Abstract
Aim:
The aim of this study was (1) To investigate the viability of bacteria within supragingival and subgingival calculus, (2) To examine motility of bacteria, and (3) To identify bacterial morphotypes in calculus.
Materials and Methods:
Supra and subgingival calculus were harvested from 30 subjects having clinical evidence of chronic inflammatory periodontal disease and were divided into two groups. Samples from both groups were immediately transported to the Department of Microbiology for gram staining, acridine orange staining, bacterial culture and to the Department of Oral Pathology for dark field microscopy.
Results:
Gram staining revealed presence of bacteria within the samples.Dark field microscopic examination revealed presence of filamentous organisms, spirochetes, and motile short bacilli. Acridine orange fluorescent stain showed that the viable bacteria appeared apple green. Bacterial culture revealed presence of a variety of aerobic organisms.
Conclusion:
From the results, it appeared that viable bacteria were present within calculus especially within internal channels and lacunae.
Keywords: Acridine orange staining, aerobic bacterial culture, gram staining, spirochetes, subgingival calculus
INTRODUCTION
Periodontitis is a chronic inflammatory process initiated by the plaque biofilm. This is mainly associated with development of destructive periodontal disease, leading to pocket formation, bone loss, and eventually tooth loss. Current understanding of initiation and progression of periodontal disease describes dental plaque to be the primary etiological factor. This biofilm is accompanied by the presence of calculus, which is virtually always covered with unmineralised plaque.[1,2] Calculus is plaque that mineralizes to form calcified deposits. Although gingivitis can develop in the absence of supragingival calculus, it is not clear to what extent the presence of mineralized deposit enhances gingival inflammation. In the past 40 years, calculus has been deposed by plaque, and the hardened criminal has come to be viewed as a fossilized remnant of minor significance.[3,4]
Regarding the significance of dental deposits, Schroeder summed up “Initial damage to the gingival margin is presumably due to enzymatic effects caused by the microorganisms of the plaque. This process is enhanced by the formation of supragingival dental calculus, which provides further retention and thus promotes new plaque accumulations.” Plaque-induced chronic inflammation eventually results in formation of periodontal pockets in which sub gingival calculus is deposited. The latter is a secondary phenomenon; it is not a cause of pocket formation but a concomitant manifestation. This subgingival dental calculus in turn favors and promotes the chronicity of the inflammation, and thus, contributes toward making it progressively worse.[5] It has also been thought that calculus is plaque retentive and may impede natural and mechanical oral hygiene activities. It also may act as a reservoir for irritating substances such as endotoxins and bacterial antibodies. About 20 percent of dental calculus is made of organic matrix, non mineralized cavities containing micro-organisms, and extra cellular substance resembling that of the covering soft plaque that are found within the body of supragingival calculus.[6] It is known that the environment within a biofilm is able to support viable bacterial communities through molecular diffusion of nutrients through channels and calculus can be permeated by substances such as endotoxins within 24 hours.[7]
AIM
The aims of this study were (1) To investigate the viability of bacteria within calculus, (2) To examine motility of bacteria, and (3) To identify bacterial morphotypes in calculus.
MATERIALS AND METHODS
After seeking an informed consent, a total of 30 subjects aged from 35 to 55 years (Mean age - 45 ± 5 years) were included in the study.
Supra and subgingival calculus were harvested from 30 subjects having clinical evidence of chronic inflammatory periodontal disease and substantial calculus deposits as a part of periodontal therapy (one sample from each patient).
Patients having history of any systemic disease, who underwent antimicrobial therapy since the past 6 months, oral prophylaxis for at least 6 months prior to harvesting the samples, pregnant, lactating women, patients with salivary gland disease, and xerostomia were excluded from the study. Care was taken to obtain large single pieces (2-5 mm) and to maintain the integrity of the calculus samples. Immediately upon procurement, the harvested calculus samples were divided into two groups [Figure 1].
Figure 1.
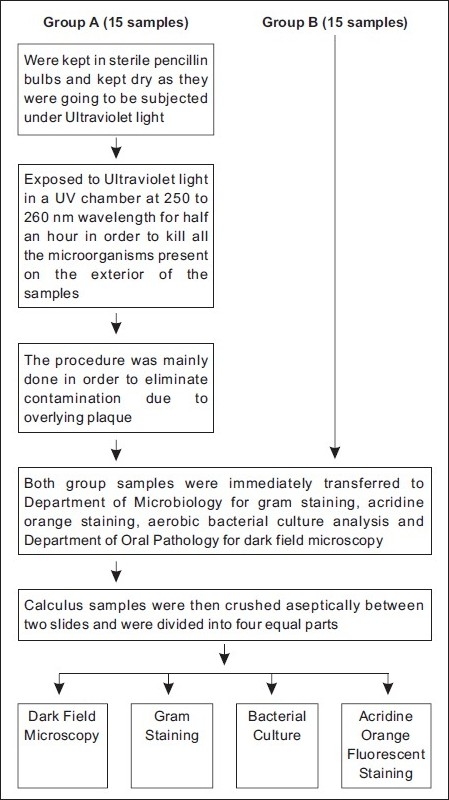
Division and treatment of calculus samples
Group A (15 samples): Harvested samples were immediatelykept in sterile penicillin bulbs to keep them dry. Samples in this group were exposed to Ultraviolet light in a UV chamber (NOVOUltraviolet germicidal chamber, Sankyo Denki, Japan) at 250 to 260 nm wavelength for 30 minutes in order to kill all the microorganisms present on the exterior of the calculus. This procedure was mainly done in order to eliminate contamination due to the overlying plaque.[7] The samples were turned over carefully at fixed intervals to provide enough exposure to UV light to all the surfaces.
Group B (15 samples): Harvested sampleswere immediately placed in sterile saline. Samples from both groups were immediately transported to the Department of Microbiology for gram staining, acridine orange staining, aerobic bacterial culture analysis and also to Department of Oral Pathology for dark field microscopy. Calculus samples were then crushed aseptically between two sterile slides and were divided into four equal parts.
Bacterial culture
Crushed calculus sample was placed in brain heart infusion broth and incubated at 37ºC for 48 hours. Samples were observed for turbidity at the end of 48 hours. The tubes showing turbidity were sub-cultured on Chocolate agar and MacConkey agar (Hi Media, Mumbai, India) [Figure 2]. The plates were incubated aerobically at 37ºC for 48 hours. Identification of growth was done as per the standard protocol.[8] All procedures were carried out in a laminar air flow to maintain sterility (Kartos International - Size 4×2×2, Model: KIB-Type A, Serial No. 1056B)
Figure 2.
Calculus samples harvested on blood agar plates
Acridine orange fluorescent staining
With a 1-microlitre loop a drop of 0.01% acridine orange was put in the centre of the slide. Crushed calculus sample was added to the stain and mixed gently with a sterile loop. A cover slip was placed over the mix and sides were sealed with petroleum jelly. The stained sample was examined with a UV fluorescence microscope (Becton-Dickinson Paralens Fibre optic illuminator Model 4715MS-23W-B10 {50/60 Htz}) at 60× oil objective.
Gram staining
The smears were stained with gram stain and viewed under microscope at 100× oil objective(Nikon Model: E200Sr. No. 763683)
Dark field microscopy
Wet calculus samples were mixed with a drop of normal saline (approximately 50 μl) and a coverslip was put on it. The preparations were observed under 40× objective. Dry samples were directly observed on slides under the microscope at 100× oil objective.
RESULTS
Gram stain [Figure 3] and bacterial culture revealed presence of following organisms in the 12 samples.
Figure 3.
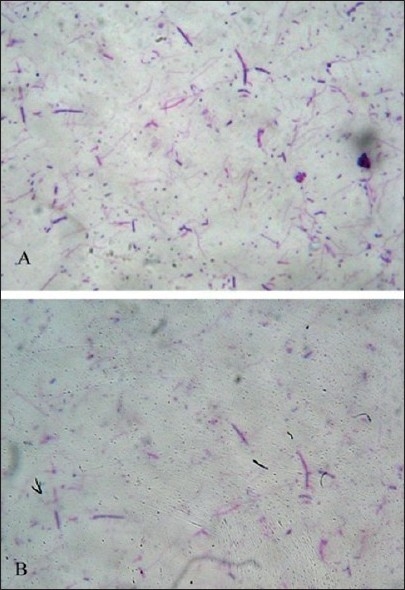
Gram staining of bacteria showing Gram positive cocci and bacilli
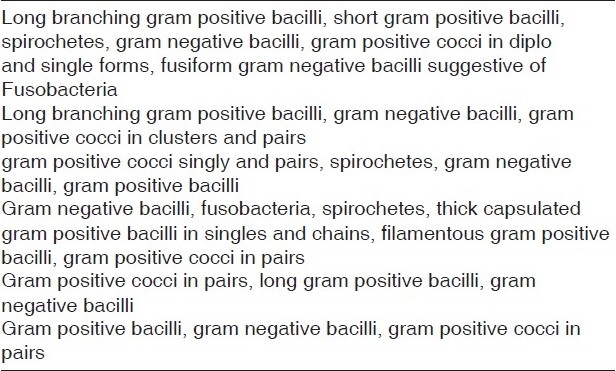
Group A samples showed the presence of Pseudomonas aeruginosa, Staphylococcus aureus, and enterococcus species, [Table 1] whereas Group B samples revealed the presence of Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, Klebsiella pneumonia, and enterococcus species [Table 2].
Table 1.
Bacterial morphotypes observed in Group A samples

Table 2.
Bacterial morphotypes observed in Group B samples
Dark field microscopic examination revealed the presence of filamentous organisms, spirochetes, and motile short bacilli. Motility of organisms was appreciated in all the 12 samples. No difference was seen between the groups [Figure 4].
Figure 4.
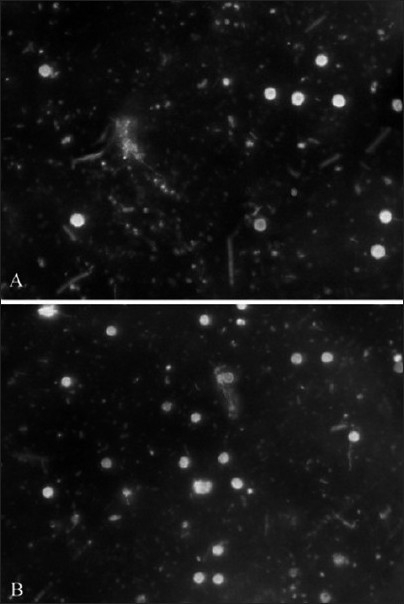
Dark field microscopic imaging showing presence of rods and cocci
After application of the acridine orange fluorescent stain both viable bacteria appeared apple green. Viable bacteria which appeared as small dots or areas of bright green fluorescence were detected in calculus samples. All 12 samples demonstrated presence of viable bacteria [Figure 5].
Figure 5.
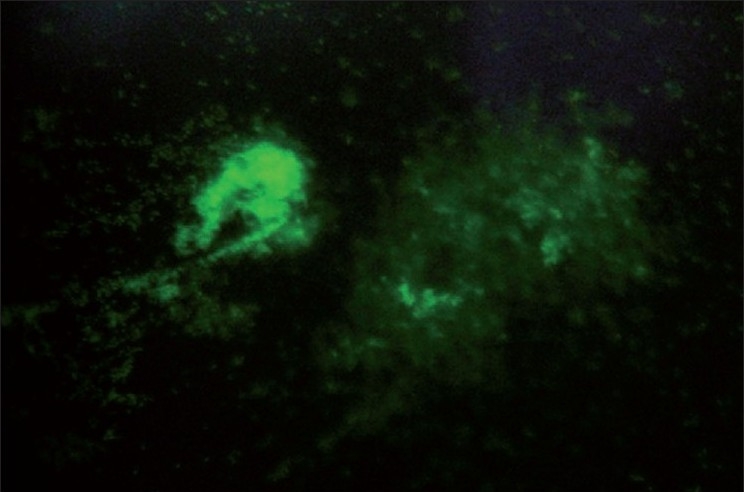
Acridine orange staining showing apple green colored appearance of bacteria
DISCUSSION
The objective of this study was to investigate the viability of bacteria within calculus using four techniques namely dark field microscopy, gram staining, acridine orange staining, and bacterial culture, and also to examine motility of bacteria as well as identify bacterial morphotypes in calculus.
Bacterial culture revealed growth of viable aerobic microorganisms from both group samples. Group B samples were first placed under ultraviolet light for half an hour each side in order to kill any viable microorganisms present on the plaque covering the calculus samples; however, it did not make a significant difference to the results as both group samples showed almost similar growth of organisms. Similar growth was obtained on the exterior as well as interior of calculus samples. It could be hypothesized that calculus is pathogenic suggesting that bacteria may reside within channels or substance of calculus. Tan et al [7] eliminated contamination due to the overlying plaque by placing calculus samples overnight under ultraviolet light on a shaker to enable all surfaces of calculus to be exposed to UV light. They too indicated the possibility of presence of pathogens within calculus. Sidaway[9] reported successful bacterial culture from samples of supragingival and subgingival calculus, although the superficial covering of plaque was probably included in the cultures. Although there are studies implying that calculus may be essentially mineralized dead organic material it has been shown that calcification can occur in a culture of live bacterial colonies. It is therefore possible that some organisms may readily calcify, while others may not leading to survival of non-mineralized bacteria within the otherwise calcified mass.[10]
Spirochetes are difficult to culture but one of the most common organisms associated with periodontitis. Dark field microscopy was an important tool for detecting presence and viability of these bacteria from plaque. Dark field microscope was mainly used to see the presence of spirochetes as well as motility of bacteria, which is an indicator of bacterial viability. With the help of dark field microscopy the presence of spirochetes could be easily demonstrated from the samples.[8]
Transmission electron microscope studies have indicated that some of the bacteria within the non mineralized channels and cavities in supragingival calculus remain intact and appear viable.[11]
Gram staining was used mainly to visualize bacteria and also to study morphology of all these microorganisms. Gram character is of considerable importance as the gram positive and negative bacteria differ not merely in staining characteristics and in structure but also in other properties such as growth requirements, susceptibility to antibiotics and pathogenecity.[8]
Acridine orange, a fluorochrome strain, is potentially superior to the gram stain in the direct microscopic examination of clinical specimens because it gives striking differential staining between bacteria and background cells and debris.[12]
Acridine orange stain has marked affinity for nucleic acids and stains both live and dead bacteria. When the samples were stained, the DNA component of the organisms appeared green. All the 12 samples gave an apple green fluorescence. This method was useful for detecting weakly staining gram negative bacteria if at all they were overlooked in gram stain.
Lauer assessed its value in clinical laboratories by testing 209 cerebrospinal fluids and 288 other body fluids, tissues, and exudates by both techniques. Smears were made in duplicate, fixed with methanol, stained, and examined without knowledge of the result of the companion smear or culture. Overall, acridine orange was slightly more sensitive than the gram stain (acridine orange, 59.9%; gram stain, 55.8%) and equally specific in detecting microorganisms. It was concluded that acridine orange staining was a sensitive method for screening clinical specimens and reviewing selected specimens that are purulent, but negative by the gram stain.[12] Overall, there were no appreciable differences between Group A and Group B samples suggesting that calculus does not just serve as a nidus for formation of plaque biofilm on the surface. Hence, it is clear that in spite of mineralization viable bacteria are present within the substance of calculus. These findings are of clinical importance as bacteria within calculus may serve as a reservoir of organisms that may play a crucial role in the etiology of periodontal disease.[13–16] If bacteria in calculus are vital, they may well be releasing toxic antigenic metabolites that may leach from calculus. If bacteria in calculus are non vital, the by-products from their degradation such as lipopolysaccharide remnants might be leached from calculus into soft tissues.[17–21] This has led Mandel and Gaffar[1] to call it as “slow releasing device releasing toxic and pathogenic products into the soft tissues.” These may initiate inflammatory responses into soft tissues. This study provides strong evidence indicating that viable microorganisms contained within the calculus probably within the lacunae and channels. Furthermore, results of the culture data imply that some of these bacteria are capable of growth when placed in a suitable environment, as might be the scenario with incomplete removal of calculus.[22] This study had a few short comings as anerobic culture would give a clear picture of the microbiota since anaerobic organisms play a vital role in the pathogenesis of periodontal disease. The presence of viable bacteria within calculus may explain some of the conflicting results of the epidemiological and clinical studies and reinforce the case for ensuring complete removal of these deposits from the tooth surface.
CONCLUSION
From the results, it appears that viable aerobic bacteria may be present within calculus specifically within internal channels and lacunae.
Clinical relevance
Scientific rationale for the study
Calculus thus plays an important role in the etiology of periodontal disease contrary to early reports that it plays an important role in just serving as a reservoir for plaque deposition and endotoxins only. Therefore, the present study was conducted to investigate the viability of bacteria within calculus, to examine motility of bacteria, and to identify bacterial morphotypes in calculus.
Principal findings
Bacteria reside within channels and lacunae in contrast to earlier studies, which stated that micro-organisms are killed once plaque calcifies to form calculus. Viable bacteria were found within calculus.
Practical implications
It has been well documented that thorough subgingival scaling and root planing will result in a reduction of periodontal bacteria. However, in the presence of supragingival calculus, rapid subgingival colonization will occur within a few weeks. Hence, a thorough mechanical debridement to ensure complete removal of this pathogenic calculus is mandatory and it will also help prevent progression of periodontitis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mandel ID, Gaffar A. Calculus revisited.A review. J Clin Periodontol. 1986;13:249–57. doi: 10.1111/j.1600-051x.1986.tb02219.x. [DOI] [PubMed] [Google Scholar]
- 2.Roberts-Harry EA, Clerehugh V. Subgingival calculus: Where are we now.A comparative review? J Dent. 2000;28:93–102. doi: 10.1016/s0300-5712(99)00056-1. [DOI] [PubMed] [Google Scholar]
- 3.Mandel ID. Biochemical aspects of calculus formation.II Comparative studies of saliva in light and heavy calculus formers. J Period Res. 1974;9:211–21. doi: 10.1111/j.1600-0765.1974.tb00675.x. [DOI] [PubMed] [Google Scholar]
- 4.Mandel ID. Calculus Update Prevalence, Pathogenecity and Prevention. J Am Dent Ass. 1995;126:575. doi: 10.14219/jada.archive.1995.0235. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder HE. Huber: Berne: Verlag H; 1969. Formation and inhibition of dentalcalculus; pp. 11–69. [Google Scholar]
- 6.Schueback P, Guggenheim B. Structural and ultrastructural features of subgingival and supragingival human dental calculus. J Dent Res (AADR abstracts) 1992;71:251. [Google Scholar]
- 7.Tan BT, Mordan NJ, Embleton J, Pratten J, Galgut PN. Study of Bacterial viability within human Supragingival dental calculus. J Periodontol. 2004;75:23–9. doi: 10.1902/jop.2004.75.1.23. [DOI] [PubMed] [Google Scholar]
- 8.Winn W, Jr, Allen S, Janda W, Koneman E, Procop G, Schreckenberger P, et al. USA: Lippincott Williams and Wilkins; 6th ed. USA: Lippincott Williams and Wilkins; 2006. Koneman's colour atlas and text book of diagnostic microbiology; pp. 2–64. [Google Scholar]
- 9.Sidaway DA. A microbiological study of dental calculus.I The microbiological flora of mature calculus. J Period Res. 1978;13:349–59. doi: 10.1111/j.1600-0765.1978.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 10.Wasserman BH, Mandel ID, Levy BM. in vitro calcification of dental calculus. J Periodontol. 1958;29:144–7. [Google Scholar]
- 11.Galgut PN, Mordan N, Newman HN. A transmission electron microscope study of supragingival calculus. J Int Acad Periodontol. 2001;3:31–7. [PubMed] [Google Scholar]
- 12.Lauer BA, Reller LB, Mirrett S. Comparison of acridine orange and Gram stains for detection of microorganisms in cerebrospinal fluid and other clinical specimens. J Clin Microbiol. 1981;14:201–5. doi: 10.1128/jcm.14.2.201-205.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Addy M, Loltai R. Control of supragingival calculus. J Clin Periodontol. 1994;21:342–6. doi: 10.1111/j.1600-051x.1994.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 14.Anerud A, Loe H, Boyser H. The natural history and clinical course of calculus formation in man. J Clin Periodontol. 1991;18:160–70. doi: 10.1111/j.1600-051x.1991.tb01128.x. [DOI] [PubMed] [Google Scholar]
- 15.Bauhammers H, Rohrbaugh EA. Permeability of human and rat dental calculus. J Periodontol. 1970;41:39–42. doi: 10.1902/jop.1970.41.5.279. [DOI] [PubMed] [Google Scholar]
- 16.Chesters RK, Huntington F, Schafer F. Relationship between dental caries and calculus. Caries Res. 1994;28:209. [Google Scholar]
- 17.Friskopp J, Hammarstrom L. A comparative Scanning electron microscopic study of supragingival and subgingival calculus. J Periodontol. 1980;1:553–8. doi: 10.1902/jop.1980.51.10.553. [DOI] [PubMed] [Google Scholar]
- 18.Friskopp J. Ultrastructure of nondecalcified supragingival and subgingival calculus. J Periodontol. 1982;9:542–50. doi: 10.1902/jop.1983.54.9.542. [DOI] [PubMed] [Google Scholar]
- 19.Pfeiffer HJ. The prevalence and incidence of dental calculus in adults. J Clin Dent. 1989;1:55–58. [PubMed] [Google Scholar]
- 20.Sewon L, Parvinen T, Sinisalo T, Larmas M, Alanen P. Dental status of adults with and without periodontitis. J Periodontol. 1988;59:595–598. doi: 10.1902/jop.1988.59.9.595. [DOI] [PubMed] [Google Scholar]
- 21.Volpe AR, Petrone ME, Davies RM. A review of calculus clinical efficacy studies. J Clin Dent. 1993;4:71–81. [PubMed] [Google Scholar]
- 22.Picozzi A, Fischman SL, Pader M, Cancro LP. Calculus inhibition in humans. J Periodontol. 1972;43:692–695. doi: 10.1902/jop.1972.43.11.692. [DOI] [PubMed] [Google Scholar]


