Abstract
N-of-1 or single subject clinical trials consider an individual patient as the sole unit of observation in a study investigating the efficacy or side-effect profiles of different interventions. The ultimate goal of an n-of-1 trial is to determine the optimal or best intervention for an individual patient using objective data-driven criteria. Such trials can leverage study design and statistical techniques associated with standard population-based clinical trials, including randomization, washout and crossover periods, as well as placebo controls. Despite their obvious appeal and wide use in educational settings, n-of-1 trials have been used sparingly in medical and general clinical settings. We briefly review the history, motivation and design of n-of-1 trials and emphasize the great utility of modern wireless medical monitoring devices in their execution. We ultimately argue that n-of-1 trials demand serious attention among the health research and clinical care communities given the contemporary focus on individualized medicine.
Keywords: clinical equipoise, early-phase trials, individualized medicine, n-of-1, remote phenotyping, single patient trial, treatment repositioning, wireless health
There is a growing acceptance that the development of medical interventions that work ubiquitously (or under most circumstances) for the majority of common chronic conditions is exceptionally difficult and all too often has proved to be fruitless [1,2]. This recognition has led to the notion that the clinical practice of medicine should acknowledge and embrace the unique characteristics of individual patients, particularly at the genetic level, and seek to individualize patient care [3–5]. In addition, there has been a great deal of emphasis on obtaining and evaluating objective criteria for claims that certain interventions work better than others. For example, initiatives to facilitate and promote ‘evidence-based’ medicine [6,7] and ‘comparative effectiveness’ research [8] have been proposed by many government and research agencies. In fact, these beliefs are so strong that legislation to promote research and practices aimed at personalizing medicine has been introduced in at least the USA [9], and the US NIH has initiated large-scale programs to facilitate comparative effectiveness research. Such initiatives have even become a rallying cry for reinvigorating the troubled US healthcare system [10–13].
The interest in evidence-based, as well as individualized medicine, has led to some very notable discoveries. For example, for individualized medicine, genetic data have been exploited to identify therapies appropriate for an individual and has led to changes in drug oversight policy and the way certain drugs have been labeled. For example, many cancer therapeutic responses have been demonstrated to be influenced by very specific tumor genetic profiles, which has led to the obvious notion that before one administers the relevant compounds, a patient’s tumor should be screened for the presence of specific genetic profiles [14]. In fact, the drug cetuximab (Erbitux®), used to treat colorectal cancer, is rendered ineffective in the presence of a specific mutation in the KRAS protein in the tumor [15]. In response, the US FDA relabeled the drug to indicate a need for genetic profiling before administering the drug. There are many other instances in which connections between the presence of genetic variations and noncancer drug effectiveness or side-effect profiles have been made that have led to FDA relabeling, such as warfarin, carbamazepine and clopidogrel [16,17]. At present, approximately 10% of labels for FDA-approved drugs contain pharmacogenomic information. In addition, the FDA is actively involved in creating a streamlined review approach to diagnostic companion tests with therapeutics where n-of-1 trials could play a role in facilitating the approval process [18].
As compelling as these studies and consequent drug administration policy changes are, they do not necessarily indicate a shift towards true individualized medicine since they only reflect attempts to fractionate or stratify the larger population into smaller groups likely and not likely to benefit from specific treatments [19]. Hence, they do not involve a true consideration of all the nuances and characteristics individual patients may have that would dictate – or be most compatible with – therapies tailored specifically to those patient characteristics. Obviously, as more insights or connections between various factors and drug responses are revealed, the more likely clinical care can be specifically directed to the unique combinations of factors that define an individual patient’s clinical presentation. Until that time, however, for many clinical conditions, a physician is faced with the dilemma of true ‘clinical equipoise’ in which the best course of therapy is unknown a priori simply because connections between individual patient characteristics, such as genetic profile, and likely response to particular therapeutic agents, have not been identified. Many physicians recognize that the practice of medicine is individualized medicine but not in a systematic manner across every patient, physician and health institution. N-of-1 trials, which focus on the objective determination of the optimal therapy for a single individual, can possibly improve outcomes by preserving some homogeneity while stratifying care among patients.
An intuitive way around this dilemma is to treat the individual patient as a study subject and objectively and empirically determine the best course of therapy. Such single subject or ‘n-of-1’ trials have great precedent in educational and behavioral settings, but have not been used to an appreciable degree within the medical and clinical communities; in fact, many such trials have been disparaged as ‘only anecdotal’ [20]. There are many reasons for this, not the least of which is cost, but n-of-1 studies are a promising way to advance individualized medicine and a method for gaining insights into comparative treatment effectiveness among a wide variety of patients. We review the design and conduct of n-of-1 studies and suggest that modern remote wireless medical devices may play a big role in their execution in the future. We also consider some of the drawbacks of such studies as well as areas for future research.
Do n-of-1 clinical trials have a role in clinical science?
Randomized controlled trials (RCTs) are considered the sine qua non of applied biomedical research. The objective evaluation of the benefits and problems associated with novel clinical interventions by directly comparing them with standard or sham (placebo) interventions allows claims to be made about the ultimate effectiveness and utility of those interventions. Although the amount of evidence one might need in order to motivate the pursuit of a clinical intervention in the absence of a clinical trial is arguable, the basic motivation and scientific foundation behind clinical trials are not in doubt, and few would argue that the positive results of a well-designed clinical trial could ever hurt the case for implementing or pursuing an intervention. The appropriateness of different designs for clinical trials, however, is highly debatable and a rich area of biostatistical research. For example, the appropriateness of certain kinds of adaptive designs, which minimize the amount of time a subject is on an inferior intervention, sequential designs that seek to reach a conclusion about an intervention prior to a fixed, prespecified lengthy data collection process, crossover designs that allow subjects to act as their own controls, and other strategies all come with challenges that need to be considered when vetting or testing particular interventions, especially for rare diseases and unique situations [21–23].
One issue that has been of immense historical and clinical importance in the design and conduct of clinical trials involves the generalizability of the results, especially if they suggest a novel intervention has utility. Addressing this issue is important because it obviously impacts on wider use, dissemination and marketing of an intervention after the completion of a successful clinical trial. Ensuring that a trial’s design and subject enrollment facilitates applicability of the results is not trivial given the tremendous heterogeneity of diseased populations. In this light, n-of-1 trials that focus exclusively on the objective, empirically determined optimal intervention for a single patient or subject clearly defy easy generalizability, but are compatible with the ultimate end point of clinical practice – the care of individual patients. In addition, clinical studies focusing on the treatment of single patients is, as noted previously, actually more consistent with the vision of individualized or personalized medicine than stratifying patients into groups more or less likely to benefit from a specific treatment on the basis of population-level association studies [24,25]. Finally, as discussed later, n-of-1 trials could be very efficient and less costly vehicles for motivating serious consideration about an intervention with respect to other patients, larger patient groups, or other clinical conditions.
N-of-1 trials have been pursued routinely in education and learning settings [26], often in behavioral and psychological assessment settings but, with the exception of studies of pain medications (Table 1) [27], rarely in medical settings (Table 2). The reasons for this are unclear but may have to do with the physician’s ability to effectively monitor relevant clinical end points easily and remotely, as well as the costs and time involved in both patient and physician conducting n-of-1 trials [28,29]. Although modern wireless health-monitoring devices may help overcome these problems, as discussed later. The ultimate benefits of n-of-1 trials may derive from the reality that interventions of whatever type rarely work in everyone. If comparable interventions have differing effects across groups of patients defined by certain characteristics, then it is highly likely that these interventions will show variation in efficacy between individuals, even within specific strata, as long as those strata are defined appropriately [30–32]. N-of-1 trials explore this variability in an objective way while simultaneously leading to an informed decision about the best way to treat an individual patient using his or her own data. Furthermore, with the rising cost of patient care (including drug costs and clinic visits), it is desirable to minimize clinic visits and patient time on a suboptimal treatment. Therefore, although outcomes must be shown on a case-by-case basis, it is possible that efficient n-of-1 trials will be comparatively more effective at identifying and minimizing the time on suboptimal interventions than standard care [33].
Table 1.
Examples of individual and combined n-of-1 studies investigating the utility of an intervention in pain and discomfort related to a disease.
| Disease | Trials (n) | Intervention (Dx) | Results | Ref. |
|---|---|---|---|---|
| Chronic neuropathic pain | 73 | Gabapentin | N-of-1 trials impacted Tx use of gabapentin | [40] |
| Childhood arthritic pain | 6 | Amitriptyline | No benefit of amitriptyline | [37] |
| Refractory neuralgia | 1 | Spinal cord stimulation | Study led to effective use of stimulation | [61] |
| Osteoarthritis | 56 | Paracetamol/celecoxib | Paracetamol more effective | [40] |
| Nausea from chemotherapy | 12 | Metopimazine | Metopimazine use is beneficial | [62] |
| Skeletal cramping | 13 | Quinine | Heterogeneity in quinine response | [63] |
| Chronic pain | 116 | Paracetamol/ibuprofen | N-of-1 trials led to many Tx changes | [64] |
| Osteoarthritis pain | 51 | NSAIDs | N-of-1 trials slightly better than standard | [57] |
| Chronic pain | 34 | Cannabis extracts | 28 out of 34 patients achieved benefit | [65] |
| Migraine | 32 | Dextroamphetamine | Improvements with dextroamphetamine | [66] |
| Osteoarthritis | 13 | NSAIDs | Heterogeneity in response to NSAIDs | [67] |
| Depression | 5 | Methylphenidate | Two patients improvement with Dx | [68] |
| Chronic pain | 21 | Ketamine | Small subgroup responded to Dx | [69] |
| Osteoarthritis pain | 25 | NSAIDs | NSAIDs are useful in pain management | [70] |
Dx: Diagnosis; Tx: Treatment.
Table 2.
Examples of individual and combined n-of-1 studies investigating the utility of an intervention in the treatment of a disease.
| Disease | Trials (n) | Intervention (Dx) | Results | Ref. |
|---|---|---|---|---|
| COPD | 26 | Ambulatory oxygen | Reported use of oxygen is biased | [71] |
| OCTD | 1 | L-arginine diet | L-arginine improved health | [72] |
| Brain injury | NR | Methylphenidate | No benefit of methylphenidate | [73] |
| Oral mucositis | 16 | Topical vitamin E | No benefit of topical vitamin E | [74] |
| Chronic fatigue | 4 | Spirulina | No effect of spirulina | [75] |
| ADHD | 86 | Stimulants | 28 out of 64 trials led to change of Tx | [56] |
| Anticoagulation | 7 | Generic/brand warfarin | No difference between generic/brand | [76] |
| Sleep disturbances | 15 | Temazepam | Temazepam is beneficial | [67] |
| Sleep disturbances | 42 | Valerian | Valerian did not improve sleep | [77] |
| COPD | 27 | Eformoteral | No effect of eformoterol | [78] |
| Cystic fibrosis | 48 | Recombinant DNase | Marginal improvements with Dx | [79] |
| Severe CM poisoning | 1 | Donepezil | No effect of donepezil on memory | [80] |
| Reflux disease | 32 | Omeprazole/ranitidine | Utility of n-of-1 trials was observed | [81] |
| Depression | 5 | Methylphenidate | Two patients improvement with Dx | [68] |
| Cystic fibrosis | 52 | Recombinant DNase | Marked improvements after Dx | [82] |
| ADHD | 43 | Methylphenidate | Improvement with methylphenidate | [83] |
| Chronic airflow limits | 68 | Theophylline | N-of-1 studies no better than standard Tx | [84] |
ADHD: Attention deficit and hyperactivity disorder; CM: Carbon monoxide; COPD: Chronic obstructive pulmonary disease; Dx: Diagnosis; NR: Not reported; OCTD: Overlap connective tissue disease; Tx: Treatment.
In light of issues surrounding the feasibility of specific types of clinical trial, there exists medical care settings, such as palliative care, that defy the successful completion of RCTs owing to substantial methodological barriers. Recruiting and retaining subjects along with maintaining distinct interventions are challenged by patient variation related to disease burden, complex needs and changing symptomology. RCTs in palliative care fail because of the inability to recruit and retain sufficient numbers of subjects to achieve necessary sample size requirements [34]. As a result of the paucity of RCTs with relevant subset analyses, RCTs as a whole have failed to inform drug selection for an individual patient requiring palliative care. Thus, with the great variability in responses exhibited among individual patient responses and the availability of multiple drugs, a ‘hit-or-miss’ approach is often used until a drug and dose with acceptable efficacy and tolerable side effects is found. Until this occurs, patients may suffer through extensive periods of suboptimal treatment [35]. N-of-1 trials have thus been proposed as an alternative method of gathering evidence to inform palliative care decision-making [27].
Although n-of-1 trials, by definition, seemingly eschew consideration of the population-level effects of an intervention, they do not necessarily have to, as discussed in the section on ‘Combining and evaluating multiple n-of-1 trials.’ Meta-analysis of the outcomes of multiple n-of-1 trials could be compared with standard treatment regimens and help put into context the utility and practicality of n-of-1 trials (see later) [36,37]. In addition, if there are many interventions that contribute to an apparent state of clinical equipoise, then leveraging insights into how individuals within populations might be stratified on the basis of genetic or clinical risk profile information from large-scale trials could lead to the study of a subset of all possible interventions in an n-of-1 trial involving a patient with a specific genetic or clinical risk profile.
As useful as n-of-1 trials are in many situations, they may not be possible or ideal for certain conditions owing to the nature of the symptoms and pathologies associated with a given condition, the clinical stability of the condition, as well as the clinical assessments necessary for conducting a trial. This has been shown to be problematic in standard crossover trials as well [38]. An example is infectious conditions that progress or regress relatively rapidly. In this context, chronic conditions for which there are easily measurable clinical end points and where the drugs or interventions that are to be tested have a relatively short half-life are the most amenable to n-of-1 trials [39].
Design issues in n-of-1 clinical trials
The design of n-of-1 trials is rooted in standard techniques and strategies used in the design of population-based clinical trials with a few caveats. For example, simple crossover designs in which the order of the administration of two compounds, one perhaps being a placebo, is randomized across different subjects enrolled in n-of-1 studies have often been used. In fact, there is a multitude of literature in the education research community on these types of designs for which one treatment or compound is labeled ‘A’ and the other is labeled ‘B’. Thus, an ABAB design would involve a four-period crossover design [24,25]. The number and length of the crossover periods would be dictated by the nature of the outcome and interventions as well as the statistical power associated with the chosen number of observations or data collection points within each period given the likely differential effect of the interventions. Although some confounding factors cannot be eliminated entirely, a greater number of periods within which different interventions are pursued, though more costly and time-consuming, can also help reduce the confounding effects of other lifestyle modifications the patient may pursue – or need to pursue – during the course of the trial in order to treat his/her condition (e.g., dietary modification and exercise regime). In addition, it is quite possible that for any n-of-1 design, not enough evidence favoring one intervention over another might occur. If both interventions did not achieve some reasonable target then the interventions might be seen as equally ineffective. If they both achieve a target but equally well, then either intervention might be appropriate for future use. Obviously, increasing the length or sophistication of the trial may help resolve issues of ambiguity like this. There is a trade-off, as with any trial design; patient retention is jeopardized with a longer trial.
The simple ABAB design raises at least four related design questions. First, should one randomize the sequence in which interventions are administered to a single patient such that they may not be alternating? For example, by randomizing the sequence in a six-period design, the order of the treatments might be AABABB or the possibly more interpretively problematic order AAABBB. An argument for the use of randomized sequencing, as opposed to simply randomizing the intervention labeled A and B, could be made if the intention was to pursue many n-of-1 trials and then assess the results via combined or meta-analysis (see later) where order effects of the treatments might be of interest. Another argument for randomizing the sequence would be if a patient’s disease or lifestyle choices exhibit periodicity.
A second question concerns the carryover effects of the interventions. Many drugs and behavioral interventions may linger in the system or influence the behavioral patterns and psyche of the patient once their administration is stopped, thereby influencing future interventions. Such effects may confound the interpretation of the effectiveness of subsequent interventions. Sequence randomization and meta-analyses may help identify and assess such effects, but to really combat them in any one study, it is important to ensure that the treatment periods are sufficiently long and that statistical methods that appropriately accommodate or consider carryover effects are used to analyze the data. A third question is directly related to the first two and it concerns the use of washout periods between administrations of interventions. Washout periods can be used to combat carryover effects, but their use may compromise patient safety since they may result in taking a patient off all treatments during the course of the trial (although such an approach is no different in orientation from large trial randomization to a placebo arm, or to the use of washout periods in a population-based trial).
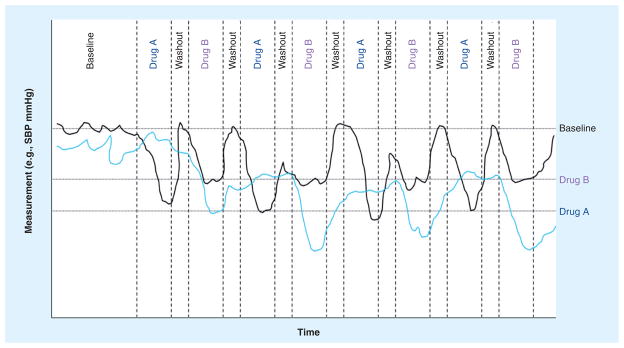
The fourth question concerns the use of blinding, baseline periods and placebo controls. As with the use of washout periods, the establishment of a baseline and the use of placebos may compromise the patient if they are completely taken off treatments. The use of blinding is arguably essential for the success of such trials and should involve blinding of the patient as well as the evaluating physicians and clinical monitoring team. Obviously, as with standard population-based clinical trials, an objective, ‘behind-the-scenes,’ unblinded research team should drive the overarching aspects of the study. Figure 1 depicts the results of two hypothetical n-of-1 trials with an ABABABAB design with baseline and washout periods after each treatment.
Figure 1. Hypothetical outcomes associated with two individual n-of-1 trials investigating the efficacy of two different antihypertensive medications.
The wavy dark and light lines reflect the SBP levels for individuals 1 and 2, respectively, during the trial. The design included a baseline period followed by four alternating periods in which two drugs, A and B, were administered with a washout period between drug administrations. Note that individual 1 had better blood pressure control while on drug A, as indicated by the horizontal lines denoting ‘drug A’ and ‘drug B’, which reflect the average blood pressure achieved while on the drugs. Individual 2 had better blood pressure control on drug B.
SBP: Systolic blood pressure.
A study by Yelland et al. provides a good example of a series of actual n-of-1 clinical trials [40]. A comparison of two treatments for osteoarthritsis, celecoxib and paracetamol, were assessed. The design of the trial was based on a double-blind, crossover comparison where a subject took either celecoxib or sustained-release paracetamol for three pairs of 2-week periods. The order of the drugs during each pairing was random. Both patients and physicians did not know the order of the drug regimens until after the study was completed and data comparing treatment response were pursued.
Analysis of n-of-1 clinical trials
Analyzing data from n-of-1 trials has parallels to the analysis of traditional population-based crossover design clinical trials, again with a few caveats. The most obvious caveat has to do with the fact that the assessment of the effects of interindividual variation (e.g., symptoms, side effects, treatment response) is of no immediate concern. Another relates to the likelihood that more intensive data collection would be associated with n-of-1 trials rather than population-based trials. Thus, the large number of observations collected on a patient in an n-of-1 trial suggests that data analysis methods more in line with time-series analysis, which assume many observations rather than methods, such as simple repeated measures of analysis of variance and related techniques, designed for a relatively few observations, are appropriate.
The actual statistical methods that have been used in the analysis of n-of-1 trials range from visual inspection techniques for making clinical decisions [41,42] to sophisticated time-series analyses [42,43]. However, two very important phenomena need to be accommodated in the analysis of n-of-1 trial data, as mentioned previously. The first is serial correlation between the measures. Since the data are to be collected on a single individual with probable short intervals between the data collections, the observations collected at adjacent or near time points will exhibit strong correlations. These correlations need to be accommodated in relevant analyses. For example, it has been shown that the use of standard t-tests comparing quantitative responses to two particular interventions collected over time in a crossover-based n-of-1 trial will lead to erroneous inferences owing to dependencies between the observations [44]. Therefore, methods that account for serial correlation in comparing the response to two or more treatments, such as certain time-series analyses, are necessary.
The second phenomenon that needs to be accounted for and/or assessed in the analysis of n-of-1 trial data is carryover effects. Even if washout periods are included in a study, it is quite likely that the influence of a prior intervention on the end points of interest will linger into the time during which a different intervention is employed. Accounting for carryover effects is not trivial as their lengths may vary from intervention to intervention and at different times in the study. More research into how to identify and accommodate carryover effects in n-of-1 trials is clearly needed.
Leveraging wireless medical devices
In order to monitor a patient’s status and response to different interventions, a specific monitoring device or reporting structure is necessary. The feasibility of n-of-1 trials could be completely undermined if the measurement of relevant clinical end points is impractical in terms of costs and the demands on a patient’s time, mobility and ability for reporting. Therefore, monitoring and reporting methods should be as invisible and labor-free to the patient as possible. Remote clinical phenotyping and wireless devices have enormous potential in this light [45]. In fact, there have been many innovations in wireless health monitoring that could be of great value in the implementation of n-of-1 clinical trials. Table 3 provides a few examples. However, it is important to note that not all clinical conditions may be amenable to n-of-1 trials with wireless devices or at least current monitoring devices.
Table 3.
Examples of remote phenotypic monitoring devices for potential use in n-of-1 clinical trials.
| Condition | Phenotype | Treatments and monitoring | Device |
|---|---|---|---|
| Type 2 diabetes | Glucose, insulin | Metformin/glitazones/sulfonylureas | Continuous glucose monitor |
| Hypertension | Blood pressure | All | Blood pressure, heart rate |
| Atrial fibrillation | Heart rhythm | Dose titration | Heart rate monitor |
| Insomnia | Sleep quality | All | Zeo |
| Osteoarthritis of the knee | Pain, mobility | NSAID, lidocaine, DMSO | Actigraph, pain diary |
| Esophageal reflux (GERD) | pH | Proton pump inhibitor | pH sensor placed via esophagela probe |
| Migraines | Pain, frequency | Triptans | Pain, occurrence diary |
| Fibromyalgia syndrome | Pain, frequency | Antiseizure, antiepileptic or placebo | Pain, occurrence diary |
| Depression | Severity, frequency | All, placebo | Actigraph, mood diary |
| Congestive heart failure | Heart rhythm | β-blockers, ACE inhibitors | Blood pressure, heart rate and oxygen saturation |
| COPD | Attack frequency | Inhaled bronchodilators/anti-inflammatories | Occurrence and severity diary |
| Obesity | Weight | Behavioral and/or antiappetitive | Actigraph, weight and energy expenditure |
| Sleep apnea | Oxygen saturation | Mechanical devices | Oxygen saturation |
| Parkinson’s disease | Tremor, mobility | L-dopa | Wrist tremor monitor |
| ADHD | Activity, focus | Behavioral, stimulants | Actigraph, behavorial diary |
ACE: Angiotensin-converting enzyme; ADHD: Attention deficit and hyperactivity disorder; COPD: Chronic obstructive pulmonary disease; DMSO: Dimethyl sulfoxide; GERD: Gastroesophageal reflux disease.
For further discussion of remote phenotypic monitoring devices please see [45].
Many of the available wireless health monitoring devices have not themselves been shown to be reliable in clinical settings, hence making their immediate use in n-of-1 clinical trials that focus on the data they produce premature. Despite this limitation, there is a great affinity between these devices and n-of-1 trials, and some may be ready for use. For example, cell-phone-based mood, activity and pain-level diaries, although not completely invisible to a patient, could be used to assess the efficacy of antidepressants, anxiolytics, analgesics and other palliative interventions. Cell phone diaries could also be used to record mild side effects of interventions, as well as compliance with an intervention, and hence complement symptom monitoring.
Other simple monitoring devices that could be of immediate use in certain n-of-1 trial settings are actigraphs or movement monitors [46]. Such devices could be used to monitor activity levels of patients undergoing interventions for obesity and depression, or assess the tremor or mobility impairment of individuals with Parkinson’s disease. Activity monitors have become very sophisticated and could be used as adjuncts to other monitoring devices in an n-of-1 trial in which such monitoring may only be secondary to a primary set of measures. For example, the use of continuous glucose or heart rate monitoring could complement an n-of-1 patient’s wearing of the Nike+iPod® shoe to record activity levels in a study of interventions for diabetes and hypertension [46–50]. The activity level information could provide insight into compliance, secondary effects of the intervention, an important covariate or confounding factor, or an additional end point relevant to the intervention.
In addition to monitoring symptoms and physiological end points, such as blood pressure or insulin levels, one can only speculate, but in the near future, it may be possible to evaluate molecular biomarkers (or ‘surrogate end points’) of disease status and progression remotely. For example, the quantity of rare cell types found in the blood, such as circulating endothelial cells and circulating tumor cells, and the expression levels of particular genes in these cells, may be indicative of the effectiveness of a treatment in eradicating pathologies or signs of pathologies [51,52].
Combining & evaluating multiple n-of-1 trials
If multiple n-of-1 trials investigating the same sets of interventions are initiated, then it is possible to pursue joint or meta-analytic studies of the data generated from those trials (Table 2). Such analyses can explore trends in the data that may shed light on the characteristics of patients found to respond to one particular intervention, side-effect profiles, and overt carryover effects and other confounders that could be accommodated in future trials. A number of statistical approaches to the combined or meta-analysis of multiple n-of-1 trials have been proposed for this purpose [36,37]. A recent paper by Zucker et al. introduces an elegant Bayesian mixed-model approach to combining n-of-1 trials for making population-level claims about the merits of different intervention strategies [53].
Of the possible motivations for combining the results of n-of-1 trials, two stand out. The first involves the assessment of the utility of n-of-1 trials in improving healthcare. Thus, one could effectively design and conduct a ‘trial’ comparing n-of-1 trials to standard care to determine whether the costs and time associated with obtaining objective information in determining optimal interventions for a patient are worth it, relative to the standard ‘hit-or-miss’ clinical care approach to identifying appropriate interventions in the face of clinical equipoise. Guyatt et al. described their experience over a 3-year period in which they compared the use of n-of-1 trials with standard care [26]. They found that not only were n-of-1 trials feasible, but that the results of a large fraction of them prompted physicians to change their ‘prior to the trial’ plan of management for a patient. Larson et al. described their 2-year experience with n-of-1 trials by rating patients’ and physicians’ confidence in treatment before and after the trials on visual analog scales [54]. The authors ultimately concluded that an n-of-1 trial service is feasible, the trial costs were comparable to other conventional services and clinicians appeared to gain confidence and precision from them [54]. Finally, Mahon et al. conducted a randomized study of n-of-1 trials versus standard practice to compare outcomes between groups of patients with irreversible chronic airflow limitation who had been given theophylline [55]. Interestingly, they found n-of-1 trials led to less theophylline use without adverse effects on exercise capacity or quality of life in patients with irreversible chronic airflow limitation [55]. The authors concluded that there was clinically important bias towards unnecessary treatment during open prescription of theophylline for irreversible chronic airflow limitation that can be mitigated through the use of objective criteria associated with n-of-1 trial designs.
Of note, in the context of combining n-of-1 trials in order to assess their utility and feasibility, is the experience of Nikles et al. in setting up a nationwide n-of-1 (or what they referred to as a ‘single patient trial’ [SPT]) service in Australia [56]. This service was designed for patients with attention deficit and hyperactivity disorder for which individual variations in intervention responses are common. Essentially, patients were referred to the service by a physician, a trial was initiated after referral, data analyzed and reports were sent back to the prescribing physician. The service had been designed to require minimal time and effort from the patient’s clinician and has been met with success and favorable responses from patients and physicians [56]. Two additional studies have examined the feasibility of n-of-1 trials from a cost perspective [57,58]. Both studies observed, as one might expect, that the operational costs of n-of-1 trials are not trivial relative to standard care and when high-cost interventions are used to contrast with other interventions, knowingly putting a patient on the intervention for prespecified periods without a favorable response is problematic from a care perspective. However, this criticism is true of all clinical trials, and ways of mitigating this problem via adaptive and sequential designs, for example, have been proposed [59].
The second important motivation for combining n-of-1 trial data and results concerns the identification of common characteristics among patients who are ultimately found to respond best to a particular intervention. For example, it might be that patients who are found to respond best to a certain intervention share genotypic, biomarker, clinical or demographic characteristics. Knowledge of these characteristics would help inform a physician as to the use of a particular intervention for future patients without having to resort to an n-of-1 trial. Obviously, the degree to which these characteristics are reliably predictive of response is incredibly important in this context.
The notion that one could analyze the results of multiple n-of-1 trials to search for patterns associated with response to an intervention contrasts with the approaches to individualized medicine that leverage the results from large population-based trials for this purpose. In the traditional approach, a large-scale population-based trial is pursued and individuals are identified that ultimately responded to an intervention. Some characteristic (e.g., genotype) is then found that distinguishes responders from nonresponders. This characteristic is then used to inform use of the intervention in the future. This approach essentially casts a wide net initially by studying a large number of patients in a unified manner, then winnows things down to what might work best in an individual patient over time and through additional studies of the subjects in the large trial.
The combined n-of-1 trials approach achieves the same goal: a number of n-of-1 trials are pursued and the best interventions for each patient are recorded. Characteristics of the patients are noted and contrasted in order to identify distinguishing features among those who did best on a specific intervention. If such a characteristic is found, it is used to inform the use of that intervention in the future. This approach essentially starts out in a small and focused manner, and then works its way towards insights that would immediately benefit a much larger group of patients.
There are some serious advantages to the n-of-1 approach to achieving personalized medicine despite the lack of immediate generalizability of the results to large numbers of patients. First, the n-of-1 trials can be somewhat heterogeneous in design as long as objective evidence is found favoring one or another intervention for subsets of patients (e.g., the number and length of crossover periods may vary from study to study). Such heterogeneity is often not tolerated in large population-based trial protocols where uniformity is emphasized in order to prevent confounding of generalizations. Second, the patients involved in the n-of-1 trials draw immediate benefit from the trial in that a determination of which intervention is likely to benefit from them is made. This is unlike many population-based trials where, depending on the protocol and design used, an individual may have been on a placebo for the entire trial. Third, the timing and costs of conducting n-of-1 trials can be varied and distributed across participating clinics or institutions. Obviously, there are many issues in vetting the utility of n-of-1 trials relative to standard population and uniform protocol-based trials. For example, just how much heterogeneity in the conduct of n-of-1 trials can be tolerated before it is impossible to draw inferences from them collectively is an open question. However, at the very least, the use of results of combined n-of-1 trials relative to standard population-based trials is an important research question.
Issues & future directions
Coordinated n-of-1 trials have the potential to radically change the way in which evidence-based and individualized medicine is pursued. The availability of relevant wireless clinical monitoring devices that are largely invisible to the user will enhance their value. These enhancements may involve the collection of data for risk factors or surrogate end points, such as continuous time heart rate or blood pressure variability that have never been considered in population-based trials and may (or may not) shed light on the efficacy of the intervention for the clinical end point. Not only are the results of n-of-1 trials of immediate benefit to the patient and the treating physician, but if enough of them are pursued, patient characteristics that ultimately differentiate those that benefit from a particular intervention from those that do not can be explored, allowing for stratification of future patient groups in a way that would further benefit patient care. Furthermore, n-of-1 trials can be used to determine if a larger trial (n-of-1 or standard RCT) is appropriate. Despite these facts, the costs associated with n-of-1 trials – though not exorbitant for any one n-of-1 trial – must be justified if they are to be pursued on a larger scale. However, this is no less true of massive population-based trials that cost tens to hundreds of millions of dollars. In this context it is arguable that there are a number of motivations for pursuing n-of-1 trials that may justify institutional and research funding investment. Brief descriptions of these are provided later.
Clinical equipoise
As noted, when a physician is faced with uncertainty over the best course of action to take for a given patient owing to the fact that many different interventions are available, all of which have been vetted at some level and for which there is little information regarding how to stratify patient populations for their differentiated use, an n-of-1 trial examining the relative merits of each for that patient is appropriate. There are many clinical settings where a state of equipoise or near equipoise exists; for example, in pain management, blood pressure control and in the treatment of depression, in which pharmacotherapy, counseling and behavioral therapy should be considered and contrasted.
Treatment repositioning
N-of-1 trials can be of value in evaluating addition indications for a drug or intervention. If, for example, it is felt that a drug originally designed for use in treating a specific clinical profile or condition may be of value in treating a patient with a different clinical profile or condition, then testing the drug against a standard treatment in individual patients with designs that may cater to the characteristics of the patient and his or her clinical condition would make sense. If evidence from such nuanced individual trials suggests that the drug has potential for treating this new condition, larger and more traditional trials could be pursued investigating the drug for wider use.
Leveraging medical records
In the future, as medical records systems become more sophisticated, the ability to capture patient data for n-of-1 trials will be much improved. Medical devices that are interoperable with electronic medical records have been shown to improve the quality, efficiency and ultimately the cost of data capture [60]. The challenges that both physicians and patients have faced in the past in weighing the benefit of n-of-1 trials compared with the effort involved [28,29], may be less of a concern when data collection and visualization is made more facile by the integration of wireless data capture with electronic medical records.
Early-phase trials
Although obvious and already pursued to some degree, well-designed and controlled n-of-1 trials can be used in early-phase trials evaluating the tolerability, dosing and potential utility of an experimental compound. The comparison of a novel intervention against a standard or placebo is often pursued in Phase II trials, but greater sophistication in design and execution may benefit such trials. In addition, even dosing studies of the type pursued in Phase I ‘first-in-human’ studies may benefit from the objective comparison of contrasting interventions, although, there are many ethical and scientific factors to consider.
Training
N-of-1 trials are excellent vehicles for physician training since they would expose a physician to objective clinical decision-making and evidence-based practice on a systematic and rigorous level. Such trials would also enhance physician sensitivity to the nuances of treating individual patients. In addition, the conduct of such trials would require familiarity and exposure to trial design and execution issues, including ethical and legal issues surrounding patient use in research.
Nationwide agenda in individualized medicine
Recognition that the USA is in the midst of a healthcare crisis has motivated serious calls for advances in biomedical research [9]. It is clear that two potential ways forward in the midst of this crisis are to promote both individualized medicine and evidence-based medicine as a way of reducing inefficiencies in clinical care, through reducing individual patients’ exposure to treatments that do not work and those that cause adverse side effects. In addition, moving towards a more individualized and evidence-based health-care system of the type built from the n-of-1 study principle and infrastructure would tap into, and build on, the creative and innovative strengths of the biomedical research community, especially in areas of relevance such as genomics and wireless devices. In this context, both the theoretical and practical issues surrounding n-of-1 trials in medical settings are as logical to think about as they are timely.
Future perspective
Coordinated n-of-1 trials have the potential to radically change the way in which evidence-based and individualized medicine is pursued. The availability of relevant wireless clinical monitoring devices that are largely invisible to the user will enhance their value. These enhancements may involve the collection of data, such as continuous time heart rate or blood pressure variability that have never been considered in population-based trials. Not only are the results of n-of-1 trials of immediate benefit to the patient and the treating physician, but if enough of them are pursued, patient characteristics that ultimately differentiate those that benefit from a particular intervention from those that do not can be explored, allowing for stratification of future patient groups in a way that would further benefit patient care.
Executive summary.
Do n-of-1 trials have a role in clinical science?
N-of-1 trials that focus exclusively on the objective, empirically determined optimal intervention for a single patient are compatible with the ultimate end point of clinical practice: the care of individual patients.
Meta-analyses of the outcomes of multiple n-of-1 trials could be compared with standard treatment regimens and help put into context the utility and practicality of n-of-1 trials.
Design issues in n-of-1 clinical trials
Randomization of treatment order, carryover effects, washout periods and blinding are key design elements that need to be considered in n-of-1 trials.
The analysis of n-of-1 clinical trials
Methods that account for serial correlation in comparing the response to two or more treatments, such as certain time-series analyses, are necessary.
More research into how to identify and accommodate carryover effects in n-of-1 trials is clearly needed.
Leveraging wireless medical devices
The feasibility of n-of-1 trials could be completely undermined if the measurement of relevant clinical end points is impractical in terms of costs and the demands on a patient’s time, mobility and ability for reporting.
Remote clinical phenotyping and wireless devices make data acquisition as transparent and labor-free to the patient as possible.
Combining & evaluating multiple n-of-1 trials
Randomized controlled trials cast a wide net initially by studying a large number of patients in a unified manner, then winnow things down to what might work best in an individual patient over time and through additional studies of the subjects in the large trial.
The n-of-1 approach essentially starts out small and focused, and then works its way towards insights that would immediately benefit a much larger group of patients by combining n-of-1 trial outcomes in a meta-analysis.
Issues & future directions
There are a number of motivations for pursuing n-of-1 trials that may justify institutional and research funding investment. These motivations include overcoming clinical equipoise, treatment repositioning, early-phase trials, physician training and the nationwide agenda in individualized medicine.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work was supported in part by the following research grants: U19 AG023122-05; R01 MH078151-03; N01 MH22005, U01 DA024417-01, P50 MH081755-01, R01 AG030474-02, N01 MH022005, R01 HL089655-02, R01 MH080134-03, U54 CA143906-01; UL1 RR025774-03, from the Price Foundation (Zurich, Switzerland) and Scripps Genomic Medicine (CA, USA). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
• of interest
- 1.Jorgensen JT. Are we approaching the post-blockbuster era? Pharmacodiagnostics and rational drug development. Expert Rev Mol Diagn. 2008;8(6):689–695. doi: 10.1586/14737159.8.6.689. [DOI] [PubMed] [Google Scholar]
- 2.Jorgensen JT. New era of personalized medicine: a 10-year anniversary. Oncologist. 2009;14(5):557–558. doi: 10.1634/theoncologist.2009-0047. [DOI] [PubMed] [Google Scholar]
- 3.Hu SX, Foster T, Kieffaber A. Pharmacogenomics and personalized medicine: mapping of future value creation. Biotechniques. 2005;39(10 Suppl):S1–S6. doi: 10.2144/000112048. [DOI] [PubMed] [Google Scholar]
- 4.Langreth R, Waldholz M. New era of personalized medicine: targeting drugs for each unique genetic profile. Oncologist. 1999;4(5):426–427. [PubMed] [Google Scholar]
- 5.Trusheim MR, Berndt ER, Douglas FL. Stratified medicine: strategic and economic implications of combining drugs and clinical biomarkers. Nat Rev Drug Discov. 2007;6(4):287–293. doi: 10.1038/nrd2251. [DOI] [PubMed] [Google Scholar]
- 6.Guyatt GH, Haynes RB, Jaeschke RZ, et al. Users’ guides to the medical literature. XXV Evidence-based medicine: principles for applying the users’ guides to patient care Evidence-Based Medicine Working Group. JAMA. 2000;284(10):1290–1296. doi: 10.1001/jama.284.10.1290. [DOI] [PubMed] [Google Scholar]
- 7.Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312(7023):71–72. doi: 10.1136/bmj.312.7023.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lauer MS, Collins FS. Using science to improve the nation’s health system. NIH’s commitment to comparative effectiveness research. JAMA. 2010;303(21):2182–2183. doi: 10.1001/jama.2010.726. [DOI] [PubMed] [Google Scholar]
- 9.Collins FS. Research agenda. Opportunities for research and NIH. Science. 2010;327(5961):36–37. doi: 10.1126/science.1185055. [DOI] [PubMed] [Google Scholar]
- 10.Ginsburg GS, Willard HF. Genomic and personalized medicine: foundations and applications. Transl Res. 2009;154(6):277–287. doi: 10.1016/j.trsl.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Kraushaar LE, Kramer A. Are we losing the battle against cardiometabolic disease? The case for a paradigm shift in primary prevention. BMC Public Health. 2009;9:64. doi: 10.1186/1471-2458-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rich EC. The policy debate over public investment in comparative effectiveness research. J Gen Intern Med. 2009;24(6):752–757. doi: 10.1007/s11606-009-0958-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snyderman R, Yoediono Z. Perspective: prospective health care and the role of academic medicine: lead, follow, or get out of the way. Acad Med. 2008;83(8):707–714. doi: 10.1097/ACM.0b013e31817ec800. [DOI] [PubMed] [Google Scholar]
- 14.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10(8):789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 15.Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 16.Flockhart DA, Skaar T, Berlin DS, Klein TE, Nguyen AT. Clinically available pharmacogenomics tests. Clin Pharmacol Ther. 2009;86(1):109–113. doi: 10.1038/clpt.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topol EJ. Pharmacy benefit managers, pharmacies, and pharmacogenomic testing: prescription for progress? Sci Transl Med. 2010;2(44):44cm22. doi: 10.1126/scitranslmed.3001067. [DOI] [PubMed] [Google Scholar]
- 18.Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med. 2010;363(4):301–304. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- 19.Tsapas A, Matthews DR. Using N-of-1 trials in evidence-based clinical practice. JAMA. 2009;301(10):1022–1023. 1023. doi: 10.1001/jama.2009.276. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt GH, Jaeschke R. N-of-1 randomized trials – where do we stand? West J Med. 1990;152(1):67–68. [PMC free article] [PubMed] [Google Scholar]
- 21.Everitt BS, Pickler A. Statistical Aspects of The Design of Clinical Trials. Imperial College Press; London, UK: 2004. [Google Scholar]
- 22.Gerss JW, Kopcke W. Clinical trials and rare diseases. Adv Exp Med Biol. 2010;686:173–190. doi: 10.1007/978-90-481-9485-8_11. [DOI] [PubMed] [Google Scholar]
- 23.Meinert CL, Tonascia S. Clinical Trials Design, Conduct, and Analysis Monographs in Epidemiology and Biostatistics. Vol. 469. Oxford University Press; NY, USA: 1986. [Google Scholar]
- 24.Barlow DH, Nock M, Hersen M. Strategies for Studying Behavior for Change. 3. Vol. 393. Pearson/Allyn and Bacon; MA, USA: 2009. Single Case Experimental Designs. [Google Scholar]
- 25•.Guyatt G, Sackett D, Taylor DW, Chong J, Roberts R, Pugsley S. Determining optimal therapy – randomized trials in individual patients. N Engl J Med. 1986;314(14):889–892. doi: 10.1056/NEJM198604033141406. Laid the foundation for n-of-1 trials in clinical medicine. Many other papers published by this research group further describe their years of experience in implementing n-of-1 trials. [DOI] [PubMed] [Google Scholar]
- 26.Guyatt GH, Keller JL, Jaeschke R, Rosenbloom D, Adachi JD, Newhouse MT. The n-of-1 randomized controlled trial: clinical usefulness. Our three-year experience. Ann Intern Med. 1990;112(4):293–299. doi: 10.7326/0003-4819-112-4-293. [DOI] [PubMed] [Google Scholar]
- 27.Nikles J, Mitchell G, Walters J, et al. Prioritising drugs for single patient (n-of-1) trials in palliative care. Palliat Med. 2009;23(7):623–634. doi: 10.1177/0269216309106461. [DOI] [PubMed] [Google Scholar]
- 28•.Kravitz RL, Duan N, Niedzinski EJ, Hay MC, Subramanian SK, Weisner TS. What ever happened to n-of-1 trials? Insiders’ perspectives and a look to the future. Milbank Q. 2008;86(4):533–555. doi: 10.1111/j.1468-0009.2008.00533.x. Good review of the history of n-of-1 trials, including interviews with researchers who have been leaders in the field providing valuable direct insight into challenges of n-of-1 trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kravitz RL, Paterniti DA, Hay MC, et al. Marketing therapeutic precision: potential facilitators and barriers to adoption of n-of-1 trials. Contemp Clin Trials. 2009;30(5):436–445. doi: 10.1016/j.cct.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Kraemer HC, Frank E, Kupfer DJ. Moderators of treatment outcomes: clinical, research, and policy importance. JAMA. 2006;296(10):1286–1289. doi: 10.1001/jama.296.10.1286. [DOI] [PubMed] [Google Scholar]
- 31.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59(10):877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 32.Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA. 2007;298(10):1209–1212. doi: 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]
- 33•.Scuffham PA, Nikles J, Mitchell GK, et al. Using n-of-1 trials to improve patient management and save costs. J Gen Intern Med. 2010;25(9):906–913. doi: 10.1007/s11606-010-1352-7. Analysis using data from the clinical trial service developed in Australia of n-of-1 trials from the cost perspective. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grande GE, Todd CJ. Why are trials in palliative care so difficult? Palliat Med. 2000;14(1):69–74. doi: 10.1191/026921600677940614. [DOI] [PubMed] [Google Scholar]
- 35.Abrahm JL. A Physician’s Guide to Pain and Symptom Management in Cancer Patients. 2. Johns Hopkins University Press; MD, USA: 2005. [Google Scholar]
- 36•.Zucker DR, Schmid CH, McIntosh MW, D’Agostino RB, Selker HP, Lau J. Combining single patient (n-of-1) trials to estimate population treatment effects and to evaluate individual patient responses to treatment. J Clin Epidemiol. 1997;50(4):401–410. doi: 10.1016/s0895-4356(96)00429-5. This paper and a recently published follow-up paper discuss the statistical methodology involved in combining n-of-1 trials. [DOI] [PubMed] [Google Scholar]
- 37.Huber AM, Tomlinson GA, Koren G, Feldman BM. Amitriptyline to relieve pain in juvenile idiopathic arthritis: a pilot study using Bayesian metaanalysis of multiple n-of-1 clinical trials. J Rheumatol. 2007;34(5):1125–1132. [PubMed] [Google Scholar]
- 38.Senn S. Cross-over Trials in Clinical Research. 2. Wiley; Chichester, UK: 2002. [Google Scholar]
- 39.Guyatt GH, Heyting A, Jaeschke R, Keller J, Adachi JD, Roberts RS. N-of-1 randomized trials for investigating new drugs. Control Clin Trials. 1990;11(2):88–100. doi: 10.1016/0197-2456(90)90003-k. [DOI] [PubMed] [Google Scholar]
- 40.Yelland MJ, Nikles CJ, McNairn N, Del Mar CB, Schluter PJ, Brown RM. Celecoxib compared with sustained-release paracetamol for osteoarthritis: a series of n-of-1 trials. Rheumatology. 2007;46(1):135–140. doi: 10.1093/rheumatology/kel195. [DOI] [PubMed] [Google Scholar]
- 41.Kazdin AE. Methods for Clinical and Applied Settings. Vol. 368. Oxford University Press; NY, USA: 1982. Single-Case Research Designs. [Google Scholar]
- 42.Barlow DH, Hersen M. Single Case Experimental Designs. 2. Allyn and Bacon; MA, USA: 1984. [DOI] [PubMed] [Google Scholar]
- 43.Spiegelhalter DJ. Statistical issues in studies of individual response. Scand J Gastroenterol Suppl. 1988;147:40–45. doi: 10.3109/00365528809099158. [DOI] [PubMed] [Google Scholar]
- 44.Rochon J. A statistical model for the “n-of-1” study. J Clin Epidemiol. 1990;43(5):499–508. doi: 10.1016/0895-4356(90)90139-g. [DOI] [PubMed] [Google Scholar]
- 45•.Topol EJ. Transforming medicine via digital innovation. Sci Transl Med. 2010;2(16):16cm4. doi: 10.1126/scitranslmed.3000484. Recent paper that paints a picture of the future of medicine and the new age of digital medical devices that we are entering. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plasqui G, Westerterp KR. Physical activity assessment with accelerometers: an evaluation against doubly labeled water. Obesity (Silver Spring) 2007;15(10):2371–2379. doi: 10.1038/oby.2007.281. [DOI] [PubMed] [Google Scholar]
- 47.Reid SC, Kauer SD, Dudgeon P, Sanci LA, Shrier LA, Patton GC. A mobile phone program to track young people’s experiences of mood, stress and coping. Development and testing of the mobiletype program. Soc Psychiatry Psychiatr Epidemiol. 2009;44(6):501–507. doi: 10.1007/s00127-008-0455-5. [DOI] [PubMed] [Google Scholar]
- 48.Sudano I, Flammer AJ, Hermann F, et al. Auricall. A new device for a non-invasive, wireless, continuous monitoring of oxygen saturation and heart rate in patients with heart failure. Int J Cardiol. 2008;129(1):141–143. doi: 10.1016/j.ijcard.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 49.Welch J, Guilak F, Baker S. A wireless ECG smart sensor for broad application in life threatening event detection. Conf Proc IEEE Eng Med Biol Soc. 2004;5:3447–3449. doi: 10.1109/IEMBS.2004.1403967. [DOI] [PubMed] [Google Scholar]
- 50.Wong LJ, Buckingham BA, Kunselman B, Istoc E, Leach J, Purvis R. Extended use of a new continuous glucose monitoring system with wireless data transmission in children with Type 1 diabetes mellitus. Diabetes Technol Ther. 2006;8(2):139–145. doi: 10.1089/dia.2006.8.139. [DOI] [PubMed] [Google Scholar]
- 51.Vlassov VV, Laktionov PP, Rykova EY. Circulating nucleic acids as a potential source for cancer biomarkers. Curr Mol Med. 2010;10(2):142–165. doi: 10.2174/156652410790963295. [DOI] [PubMed] [Google Scholar]
- 52.Wu H, Chen H, Hu PC. Circulating endothelial cells and endothelial progenitors as surrogate biomarkers in vascular dysfunction. Clin Lab. 2007;53(5–6):285–295. [PubMed] [Google Scholar]
- 53.Zucker DR, Ruthazer R, Schmid CH. Individual (n-of-1) trials can be combined to give population comparative treatment effect estimates: methodologic considerations. J Clin Epidemiol. 2010;63(12):1312–1323. doi: 10.1016/j.jclinepi.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54•.Larson EB, Ellsworth AJ, Oas J. Randomized clinical trials in single patients during a 2-year period. JAMA. 1993;270(22):2708–2712. Another research group’s experience of conducting n-of-1 trials is described here. They review the feasibility of n-of-1 trials from a patient, physician and cost perspective. [PubMed] [Google Scholar]
- 55.Mahon J, Laupacis A, Donner A, Wood T. Randomised study of n-of-1 trials versus standard practice. BMJ. 1996;312(7038):1069–1074. doi: 10.1136/bmj.312.7038.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56•.Nikles CJ, Mitchell GK, Del Mar CB, Clavarino A, McNairn N. An n-of-1 trial service in clinical practice: testing the effectiveness of stimulants for attention-deficit/hyperactivity disorder. Pediatrics. 2006;117(6):2040–2046. doi: 10.1542/peds.2005-1328. Published by a research group that implemented an n-of-1 service in Australia to treat attention deficit and hyperactivity disorder. [DOI] [PubMed] [Google Scholar]
- 57.Pope JE, Prashker M, Anderson J. The efficacy and cost effectiveness of n-of-1 studies with diclofenac compared with standard treatment with nonsteroidal antiinflammatory drugs in osteoarthritis. J Rheumatol. 2004;31(1):140–149. [PubMed] [Google Scholar]
- 58.Scuffham PA, Yelland MJ, Nikles J, Pietrzak E, Wilkinson D. Are n-of-1 trials an economically viable option to improve access to selected high cost medications? The Australian experience. Value Health. 2008;11(1):97–109. doi: 10.1111/j.1524-4733.2007.00218.x. [DOI] [PubMed] [Google Scholar]
- 59.Chow SC, Chang M. Adaptive Design Methods in Clinical Trials. Chapman Hall; FL, USA: 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rausch TL, Judd TM. The development of an interoperable roadmap for medical devices. Conf Proc IEEE Eng Med Biol Soc. 2006;(Suppl):6740–6743. doi: 10.1109/IEMBS.2006.260935. [DOI] [PubMed] [Google Scholar]
- 61.Cepeda MS, Acevedo JC, Alvarez H, Miranda N, Cortes C, Carr DB. An n-of-1 trial as an aid to decision-making prior to implanting a permanent spinal cord stimulator. Pain Med. 2008;9(2):235–239. doi: 10.1111/j.1526-4637.2007.00329.x. [DOI] [PubMed] [Google Scholar]
- 62.Nathan PC, Tomlinson G, Dupuis LL, et al. A pilot study of ondansetron plus metopimazine vs. ondansetron monotherapy in children receiving highly emetogenic chemotherapy: a Bayesian randomized serial n-of-1 trials design. Support Care Cancer. 2006;14(3):268–276. doi: 10.1007/s00520-005-0875-7. [DOI] [PubMed] [Google Scholar]
- 63.Woodfield R, Goodyear-Smith F, Arroll B. N-of-1 trials of quinine efficacy in skeletal muscle cramps of the leg. Br J Gen Pract. 2005;55(512):181–185. [PMC free article] [PubMed] [Google Scholar]
- 64.Nikles CJ, Yelland M, Glasziou PP, Del Mar C. Do individualized medication effectiveness tests (n-of-1 trials) change clinical decisions about which drugs to use for osteoarthritis and chronic pain? Am J Ther. 2005;12(1):92–97. doi: 10.1097/00045391-200501000-00012. [DOI] [PubMed] [Google Scholar]
- 65.Notcutt W, Price M, Miller R, et al. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 ‘n-of-1’ studies. Anaesthesia. 2004;59(5):440–452. doi: 10.1111/j.1365-2044.2004.03674.x. [DOI] [PubMed] [Google Scholar]
- 66.Haas DC, Sheehe PR. Dextroamphetamine pilot crossover trials and n-of-1 trials in patients with chronic tension-type and migraine headache. Headache. 2004;44(10):1029–1037. doi: 10.1111/j.1526-4610.2004.04199.x. [DOI] [PubMed] [Google Scholar]
- 67.Wegman AC, van der Windt DA, de Haan M, Deville WL, Fo CT, de Vries TP. Switching from NSAIDs to paracetamol: a series of n-of-1 trials for individual patients with osteoarthritis. Ann Rheum Dis. 2003;62(12):1156–1161. doi: 10.1136/ard.2002.002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jansen IH, Olde Rikkert MG, Hulsbos HA, Hoefnagels WH. Toward individualized evidence-based medicine: five ‘n-of-1’ trials of methylphenidate in geriatric patients. J Am Geriatr Soc. 2001;49(4):474–476. doi: 10.1046/j.1532-5415.2001.49092.x. [DOI] [PubMed] [Google Scholar]
- 69.Haines DR, Gaines SP. N-of-1 randomised controlled trials of oral ketamine in patients with chronic pain. Pain. 1999;83(2):283–287. doi: 10.1016/s0304-3959(99)00117-7. [DOI] [PubMed] [Google Scholar]
- 70.March L, Irwig L, Schwarz J, Simpson J, Chock C, Brooks P. N-of-1 trials comparing a non-steroidal anti-inflammatory drug with paracetamol in osteoarthritis. BMJ. 1994;309(6961):1041–1045. doi: 10.1136/bmj.309.6961.1041. discussion 1045–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nonoyama ML, Brooks D, Guyatt GH, Goldstein RS. Ambulatory gas usage in patients with chronic obstructive pulmonary disease and exertional hypoxemia. J Cardiopulm Rehabil Prev. 2008;28(5):323–329. doi: 10.1097/01.HCR.0000336144.79192.5e. [DOI] [PubMed] [Google Scholar]
- 72.Hackett A, Gillard J, Wilcken B. N-of-1 trial for an ornithine transcarbamylase deficiency carrier. Mol Genet Metab. 2008;94(2):157–161. doi: 10.1016/j.ymgme.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 73.Martin RT, Whyte J. The effects of methylphenidate on command following and yes/no communication in persons with severe disorders of consciousness: a meta-analysis of n-of-1 studies. Am J Phys Med Rehabil. 2007;86(8):613–620. doi: 10.1097/PHM.0b013e3181154a84. [DOI] [PubMed] [Google Scholar]
- 74.Sung L, Tomlinson GA, Greenberg ML, et al. Serial controlled n-of-1 trials of topical vitamin E as prophylaxis for chemotherapy-induced oral mucositis in paediatric patients. Eur J Cancer. 2007;43(8):1269–1275. doi: 10.1016/j.ejca.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 75.Baicus C, Baicus A. Spirulina did not ameliorate idiopathic chronic fatigue in four N-of-1 randomized controlled trials. Phytother Res. 2007;21(6):570–573. doi: 10.1002/ptr.2114. [DOI] [PubMed] [Google Scholar]
- 76.Pereira JA, Holbrook AM, Dolovich L, et al. Are brand-name and generic warfarin interchangeable? Multiple n-of-1 randomized, crossover trials. Ann Pharmacother. 2005;39(7–8):1188–1193. doi: 10.1345/aph.1G003. [DOI] [PubMed] [Google Scholar]
- 77.Coxeter PD, Schluter PJ, Eastwood HL, Nikles CJ, Glasziou PP. Valerian does not appear to reduce symptoms for patients with chronic insomnia in general practice using a series of randomised n-of-1 trials. Complement Ther Med. 2003;11(4):215–222. doi: 10.1016/s0965-2299(03)00122-5. [DOI] [PubMed] [Google Scholar]
- 78.Smith BJ, Appleton SL, Veale AJ, McElroy HJ, Veljkovic D, Saccoia L. Eformoterol n-of-1 trials in chronic obstructive pulmonary disease poorly reversible to salbutamol. Chron Respir Dis. 2004;1(2):63–69. doi: 10.1191/1479972304cd028oa. [DOI] [PubMed] [Google Scholar]
- 79.Suri R, Metcalfe C, Wallis C, Bush A. Predicting response to rhDNase and hypertonic saline in children with cystic fibrosis. Pediatr Pulmonol. 2004;37(4):305–310. doi: 10.1002/ppul.10442. [DOI] [PubMed] [Google Scholar]
- 80.Price JD, Grimley Evans J. An n-of-1 randomized controlled trial (‘n-of-1 trial’) of donepezil in the treatment of non-progressive amnestic syndrome. Age Ageing. 2002;31(4):307–309. doi: 10.1093/ageing/31.4.307. [DOI] [PubMed] [Google Scholar]
- 81.Wolfe B, Del Rio E, Weiss SL, et al. Validation of a single-patient drug trial methodology for personalized management of gastroesophageal reflux disease. J Manag Care Pharm. 2002;8(6):459–468. doi: 10.18553/jmcp.2002.8.6.459. [DOI] [PubMed] [Google Scholar]
- 82.Bollert FG, Paton JY, Marshall TG, Calvert J, Greening AP, Innes JA. Recombinant DNase in cystic fibrosis: a protocol for targeted introduction through n-of-1 trials. Scottish Cystic Fibrosis Group. Eur Respir J. 1999;13(1):107–113. doi: 10.1183/09031936.99.13105399. [DOI] [PubMed] [Google Scholar]
- 83.Kent MA, Camfield CS, Camfield PR. Double-blind methylphenidate trials: practical, useful, and highly endorsed by families. Arch Pediatr Adolesc Med. 1999;153(12):1292–1296. doi: 10.1001/archpedi.153.12.1292. [DOI] [PubMed] [Google Scholar]
- 84.Mahon JL, Laupacis A, Hodder RV, et al. Theophylline for irreversible chronic airflow limitation: a randomized study comparing n-of-1 trials to standard practice. Chest. 1999;115(1):38–48. doi: 10.1378/chest.115.1.38. [DOI] [PubMed] [Google Scholar]