Abstract
Background
Free gingival grafts have been used extensively for gingival augmentation procedures, but are associated with postoperative morbidity because of the open palatal wound. This study compares the clinical efficiency of two dressing materials, a non-eugenol-based dressing (Coe-Pak™) and a collagen dressing (Colla Cote®) on palatal wound healing.
Materials and Methods
Thirty-two patients in the age group of 25−50 years, who required gingival augmentation, were selected. Free gingival graft was harvested from the palatal mucosa and the wound was then protected using Coe-pak® in control group and Colla Cote® in test group. The subjective parameters pain and burning sensation were recorded on the 2nd and 7th day and the objective parameters colour and consistency were recorded on the 7th and 42nd day, using a visual analog scale. Thickness of the mucosa was measured using K file at baseline and 42nd day. Histological examination was done on 42nd day.
Results
The subjective and objective parameters showed significant improvement in the test group when compared to control group. Histologically, there was a greater evidence of collagen formation and turn over in the test group than control group.
Conclusions
Collagen-based dressing may thus offer significantly greater advantages over the traditional non-eugenol dressings.
Keywords: Collagen dressing, periodontal dressings, visual analog scale
INTRODUCTION
Free gingival grafts have been used as a predictable and reproducible technique for increasing the width of keratinised gingiva around natural teeth and implants.[1] The postoperative morbidity associated with an open secondary wound site includes inconvenience caused by pain and post surgical bleeding.[2] The necessity for enhancement of healing time arises when the palatal donor site is required for repeated procedures.
A variety of dressing materials have been used to cover and protect the wound surface from the external environment after periodontal surgery.[3] Conventional periodontal dressings provide an inert mechanical barrier, thereby assisting healing by prevention of external influences on the wound area.[4] However, these dressings neither influence cellular behavior nor play a major role in the biological events that take place during wound healing. Biological dressings on the other hand may help accelerate the cascade of events involved in the healing process.
Collagen dressings have been extensively used in the field of medicine and dentistry due to its ability to achieve hemostasis, chemotactic to fibroblasts and platelets, and inducing mesenchymal proliferation and differentiation.[5,6]
A porous, non-friable collagen sponge (Colla Cote®) is currently available as a dressing material for covering palatal donor sites. It is stated to help adsorb blood and wound exudates and promote hemostasis, thus aiding wound healing while concurrently enhancing patient's comfort. The clinical efficacy of these dressings can however be fully ascertained only when it is compared to the time tested non-eugenol dressing material (Coe Pak™).[7]
The objective of the study was to compare the effects of a collagen-based dressing material (Colla Cote®) and a non- eugenol pack (Coe Pak™) on palatal wounds using various subjective and objective clinical parameters.
MATERIALS AND METHODS
Study subjects
Thirty-two patients in the age group of 25–50 years, who required gingival augmentation, were selected from those seeking care at the Department of Periodontics, Ragas Dental College and Hospital, during the period April 2005 to December 2005. The patients signed an informed consent approved by the institutional review board. Care was taken in selecting non-smoking patients in good general health. The patients were randomly assigned into control group (Coe Pak™) or test group (Colla Cote®) with sixteen patients in each group.
Surgical procedure
One hour prior to the surgery, the patients were given 0.12% chlorhexdidine gluconate solution to swish with for 30 seconds. Lidocaine 2% with 1:80,000 epinephrine was administered at recipient and palatal donor surgical site. After preparing the recipient site for accepting the graft, palatal donor tissue of required dimension was procured. In the control group, non-eugenol, non-resorbable periodontal dressing (Coe-Pak™)* was placed on the palatal donor site. In the test group, a collagen-based wound dressing (Colla Cote)† was placed. The palatal donor site was then protected with a custom made acrylic stent. The area was examined for 2−3 minutes for any fresh bleeding
Following surgery, patients were instructed not to remove the stent and avoid hot food and beverages for two days. Patients were prescribed Cap. Amoxicillin (500 mg thrice a day for five days) in addition to anti-inflammatory analgesics.
At the 2nd postoperative day patients were reviewed for any complications. The patients were evaluated post surgically at 2nd , 7th , 14th , and 42nd day by two independent investigators. Clinical parameters were assessed on subjective and objective basis.
Subjective assessment
Patients were asked to assess pain and burning sensation at the end of 2nd and 7th day using a Visual Analog Scale (VAS). The VAS score for pain ranged from 0 (no pain) to 10 (severe pain); while the burning sensation was scored as follows: 0 (absent) and 1 (present).
Objective assessment
Consistency
Consistency of the palatal mucosa was assessed on the 7th and 42nd day by palpation with a blunt instrument and scored as soft or firm.
Color match
On the 7th and 42nd day, the color of the palatal mucosa was assessed by comparing it to that of the adjacent and opposite side using the objective VAS (VAS score 0−10). Score 0 indicated no color match and score 10 indicated very good color match with the adjacent tissues.
Thickness
Thickness of the palatal mucosa was measured using a sterile standard endodontic K file of size 15 from the surface of the mucosa to the bone. The measurement was made 1 cm from the marginal gingiva between the premolars and rounded off to the nearest 0.5 mm.
Histological examination
To evaluate and compare the tissue response to both dressing materials at the microscopic level, standardized punch biopsy was taken at the end of 42nd day from both the control and test sites using a Tru-cut biopsy needle and compared with healthy tissues. The epithelial and connective tissue characteristics were studied by Hematoxyilin and Eosin (H and E) staining method, and the amount of mature collagen in the stromal area was studied by Masson's Trichrome staining method in selected 5 sites in each group. The interpretation was done at ×100 magnification by an independent examiner [Figure 1a–d].
Figure 1.
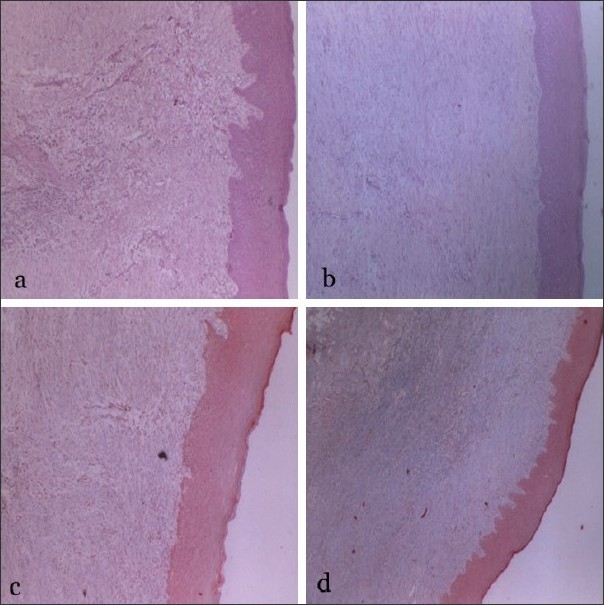
(a) H & E staining on 42nd day demonstrating complete epithelialisaton with extensive retepeg formation in control group (×10 magnification); (b) H & E staining on 42nd day demonstrating greater connective tissue formation with less inflammatory cell infiltration in test group; (c) Masson's trichrome staining exhibiting mature collagen in control group; (d) Masson's trichrome staining exhibiting greater amount of mature collagen in test group when compared to control group (1c)
Statistical analysis
Chi-Square test was employed to test the percentage changes of consistency and burning sensation at different time points between the study groups. Student's t-test and Student's Paired t-test was employed to test the changes in the mean scores of thickness, colour match and pain between and within the groups at different time points, respectively. In the present study, P<0.05 was considered as the level of significance.
RESULTS
The results of various clinical parameters are as follows:
Objective assessment
Consistency
On the 7th day, 93.3% of the sites in the control group and 100% of the sites in the test group were soft. On the 42nd day, 53.3% of the sites in the control group and 29.3% in the test group were moderately firm in consistency [Table 1]. While comparing the changes in consistency between the test and control groups, there was a statistically significant difference present on the 42nd day (P=0.309), but on the 7th day, it was not significant (P=0.005) [Table 1].
Table 1.
Clinical parameters consistency and colour evaluated on 7th and 42nd day among control and test groups

Color match
On the 7th day, the VAS for color was 2.40±0.91 and 2.60±0.83 for the control and test groups, respectively, whereas on the 42nd day it was 6.20±1.27 and 7.27±1.16 [Table 1]. The difference between the mean scores of test and control group on the 7th and 42nd day was not statistically significant (P=0.534).
Thickness
In the test group, at baseline, mean thickness was 4.13±0.69 mm and on the 42nd day, 3.07±0.96 mm [Table 2]. The mean difference in thickness between the baseline and 42nd day was 0.43±0.42mm, and the percentage of deficiency was 10.4%, which was statistically significant (P=0.001).
Table 2.
Comparison of the thickness of palatal mucosa among the control and test groups at baseline and on 42nd day

In the control group, at baseline, mean thickness was 4.40±0.83 mm and on the 42nd day was 4.37±0.73 mm [Table 2]. The mean difference in thickness between base line and 42nd day was 0.03±0.23 mm, and the percentage of deficiency was 0.7%, which was not statistically significant (P=0.582).
Subjective assessment
Pain
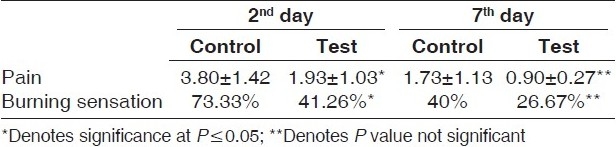
Using the VAS for pain, on the 2nd day the control group patients showed a mean score of 3.80±1.42, whereas the test group patients exhibited a mean score of 1.93±1.03. Similarly, on the 42nd day it was 1.73±1.13 and 0.90±0.27 for control and test groups, respectively [Table 3]. The difference between the mean scores of test and control group was statistically significant on the 2nd (P<0.001), but not significant on 7th day (P=0.006).
Table 3.
Subjective parameters pain and burning sensation evaluated among the test and control groups on 2nd and 7th day
Burning sensation
On the 2nd day, burning sensation was present in 73.33% of the control sites and 41.26% of the test sites, whereas on the 7th day, burning sensation was present in 40% of the control sites and in 26.67% of the test sites [Table 3]. On comparison, the test and control groups showed statistically significant difference (P=0.271) on the 2nd day, whereas on the 7th day there was no statistically significant difference (P=0.031) [Table 3].
Histological evaluation
Epithelialisation was complete in both the groups. However, rete peg formation was found to be more extensive and deeper in the control sites than the test sites. In the stromal connective tissue area, the defect was completely filled by fibrovascular tissue in both the test and control sites with more amount of mature collagen in the test group when compared with control group. While the inflammatory cell infiltration in the perivascular region was moderate in the control sites, the test sites showed mild mononuclear cell infiltration. All the above changes were significant in the healed tissues when compared to the healthy tissues.
DISCUSSION
Even with the advent of other mucogingival procedures, free gingival graft continues to be the most predictable method of increasing apico-occlusal width of keratinised gingiva.[8] The need for keratinized mucosa around teeth and implant arises from its ability to resist mechanical stress and retard spread of inflammation. Free gingival graft is therefore still in vogue as mucogingival surgical procedure directed towards augmenting the existing gingival dimensions. Postoperative morbidity is however an important limitation of this procedure.[9] This postoperative morbidity is related to the palatal wound that is left to heal by secondary intention.
Several periodontal dressings have been employed to cover the open palatal wound so as to ensure uncomplicated healing. These dressings have shown satisfactory clinical results. But their ability to assist healing has been largely through mechanical protection of the wound area.[4] Biologic dressings have been introduced with a view to accelerate the healing process and supposedly provide a more comfortable healing phase to the patient through their ability to influence cellular behavior.[10,11] However, these dressings need to be evaluated in comparison with the traditional non-eugenol dressings to determine if there is any actual benefit accrued to the patient.
In this study, therefore, palatal healing process was evaluated subjectively and objectively by the patient and the investigator. Pain and burning sensation were analyzed by the patient using the VAS, which could provide a mathematical scale for human judgments. The subjective parameters, pain and burning sensation are significantly less on the 2nd day in the test group, when compared to the control group. However, no difference was observed between the groups on the 7th day. These findings were similar to those of Jorkjend 1990, which showed that Coe Pak™ dressings were associated with greater pain.[12] The reduced pain sensation observed in this study could be a result of collagen dampening the acute inflammatory process during healing.[10]
Although Coe Pak™ is eugenol free, and thus, without any irritant that may contribute to substantial inflammation and pain, it has very little biological effect on the palatal tissues. Collagen is a natural substrate of the extracellular matrix, is chemotactic to various cell types such as endothelial cells, fibroblasts, and osteoblasts.[11] The collagen dressing used in this study would therefore have contributed to the dampening of the inflammatory process that occurs during healing. The lesser degree of inflammation could probably be directly related to the lesser pain and burning sensation observed in the test group.[13]
There was no significant difference in the color in the postoperative phase between the two groups. This may be interpreted as evidence of good healing in both the groups. Collagen dressing no doubt has accelerated the healing process as evidenced by lesser pain, but eventually satisfactory healing may be obtained even with placement of Coe Pak™.
Evidence of acceleration of early healing process, due to the presence of collagen was obtained from the greater firmness of the tissue at the end of the 2nd week in the test group. This is in accordance with studies, which have demonstrated transformation of absorbable collagen material to look more like normal tissue with no collagen material seen after 4 days.[14] Similarly when the thickness was examined at the end of the study period there was a significant increase in the test group when compared to the control group. This may be interpreted as evidence of integration of collagen dressing with the host palatal tissue.
The collagen used in this study is predominantly of type IV and type I collagen. Type IV collagen is conducive to re-epithelialisation and the accelerated healing may be associated with the firm consistency and pink color of the palatal mucosa observed in the test sites. The increased thickness in the collagen group could be a reflection of the greater bulk of the connective tissue that is formed after healing in the palatal mucosa.
The histological evidence of the wound area in the control group exhibits greater inflammatory cells and less collagen turnover similar to previous studies.[14,15] Test group demonstrated greater connective tissue turn over with more mature collagen [Figure 1]. The greater collagen turnover observed in the test group would translate clinically to firmer connective tissue in the palate. When multiple teeth are being treated or revision surgery is required it is sometimes necessary to use the palatal donor site for a second time. The increased firmness and greater connective tissue formation means that the palate can be again used as a donor site within a short interval of time.
In conclusion, the palatal wound covered with the collagen dressing Colla Cote® showed evidence of earlier healing and provided better symptomatic relief to the patients when compared to those covered with Coe Pak™ .
Footnotes
*GC America Inc. USA
†Zimmer Dental CA USA
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Matter J. Free gingival grafts for the treatment of gingival recession- A review of some techniques. J Clin Periodontol. 1982;9:103–14. doi: 10.1111/j.1600-051x.1982.tb01226.x. [DOI] [PubMed] [Google Scholar]
- 2.Brasher WJ, Rees TD, Boyce WA. Complications of free grafts of masticatory mucosa. J Periodontol. 1975;46:133–8. doi: 10.1902/jop.1975.46.3.133. [DOI] [PubMed] [Google Scholar]
- 3.Farnoush A. Techniques for the protection and coverage of the donor sites in free soft tissue grafts. J Periodontol. 1978;39:403–5. doi: 10.1902/jop.1978.49.8.403. [DOI] [PubMed] [Google Scholar]
- 4.Rubinoff CH, Greener EH, Robinson PJ. Physical properties of periodontal dressing materials. J Oral Rehabil. 1986;13:575–86. doi: 10.1111/j.1365-2842.1986.tb00681.x. [DOI] [PubMed] [Google Scholar]
- 5.Doillon CJ, Silver FH. Collagen-based wound dressing: effects of hyaluronic acid and fibronectin on wound healing. Biomaterials. 1986;7:3–8. doi: 10.1016/0142-9612(86)90080-3. [DOI] [PubMed] [Google Scholar]
- 6.Levin MP, Tsaknis PJ, Cutright DE. Healing of the oral mucosa with the use of collagen artificial skin. J Periodontol. 1979;50:250–3. doi: 10.1902/jop.1979.50.5.250. [DOI] [PubMed] [Google Scholar]
- 7.Cheshire PD, Griffiths GS, Griffiths BM, Newman HN. Evaluation of the healing response following placement of Coe-Pak and an experimental pack after periodontal flap surgery. J Clin Periodontol. 1996;23:188–93. doi: 10.1111/j.1600-051x.1996.tb02075.x. [DOI] [PubMed] [Google Scholar]
- 8.Camargo PM, Melnick PR, Kenney B. The use of free gingival grafts for aesthetic purposes. Periodontology. 2001;27:72–96. doi: 10.1034/j.1600-0757.2001.027001072.x. [DOI] [PubMed] [Google Scholar]
- 9.Terrence J, Griffin, Wai S, Cheung, Athanasios I, Zavras, Damoulis Petros D. Postoperative Complications Following Gingival Augmentation Procedures. J Periodontol. 2006;77:2070–9. doi: 10.1902/jop.2006.050296. [DOI] [PubMed] [Google Scholar]
- 10.Mian M, Beghe F, Mian E. Collagen as a pharmacological approach in wound healing. Int J Tis react. 1992;14:1–9. [PubMed] [Google Scholar]
- 11.Cullen B, Watt PW, Lundqvist C, Silcock D, Schmidt RJ, Bogan D, et al. The role of oxidised regenerated cellulose/collagen in chronic wound repair and its potential mechanism of action. Int J Biochem Cell Biol. 2002;34:1544–56. doi: 10.1016/s1357-2725(02)00054-7. [DOI] [PubMed] [Google Scholar]
- 12.Jorkend L, Skoglund LA. Effect of non-eugenol and eugenol containing periodontal dressings on the incidence and severity of pain after periodontal soft tissue surgery. J Clin Period. 1990;17:341–4. doi: 10.1111/j.1600-051x.1990.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 13.Rossmann JA, Rees TD. A comparative evaluation of hemostatic agents in the management of soft tissue graft donor site bleeding. J Periodont. 1999;70:1369–75. doi: 10.1902/jop.1999.70.11.1369. [DOI] [PubMed] [Google Scholar]
- 14.Saroff SA, Chasens AL, Eisen SF, Lavey SH. Free soft tissue autografts.Hemostasis and protection of the palatal donor site with a microfibrillar collagen preparation. J Periodont. 1982;53:425–8. doi: 10.1902/jop.1982.53.7.425. [DOI] [PubMed] [Google Scholar]
- 15.Nezwek RA, Caffesse RG, Bergenholtz A, Nasjleti CE. Connective tissue response to periodontal dressing. J Periodontol. 1980;51:521–9. doi: 10.1902/jop.1980.51.9.521. [DOI] [PubMed] [Google Scholar]


