Abstract
Background:
Resistance to broad-spectrum β lactams, mediated by extended-spectrum β lactamases (ESBLs), is an increasing problem world wide. This resistance poses problems for in vitro testing and reporting. Increased prevalence of ESBLs among Enterobacteriaceae creates a great need for laboratory testing methods that will accurately identify their presence.
Materials and Methods:
During the study, the Enterobacteriaceae isolated were tested for the presence of ESBL by the National Committee for Clinical Laboratory Standards (NCCLS) screening test, Jarlier double disc synergy (approximation) test (DDST) and NCCLS phenotypic confirmatory test (PCT), and compared their efficiency in detection.
Results:
A total of 313 Enterobacteriaceae were isolated and tested for the presence of ESBL. NCCLS PCT identified 200 (63.89%) as ESBL producers and DDST identified 176 (56.23%), with a P-value of <0.001. Among the screening agents, ceftazidime had a better sensitivity (89.49%) and specificity (95.74%).
Conclusions:
Close monitoring of the susceptibility pattern of isolates and careful spacing with specific discs can identify many ESBL producers. Ceftazidime has a better sensitivity and specificity as a screening agent. A combination of different tests can be useful for accurate identification.
Keywords: DDST, Enterobacteriaceae, ESBLs, NCCLS screening, PCT
INTRODUCTION
Extended-spectrum β-lactamases (ESBLs) are Ambler class A penicillinases, which confer resistance to and hydrolyze the expanded-spectrum cephalosporins like ceftazidime, cefotoxime, monobactam-azteronam and related oxyimino β-lactams as well as older penicillins and cephalosporins.[1–3] They arise from mutations in the genes for common plasmid-mediated β-lactamases, especially Temoniera (TEM) and sulfhydryl variable (SHV) enzymes, which alter the configuration of the enzyme near its active site to increase the affinity and hydrolytic ability of the β-lactamase for oxyimino compounds while simultaneously weakening the overall enzyme efficiency. Widespread use of third-generation cephalosporins and aztreonam is the major cause of the mutations leading to emergence of ESBLs.[4]
Organisms possessing genes for inducible β-lactamases show false susceptibility if tested in the uninduced state.[4,5]
ESBL-mediated resistance poses problems for in vitro susceptibility testing and reporting.[3] The sensitivity and specificity of a susceptibility test to detect ESBLs vary with the cephalosporin tested.[6]
A significant proportion of ESBL-producing organisms may have elevated mean inhibitory concentrations (MICs) to ceftazidime that do not reach the National Committee for Clinical Laboratory Standards (NLCLS) break point for resistance (MIC >8 μg/ml) and, therefore, may be incorrectly dismissed as non-ESBL producing organisms.[7]
The increased prevalence of Enterobacteriaceae producing ESBLs creates a great need for laboratory testing methods that will accurately identify the presence of these enzymes in clinical isolates.[6] Hence, this study was undertaken to detect ESBL producers by using NCCLS screening test, Jarlier double disc synergy (approximation) test (DDST) and NCCLS phenotypic confirmatory test (PCT), and to compare their efficiency in detection.
MATERIALS AND METHODS
A total of 313 consecutive non-repeat culture isolates of Enterobacteriaceae were obtained from 280 different specimens from different specialty wards. The isolates were identified on the basis of conventional microbiological procedures like their growth pattern on blood agar and Mac Conkey's agar. The characters assessed included morphology on Gram's staining, motility, methyl red test, Vogues-Proskauer test, citrate utilization, catalase, indole and urease production, nitrate reduction, sugar fermentation and amino acid decarboxylation and arginine dihydrolase test. Antimicrobial susceptibility testing was determined by the Kirby-Bauer disc diffusion method as per the NCCLS recommendations.[8]
NCCLS screening test
Isolates showing an inhibition zone size of ≤22 mm with ceftazidime (30 μg), ≤25 mm with cefriaxone (30 μg) and ≤27 mm with cefotaxime (30 μg) were identified as potential ESBL producers and were short listed for confirmation of ESBL production.
Double disc approximation test/DDST
First, using the detection test described by Jarlier et al,[6,9] synergy was determined between a disc of amoxicillin-clavulanate (20 μg/10 μg) (augmentin) and a 30-μg disc of each third-generation cephalosporin test antibiotic placed at a distance of 20 mm from center to center on a Mueller-Hinton Agar (MHA) plate swabbed with the test isolate. Clear extension of the edge of the inhibition zone of cephalosporin toward the augmentin disc was interpreted as positive for ESBL production.[4,6,9–11]
NCCLS phenotypic confirmatory combination disc diffusion test
A disc of ceftazidime (30 μg) alone and ceftazidime + clavulanic acid (30 μg/10 μg) were placed at a distance of 25 mm, center to center, on a MHA plate inoculated with a bacterial suspension of 0.5 McFarland turbidity standards and incubated overnight at 37°C.
An increase in the inhibition zone diameter of ?5 mm for a combination disc versus ceftazidime disc alone confirmed ESBL production.[7,8,12]
The Kirby Bauer plates for the susceptibility tests were prepared in the laboratory. All of the antibiotic discs and dehydrated media were obtained from Hi-Media Laboratories Pvt. Limited, Mumbai, India.
Statistical analysis
The chi-square test was used with appropriate correction for the observation using EPI 6 software.
RESULTS
Among the 313 Enterobacteriaceae isolates, Klesbsiella sp. was the most common pathogen isolated from the tested samples, constituting 115/313 (36.74%) of the total isolates, followed by Escherichia coli 82/313 (26.17%), Providencia sp. 25/313 (7.98%), Proteus vulgaris 24/313 (7.66%) and Proteus mirabilis 18/313 (5.75%).All the 313 Enterobacteriaceae isolates were subjected to the initial NCCLS screening test, followed by the DDST and the NCCLS PCT. The PCT identified 200/313 (63.89%) as ESBL producers, whereas the DDST at a distance of 20 mm between center to center identified only 176/313 (56.23%), with a P-value of <0.001, which was highly significant. The DDST had a sensitivity of 94.89% (167/176, 95% confidence interval [CI] 91.57–98.21), a specificity of 75.91% (104/137, 95% CI 72.26–79.56) and a positive predictive value of 83.55% (167/200) and negative predictive value of 92.03% (104/113).
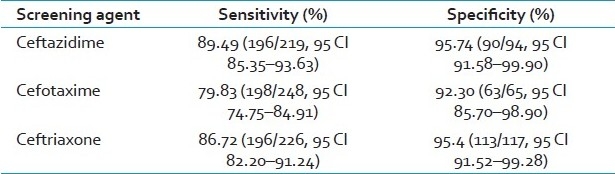
Among the third-generation cephalosporins used for screening, ceftazdime had a better sensitivity and specificity, followed by ceftriaxone. Even though cefotaxime had a good specificity, it lacked sensitivity [Table 1]. During the screening procedure, ceftazidime, cefotaxime and ceftriaxone identified 219, 248 and 226 isolates, respectively, as potential ESBL producers.
Table 1.
Test results of three screening agents as compared with the phenotypic confirmatory test (CI, confidence interval)
The distribution of ESBL producers varied among different species of Enterobacteriaceae. The rates were high among Klebsiellae oxytoca (89.47%), Klebsiellae pneuminae (71.87%), Escherichia coli (62.19%), Proteus mirabilis (61.11%), Proteus vulgaris (50.00%) and Providencia sp. (44.00%), which were the major isolates in the group.
DISCUSSION
ESBLs are a problem in hospitalized patients throughout the world. The prevalence of ESBL among clinical isolates varies greatly worldwide and within geographic areas, and is rapidly changing over time.[13] This increased prevalence of Enterobacteriaceae producing ESBLs creates a great need for laboratory testing methods that will accurately identify the presence of these enzymes in clinical isolates.[6]
The various susceptibility testing methods differ in their ability to detect cephalosporin resistance in the ESBL-producing strains.[6]
In a study by Singhal et al.,[14] four different tertiary care hospitals from across India identified a prevalence rate of 63.60% by NCCLS methods and Mathur et al.,[15] from AIIMS, New Delhi, reported a prevalence rate of 68% for ESBL production among Gram negative bacilli by the NCCLS confirmatory tests. The present study, with a prevalence rate of 63.89% by NCCLS PCT, correlates well with these studies.
ESBL-producing bacteria may appear falsely susceptible to certain extended-spectrum cephalosporins in in vitro susceptibility testing when National guidelines are used. A second test has therefore been recommended for the detection of ESBL activity. The DDST is the most widely used test due to its simplicity and ease of interpretation.[11] It is a reliable method for the detection of ESBLs.[6] However, the sensitivity of the DDST in different studies ranges from 79% to 96%. This lack of sensitivity results from the fact that DDST is not a standardized procedure.[16]
The sensitivity of DDST varies with the distance between the discs. Ho PL et al.[11 reported the sensitivity of DDST to be 83.8% at a single interdisc width of 30 mm compared with the inhibitor-potentiated disc diffusion test. They also reported that the sensitivity can be increased to 97.9% by decreasing the interdisc width to 20 mm. Vercauteren et al.[17] reported sensitivities of the double disc and three-dimensional tests at 96.9% and 90.6%, respectively. The DDST sensitivity in the present study correlates well with those studies.
Zali[18] reported that the clinical strains producing SHV-6 ESBL and Amp C type β-lactamase producers would not be detected by double disc diffusion tests or the MAST (MAST Laboratories Ltd., Bootle, Merseyside, UK) double disc test (MDD). They also noted that Amp C type β lactamase producers give negative results with the double disc, MDD and Epsiloemeter (E) test methodologies.
The commonly used disc diffusion method was insufficient for the detection of ESBL activity using ceftazidime alone as an indicator. In contrast, reduced susceptibility to aztreonam and other cephalosporins resulted in an acceptable ESBL detection rate. This reflects the common occurrence of ESBL with a low specificity for ceftazidime.[11] Recent data suggest that susceptibility testing with cefpodoxime can lead to a high number of false-positives if the current NCCLs interpretative criteria are applied.[6]
While studying ESBLs using the NCCLS criteria for screening tests, Ho PL et al.[11] reported sensitivities of 57.7% for caftazidime, 98.6% for ceftriaxone, 99.3% for cefpodoxime and 93% for Aztreonam using the Kirby Baeur disc diffusion method.
However, Vercauteren et al.[17 detected only 48% of the ESBL-producing reference strains by their reduced susceptibility to ceftazidime and by combining ceftriaxone, ceftazidime and aztreonam, they detected only 52% of the ESBL-producing strains. In the present study, ceftazdime had a better sensitivity (89.49%) and specificity (95.74%) compared with ceftriaxone (79.83% and 92.30%) as a screening agent. Even though cefotaxime has a good specificity (95.4%), it lacks sensitivity (86.72%).
Although molecular methods and automated systems appear to be sensitive in ESBL detection, they are expensive, time consuming and require specialized equipment and expertise. Commercially available E tests have been proposed as simple techniques for the detection of ESBL production, but they are costly and need extra MHA plates. In places where resources are minimal and workloads are high, close monitoring of susceptibility patterns of members of Enterobacteriaceae isolates by screening methods such as DDST and NCCLS PCT can detect many ESBL producers.[18] However, careful spacing and use of specific discs are required for accurate results. The use of these tests may contribute to wider recognition and more scrupulous monitoring for the presence of emerging drug-resistant organisms.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Jacoby GA, Han P. Detection of Extended–Spectrum β-Lactamases in clinical isolates of Klebsiella pneumonia and Escherichia coli. J Clin Microbiol. 1996;4:908–11. doi: 10.1128/jcm.34.4.908-911.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coudron PE, Molland ES, Sanders CC. Occurrence and Detection of Extended Spectrum β-Lactamases in members of he family Enterobacteriaceae at A Veterans Medical Centre: Seek and You May Find. J Clin Microbiol. 1997;35:2593–7. doi: 10.1128/jcm.35.10.2593-2597.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emery CL. Weymouth LA.Detection and Clinical Significance of Extended – Spectrum beta-Lactamases in a Tertiary care Medical Center. J Clin Microbiol. 1997;35:2061–7. doi: 10.1128/jcm.35.8.2061-2067.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhary U, Aggarwal R. Extended Spectrum β-Lactamases-An Emerging threat to Clinical therapeutics. Ind J Med Microbiol. 2004;22:75–80. [PubMed] [Google Scholar]
- 5.Revathi G, Singh S. Detection of Expanded spectrum cephalosporin resistance due to inducible lastamases in hospital isolates. Indian J Med Microbiol. 1997;15:113–5. [Google Scholar]
- 6.Bradford PA. Extended Spectrum β Lactamases in the 21st century: Characterization, Epidemiology and Detection of This Important Resistance Threat. Clin Microbiol Rev. 2001;14:933–51. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillipon A, Labia R, Jacoby G. Extended Spectrum β-Lactamases (MINIREVIEW) Antimicrob Agents Chemother. 1989;33:1131–6. doi: 10.1128/aac.33.8.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. Wayne, PA, USA: NCCLS; 2002. Performance standards for Antimicrobial disk susceptibility testing; 12th Informational Spplement; pp. M100–S12. [Google Scholar]
- 9.Jarlier V, Nicolas M, Fournier G, Phillipon A. ESBLs conferring transferable resistance to newer β- lactam agents in Enterobacteriaceae; hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988;10:867–78. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- 10.Shukla I, Tiwari R, Agrawal M. Prevalence of ESBL producing K. pneumoniae in a tertiary care hospital. Indian J Med Microbiol. 2004;22:87–97. [PubMed] [Google Scholar]
- 11.HO PL, Tsang DN, Que TL, Ho M, Yuenky Comparison of screening methods for detection of Extended Spectrum β-Lactamases and their prevalence among E.coli and Klebsiella spp. in Hongkong. APMIS. 2000;108:237–40. doi: 10.1034/j.1600-0463.2000.d01-50.x. [DOI] [PubMed] [Google Scholar]
- 12.Tenover FC, Ranxey PM, Williams PP, Rasheed JK, Biddle JW, Oliver A, et al. Evaluation of he NCCLS ESBL confirmation methods for E.coli with isolates collected during project ICARE. J Clin Microbiol. 2003;41:3142–6. doi: 10.1128/JCM.41.7.3142-3146.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babypadmini S, Apparajalu B. ESBLs in Urinary isolates of E. coli and K. pneumoniae. Prevalence and susceptibility pattern in a tertiary care hospital. Indian J Med Microbiol. 2004;22:172–4. [PubMed] [Google Scholar]
- 14.Singhal S, Mathur T, Khan S, Upadhyay DJ, Chug S, Govind R, et al. Evaluation of methods for Amp C β lactamases in Gram Negative clinical isolates from Tertiary care hospitals. Ind J Clin Microbiol. 2005;23:120–4. doi: 10.4103/0255-0857.16053. [DOI] [PubMed] [Google Scholar]
- 15.Mathur P, Kapil A, Das B, Dhawan B. Prevalence of Extended Spectrum β Lactamase producing GNB in a tertiary care hospital. Indian J Med Res. 2000;15:153–7. [PubMed] [Google Scholar]
- 16.Datta P, Thakur A, Mishra B, Gupta V. Prevalence of clinical strains Resistant to various β-Lactamas in a Tertiary Care Hospital in India. Jpn J Infect Dis. 2004;57:146–9. [PubMed] [Google Scholar]
- 17.Vercauteren E, Deschecmaeker P, Ieven M, Sanders CC, Goossens H. Comparison of Screening Methods for Detection of Extended Spectrum β-Lactamases and their Prevalence among Blood Isolates of E.coli and Klebsiella spp. In a Belgian Teaching Hospital. J Clin microbial. 1997;35:2191–2. doi: 10.1128/jcm.35.9.2191-2197.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zali FN, Chanawaong A, Kerr KG, Birkenhead D, Hawkey PM. Detection of Extended Spectrum β-Lactamases in members of the family Enterobacteriaceae; Comparison of MAST DD test, the double disc and E test ESBL. J Antimicrobial chemother. 2000;45:881–5. doi: 10.1093/jac/45.6.881. [DOI] [PubMed] [Google Scholar]


