coefficient. Interestingly, a negative correlation was also found between decreasing rCBV and increasing degree of vasospasm. The fact that rCBV did not increase in territories with vasospasm to maintain rCBF indicates dysfunctional vascular autoregulation. Other studies have also supported evidence for an association between reduced rCBF and dysfunctional vascular autoregulation in A-SAH patients [23, 24]. Patients with intact autoregulatory vasodilation, the rCBV would increase in relation to the degree of vasospasm to maintain the CBF. However, Hattingen et al. demonstrates a contrary reaction being most pronounced in the anterior cerebral artery territory and basal ganglia. This indicates a severe failure in cerebral autoregulation resulting in a decrease in rCBF. In addition, the study also reports that the rCBV in the middle cerebral artery territories were elevated in patients with mild cerebral vasospasm suggesting intact vascular autoregulation. Thereby, a complete discussion of cerebral vascular autoregulation is complex and beyond the scope of this paper as it may be preserved or impaired in A-SAH patients.
Based on this premise, our study focused on evaluating patients during the early baseline period (between days 0–3 following A-SAH) to assess perfusion deficits on CTP indicating patients at high risk for developing vasospasm. These initial results from our study support the hypothesis that A-SAH patients who develop vasospasm demonstrate early alterations in cerebral perfusion on baseline CTP with statistically significant CBF reduction and MTT prolongation. Our data reveals that CTP is not considered a sensitive modality to detect vasospasm during the baseline period. A plausible explanation is that the sensitivity is low due to many false-negative CTP interpretations from patients that have not yet manifested perfusion changes, but do later develop vasospasm during their hospital course. On the otherhand, the presence of perfusion deficits on baseline CTP has a high specificity of 92% for developing vasospasm. Importantly, these findings suggest that baseline CTP can identify A-SAH patients that are at high risk for developing vasospasm during their hospital course.
The quantitative analysis reveals statistically significant CBF reduction and MTT prolongation in the patients who subsequently developed vasospasm. The lack of statistical significance in CBV with a borderline p value of 0.058 may be partly related to its relationship with CBF and MTT via the central volume principle. It is plausible that the differences in CBV between the vasospasm and no vasospasm groups may be obscured by the counterbalance effect of decreased CBF and increased MTT. In the subgroup analysis using DSA as the reference standard, CBV reduction and MTT prolongation were also statistically significant in the vasospasm group. However, CBF reduction neared statistical significance (p=0.052) with similar quantitative values in the study population and subgroup, with the exception of a slightly wider 95% confidence interval in the subgroup vasospasm patients. Therefore, the lack of statistical significance for CBF in the subgroup may be attributed to its smaller sample size. Few publications in the literature have reported similar findings suggesting that CBF is decreased during the baseline period in patients who develop vasospasm during their hospital course [5, 6]. An example from our study is illustrated in Fig. 2. Nabavi et al. observed an initial decrease in mean CBF on days 1–3 following A-SAH in a small subset of patients who later developed moderate/severe vasospasm [6]. Knuckey et al. also noted a similar decrease in CBF early during the course of A-SAH utilizing the 133xenon inhalation method [5].
There is even less known about changes in MTT during the baseline period and its relationship with the development of vasospasm. Wintermark et al. found a statistically significant prolongation in MTT during the typical time-point of vasospasm on days 5–14 in patients with vasospasm [8]. However, the role of early prolongation of MTT in the development of vasospasm remains less clear. Early studies examining angiographic cerebral circulation time (CCT), generally defined as the time it takes for contrast to flow from the supraclinoid internal carotid artery to maximally concentrate in the parietal cortical veins, showed that CCT was prolonged on initial presentation in patients who developed vasospasm [25]. More recently, Laslo et al. studied early changes in cerebral perfusion in a rabbit model and found that MTT prolongation on day 2 predicted the development of moderate/severe vasospasm [26]. Our study supports these findings that alterations in MTT can be detected during the baseline period in A-SAH patients who later develop vasospasm during their hospital course (Fig. 3).
Quantitative analysis in our study also included defining a threshold value for each CTP parameter to determine the optimal cut-off point for identifying A-SAH patients who are at high risk for developing vasospasm. The optimal cut-off for CBF is 24–25 mL/100 g/min which yields a high specificity of 91%, CBV is 1.7 mL/100 g with 89% specificity and MTT is 5.5 s with 79% specificity. However, these threshold values are only valid for the constellation of the type of hardware equipment and software program used in our study. Careful interpretation of absolute values is suggested as the quantitation of the perfusion parameters rely on the assumption that the selected arterial input and venous outflow functions represent the true inflow and outflow measures for the tissue bed being analyzed. The subgroup analysis revealed similar threshold values, however with higher specificity values, likely due to fewer false positive CTP interpretations. Contrary to the study population, the subgroup was comprised of more than two thirds vasospasm patients.
The major limitations of this study are the retrospective study design and the use of a multi-stage hierarchical reference standard for vasospasm which is validated [9] but has not been commonly employed. The advantages and limitations of this multi-stage reference standard are discussed in detail by Reichman et al. [10]. To address this limitation in our study, a subgroup analysis (n=38) was also performed using DSA as a single reference standard. Overall, the findings of early perfusion changes demonstrated on baseline CTP in patients who developed vasospasm were not considerably affected using different reference standards, comparing the subgroup analysis (DSA) with the comprehensive analysis (multi-stage design). However, a larger prospective study is currently ongoing and is designed to address these issues further. An important advantage of using this multi-stage design as the reference standard is the ability to evaluate CTP in a study population that is comparable to the clinical population for which CTP is indicated. In clinical practice, CTP is intended for all A-SAH patients, those with and without DSA. In our study, nearly half (49%) of A-SAH patients did not have DSA performed and 95% of these patients were in the no vasospasm group. Thereby, using DSA alone as a reference standard may lead to selection bias and incorrect conclusions of the true sensitivity and specificity of CTP. Consequently, these biased results may negatively impact on decision-making in A-SAH.
In summary, these initial results support our hypothesis that A-SAH patients who develop vasospasm may demonstrate early alterations in cerebral perfusion, with statistically significant CBF reduction and MTT prolongation on baseline CTP. The baseline CTP exam has high specificity for development of vasospasm when a qualitative perfusion deficit is present. In addition, the quantitative thresholds of CBF, CBV, and MTT also yield high specificity for development of vasospasm. These findings are promising in that baseline CTP may assist in the early identification of A-SAH patients who are at high risk for development of vasospasm. Early identification of these patients has important clinical implications for implementing robust preventative measures and treatment to limit the complications of vasospasm, such as permanent neurologic deficit, stroke, and death.
Acknowledgements
This publication was made possible by Grant Number 5K23NS058387-02 from the National Institute of Neurological Disorders and Stroke (NINDS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NINDS or NIH.
Footnotes
Conflict of interest statement We declare that we have no conflicts of interest.
References
- 1.Kassell NF, Sasaki T, Colohan AR, Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985;16:562–572. doi: 10.1161/01.str.16.4.562. [DOI] [PubMed] [Google Scholar]
- 2.Biller J, Godersky JC, Adams HP. Management of aneurysmal subarachnoid hemorrhage. Stroke. 1988;19:1300–1305. doi: 10.1161/01.str.19.10.1300. [DOI] [PubMed] [Google Scholar]
- 3.Meyer CH, Lowe D, Meyer M, Richardson PL, Neil-Dwyer G. Progressive change in cerebral blood flow during the first three weeks after subarachnoid hemorrhage. Neurosurgery. 1983;12:58–76. doi: 10.1227/00006123-198301000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Geraud G, Tremoulet M, Guell A, Bes A. The prognostic value of noninvasive CBF measurement in subarachnoid hemorrhage. Stroke. 1984;15:301–305. doi: 10.1161/01.str.15.2.301. [DOI] [PubMed] [Google Scholar]
- 5.Knuckey NW, Fox RA, Surveyor I, Stokes BA. Early cerebral blood flow and computerized tomography in predicting ischemia after cerebral aneurysm rupture. J Neurosurg. 1985;62:850–855. doi: 10.3171/jns.1985.62.6.0850. [DOI] [PubMed] [Google Scholar]
- 6.Nabavi DG, LeBlanc LM, Baxter B, et al. Monitoring cerebral perfusion after subarachnoid hemorrhage using CT. Neuroradiology. 2001;43:7–16. doi: 10.1007/s002340000434. [DOI] [PubMed] [Google Scholar]
- 7.Harrigan MR, Magnano CR, Guterman LR, Hopkins LN. Computed tomographic perfusion in the management of aneurysmal subarachnoid hemorrhage: new application of an existent technique. Neurosurgery. 2005;56:304–317. doi: 10.1227/01.neu.0000148902.61943.df. [DOI] [PubMed] [Google Scholar]
- 8.Wintermark M, Ko NU, Smith WS, Liu S, Higashida RT, Dillon WP. Vasospasm after subarachnoid hemorrhage: utility of perfusion CT and CT angiography on diagnosis and management. AJNR Am J Neuroradiol. 2006;27:26–34. [PMC free article] [PubMed] [Google Scholar]
- 9.Reichman M, Gold RL, Greenberg E, Ivanidze J, Elias E, Comunale J, Tsiouris AJ, Johnson CE, Sanelli PC. Validation of a New Reference Standard for the Diagnosis of Vasospasm. Acad Radiol. 2010 doi: 10.1016/j.acra.2010.04.025. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reichman MB, Greenberg ED, Gold RL, Sanelli PC. Developing patient-centered outcome measures for evaluating vasospasm in aneurysmal subarachnoid hemorrhage. Acad Radiol. 2009;16:541–545. doi: 10.1016/j.acra.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suarez JI, Qureshi AI, Yahia AB, et al. Symptomatic vasospasm diagnosis after subarachnoid hemorrhage: evaluation of transcranial Doppler ultrasound and cerebral angiography as related to compromised vascular distribution. Crit Care Med. 2002;30:1348–1355. doi: 10.1097/00003246-200206000-00035. [DOI] [PubMed] [Google Scholar]
- 12.Powsner RA, O'Tuama LA, Jabre A, Melhem ER. SPECT imaging in cerebral vasospasm following subarachnoid hemorrhage. J Nucl Med. 1998;39:765–769. [PubMed] [Google Scholar]
- 13.Frontera JA, Fernandez A, Schmidt JM, et al. Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke. 2009;40:1963–1968. doi: 10.1161/STROKEAHA.108.544700. [DOI] [PubMed] [Google Scholar]
- 14.Rabinstein AA, Weigand S, Atkinson JLD, Wijdicks EFM. Patterns of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke. 2005;36:992–997. doi: 10.1161/01.STR.0000163090.59350.5a. [DOI] [PubMed] [Google Scholar]
- 15.Shimoda M, Takeuchi M, Tominaga J, Oda S, Kumasaka A, Tsugane R. Asymptomatic versus symptomatic infarcts from vasospasm in patients with subarachnoid hemorrhage: serial magnetic resonance imaging. Neurosurgery. 2001;49:1341–1350. doi: 10.1097/00006123-200112000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Wintermark M, Maeder P, Thiran JP, Schnyder P, Meuli R. Quantitative assessment of regional blood flows by perfusion CT studies at low injection rates: a critical review of the underlying theoretical models. Eur Radiol. 2001;11:1220–1230. doi: 10.1007/s003300000707. [DOI] [PubMed] [Google Scholar]
- 17.Wintermark M, Lau BC, Chien J, Arora S. The anterior cerebral artery is an appropriate arterial input function for perfusion-CT processing in patients with acute stroke. Neuroradiology. 2008;50:227–236. doi: 10.1007/s00234-007-0336-8. [DOI] [PubMed] [Google Scholar]
- 18.Sanelli PC, Nicola G, Tsiouris AJ, Ougorets I, Knight C, Zimmerman RD. Reproducibility of post-processing quantitative CT perfusion maps. AJR Am J Roentgenol. 2007;188:213–218. doi: 10.2214/ajr.05.2188. [DOI] [PubMed] [Google Scholar]
- 19.Sanelli PC, Nicola G, Johnson R, et al. Effect of training and experience on qualitative and quantitative CT perfusion data. AJNR Am J Neuroradiol. 2007;28:428–432. [PMC free article] [PubMed] [Google Scholar]
- 20.Aralasmak A, Akyuz M, Ozkaynak C, Sindel T, Tuncer R. CT angiography and perfusion imaging in patients with subarachnoid hemorrhage: correlation of vasospasm to perfusion abnormality. Neuroradiology. 2009;51:85–93. doi: 10.1007/s00234-008-0466-7. [DOI] [PubMed] [Google Scholar]
- 21.Dankbaar JW, Rijsdijk M, van der Schaaf IC, Velthuis BK, Wermer MJH, Rinkel GJE. Relationship between vasospasm, cerebral perfusion, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Neuroradiology. 2009;51:813–819. doi: 10.1007/s00234-009-0575-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hattingen E, Blasel S, Dettmann E, Vatter H, Pilatus U, Seifert V, Zanella FE, Weidauer S. Perfusion-weighted MRI to evaluate cerebral autoregulation in aneurysmal subarachnoid hemorrhage. Neuorradiology. 2008;50:929–938. doi: 10.1007/s00234-008-0424-4. [DOI] [PubMed] [Google Scholar]
- 23.Carpenter DA, Grubb RL, Jr, Tempel LW, Powers WJ. Cerebral oxygen metabolism after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab. 1991;11:837–844. doi: 10.1038/jcbfm.1991.143. [DOI] [PubMed] [Google Scholar]
- 24.Yundt KD, Grubb RL, Jr, Diringer MN, Powers WJ. Autoregulatory vasodilatation of parenchymal vessels is impaired during cerebral vasospasm. J Cereb Blood Flow Metab. 1998;18:419–424. doi: 10.1097/00004647-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Milburn JM, Moran CJ, DeWitte TC, Diringer MN, Pilgram TK, Dacey RG. Effect of intraarterial papavarine on cerebral circulation time. Am J Neuroradiol. 1997;18:1081–1085. [PMC free article] [PubMed] [Google Scholar]
- 26.Laslo AM, Eastwood JD, Pakkiri P, Chen F, Lee TY. CT perfusion-derived mean transit time predicts early mortality and delayed vasospasm after experimental subarachnoid hemorrhage. Am J Neuroradiol. 2008;29:79–85. doi: 10.3174/ajnr.A0747. [DOI] [PMC free article] [PubMed] [Google Scholar]