Abstract
Context
With the exception of the American Recovery and Reinvestment Act, funding support for biomedical research in the United States has slowed after a decade of doubling. However, the extent and scope of slowing are largely unknown.
Objective
To quantify funding of biomedical research in the United States from 2003 to 2008.
Design
We used publicly available data to quantify funding from federal, state and local government, private, and industry sources. We used regression models to compare financial trends between 1994–2003 and 2003–2007 (the last year complete data were available). We also evaluated the number of new drug and device approvals by the US Food and Drug Administration (FDA) over the same time period.
Main Outcome Measures
Funding and growth rates by source. Number of FDA approvals.
Results
Biomedical research funding increased from $75.5 billion in 2003 to $101.1 billion in 2007. In 2008 funding from the National Institutes of Health (NIH) and industry totaled $88.8 billion. In 2007 funding from these sources, adjusted for inflation, was $90.2 billion. Adjusted for inflation, funding from 2003 to 2007 increased by 14% for a compound annual growth rate of 3.4%. By comparison, funding from 1994 to 2003 increased at an annual rate of 7.8% (P<0.001). In 2007, industry (58%) was the largest funder followed by the federal government (33%). The modest increase in funding was not accompanied by an increase in approvals for drugs or devices. In 2007 the United States spent an estimated 4.5% of its total health expenditures on biomedical research and 0.1% on health services research.
Conclusions
After a decade of doubling, the rate of increase in biomedical research funding slowed from 2003 to 2007, and after adjusting for inflation, the absolute level of funding from NIH and industry appears to have decreased by 2% in 2008.
INTRODUCTION
Biomedical research is valued highly by individuals, governments, foundations, and corporations. They see research not only as a source of more effective treatments and preventive measures, but also as a route to political policy, economic development, and new commercial products.
In 2005, we reported that total public and private financial support of US biomedical research grew substantially during the preceding decade, with a tripling in nominal amount and doubling after adjustment for inflation, between 1994 and 2003.1 However, as measured by new drugs approved by the US Food and Drug Administration (FDA), productivity has been stagnant. The decline in number of novel drugs (new molecular entities) approved was striking. Medical devices, especially implantable and diagnostic devices, fared better during this interval, as reflected in number of approvals, companies’ financial performance, and their growth in investment in research and development.
The 2007–2009 severe global recession has renewed focus on public spending and has caused companies and foundations to reexamine their priorities. Also, debate in the US about the federal government’s role in providing health insurance has cast attention on the allocation of research support, especially between discovery of new clinical interventions and evaluation of their effect, value, and cost. The US government is following Europe in providing incentives for health services research and information technology, which were incorporated in the American Recovery and Reinvestment Act of 2009.
Researchers and sponsors alike have growing awareness that financing is necessary, but not sufficient, to sustain progress. While the promise of new drugs for refractory common or devastating diseases continues to capture the public’s imagination and enjoys very strong support, policy-makers are also aware that new beneficial technology often spawns new cost. Consequently, timely and accurate information about the sources of public and private research funds is important.
We, therefore, have revised our earlier analysis of 1994–2003 and brought it current to 2007, the last year complete information is available. This reexamination has allowed us to confirm some trends seen earlier, gauge the impact of the recession, and improve our estimates.
METHODS
Scope of Investigation
Our goal was to replicate and extend our 2005 study1 by providing updated and improved data through 2007 for all sources and 2008 data where available. We generally used similar data sources to our previous study with the notable exception that we used different sources for research support from biotechnology and medical device firms to minimize double counting support from life sciences firms.1 We again considered four major sponsors of biomedical research: federal government, state and local governments, private not-for-profit entities including foundations, and industry. As in our previous study, biomedical research was defined as the life sciences excluding the agricultural science plus the addition of psychology. We also aimed to update our data regarding subsidies from colleges and universities. We used publicly available information wherever possible. As in our previous study, we adjusted figures using the biomedical research and development price index (BRDPI)2 to 2008 dollars. A preliminary value for 2008 BRDPI was used. The BRDPI “measures changes in the weighted-average of the prices of all the inputs (e.g., personnel services, various supplies, and equipment) purchased with the NIH budget to support research” and in theory indicates how much NIH expenditures would need to increase to maintain research activity at a constant level.3
Federal Funding
As in our previous study, NIH funding was taken from the National Science Foundation Division of Science Resources Statistics data on obligations for research, development and research and development plant, 2003–2008.4 The NIH defines obligation “based on NIH funds that have been awarded by an NIH Institute/Center.”5 Similar to our earlier study, federal funding was determined using data from the National Health Expenditure Accounts (NHEA).6 NHEA has revised its method of data collection and has updated all data from 1976 forward using data directly from the NIH instead of from the National Science Foundation.7
State and Local Government Funding
We used NHEA data to determine state and local government support for biomedical research.
Private Not-For-Profit Support
Private entities that we considered included foundations, public charities, medical research organizations (e.g., Howard Hughes Medical Institute), and voluntary health organizations (e.g., American Cancer Society). We determined funding from these organizations using NHEA data.
Industry Support
Industry contribution to biomedical research comes from three major sources: pharmaceutical, biotechnology, and medical device firms. We used publicly available data, including that from trade organizations and firms’ financial reports, to determine industry support. For pharmaceutical companies, we used data from the Pharmaceutical Research and Manufacturers of America (PhRMA) trade organization8 and considered only domestic research and development expenditures by its member companies. Our previous study was limited by double counting research support from firms that were members of PhRMA and Biotechnology Industry Organization. In this analysis, we used data from Burrill & Company on research and development expenditures of biopharmaceutical companies that were not members of PhRMA.9–12 Such estimates were available for 2004–2008 but not for 2003. We, therefore, used linear regression from 2004–2008 to estimate funding for 2003.
We estimated incremental funding from medical device firms by totaling research and development expenditures in the annual reports13 of the twenty largest (measured by 2008 revenues) US-based firms that were not members of PhRMA. In our previous study, we relied on an external report that has not been updated for support from medical device firms. We also used FDA data to quantify the number of novel treatments, including small molecule biologics,14 and devices,15 that were approved for use between 2003–2008.
Expenditures at Colleges and Universities
As in our previous study, we estimated biomedical research funding at academic institutions from the National Science Foundation’s Academic Research and Development Expenditures report.16
Funding for Health Policy and Health Services Research
In this study we derived health services research expenditures from 2003–2008 from the Coalition of Health Services Research annual reports,17 which includes health services research funding from the Agency for Healthcare Research and Quality, the Center for Disease Control and Prevention, the Center for Medicare and Medicaid Services, NIH, and the Veterans Health Administration. Because updated reports on health policy grantmaking, which we used in our previous study, were not available, we estimated foundation support for health services research solely from the annual reports of the Robert Wood Johnson Foundation,18 which accounted for 63% of health policy funding by foundations in 2002.
Statistical Analysis
We used polynomial regression models to assess financial trends, allowing for a quadratic shape over time. Model parameters were estimated separately for early (1994–2003) and later (2003–2007) time periods, and they were used to estimate mean annual growth rates throughout each period. Mean rates were compared using t-tests at a two-sided significance level of 5%, with model-based standard error estimates obtained from the delta method.
A single imputation was used to estimate funding for biotechnology firms in 2003. A linear regression model was used to predict this missing data based on available data in this time period (2004–2008). Analyses were conducted using SAS version 9.2 (SAS Institute Inc, Cary, North Carolina).
RESULTS
Overall Funding for Biomedical Research
Total funding for biomedical research increased from $75.5 billion in 2003 to $101.1 billion in 2007 (Table 1 and Figure 1). Adjusted for inflation using the Biomedical Research and Development Price Index, this represents a rise of 14% over four years from $92.3 billion in 2003 to $105.6 billion in 2007. By comparison, the gross domestic product (GDP) of the United States, adjusted for inflation, grew by 12% in the same time period.19 In our previous study, funding grew at a compound annual growth rate of 7.8% for 1994–2003 compared to a compound annual rate of 3.4% for 2003–2007 (P<0.001). Funding data for 2008 was only available for NIH and industry and totaled $88.8 billion. The corresponding total for 2007 for NIH and industry was $86.4 billion and when adjusted to 2008 dollars is $90.2 billion indicating that real (adjusted for inflation) funding for biomedical research from NIH and industry decreased from $90.2 billion in 2007 to $88.8 billion in 2008.
Table 1.
Funding for Biomedical Research by Source, 2003–2008
| Data Source | US $ in Billions | ||||||
|---|---|---|---|---|---|---|---|
| 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | ||
| National Institutes of Health | National Science Foundation | 26.0 | 27.3 | 27.9 | 27.7 | 27.8 | 27.9 |
| Other federal | Calculationa | 2.0 | 3.6 | 4.0 | 4.8 | 5.2 | NA |
| State and local government | National Health Expenditure Accounts | 4.2 | 4.5 | 4.6 | 4.8 | 5.2 | NA |
| Foundations, charities, and other private funds | National Health Expenditure Accounts | 3.3 | 3.4 | 3.7 | 4.0 | 4.3 | NA |
| Pharmaceutical firms | Pharmaceutical Research and Manufacturers of America | 27.1 | 29.6 | 31.0 | 34.0 | 36.6 | 38.4 |
| Biotechnology firms | Burrill & Company | 9.3b | 10.5 | 11.9 | 12.2 | 15.3 | 14.9 |
| Medical device firms | US Securities and Exchange Commission filings | 3.6 | 4.2 | 4.8 | 5.9 | 6.7 | 7.6 |
| Total | 75.5 | 83.1 | 87.9 | 93.4 | 101.1 | 88.8 | |
| Adjusted totalc | 92.3 | 97.9 | 99.7 | 101.2 | 105.6 | 88.8 | |
Estimated as the difference between total federal funding and funding for the National Institutes of Health.
Burrill & Company reports on biotechnology companies that are not members of the Pharmaceutical Research and Manufacturers of America were not available in 2003. We used linear regression to generate an estimate for 2003.
Adjusted for inflation to 2008 dollars by the Biomedical Research and Development Price Index. Totals for 2008 reflect funding from the National Institute Health, pharmaceutical, biotechnology, and medical device firms.
Figure 1.
Funding for Biomedical Reserach by Source, 2003 – 2007
As in our previous study, industry remained the largest contributor to biomedical research, accounting for 58% of all expenditures in 2007. NIH remained the second-largest contributor, accounting for 27% of expenditures, followed by state and local governments (5%), non-NIH federal sources (5%), and private not-for-profit support (4%).
Federal Funding
NIH remains the largest federal contributor to biomedical research, accounting for 84% of total federal funding in 2007. Adjusted for inflation, NIH contributions decreased by 8.6%, from $31.8 billion in 2003 to $29.0 billion in 2007 (P<0.001 for the funding trend from 2003–2007 compared to 1994–2003). When adjusted for inflation, total federal funding increased by $200 million (or 0.7%) from 2003–2007. In our previous study, total federal funding increased by nearly 100% from 1994–2003 (P<0.001).
Due to methodological changes in NHEA’s data collection,7 our calculated federal expenditures, state and local funding, and private not-for-profit funding for the years 2003–2004 are modestly different than those in our previous paper.
State and Local Government Funding
State and local government spending on biomedical research increased from $4.2 billion in 2003 to $5.2 billion in 2007, or 6% after adjusting for inflation. State and local government expenditures had increased 45% from 1994–2003 after inflation adjustment (P<0.001).
Private Not-For-Profit Support
Foundations, public charities, medical research organizations, and voluntary health organizations contributed $4.3 billion in 2007, an increase of 11% from 2003 when adjusted for inflation (P=0.42 for the funding trend from 2003–2007 compared to 1994–2003).
Industry Support
Support from pharmaceutical, biotechnology, and medical device companies increased from $40.0 billion in 2003 to $58.6 billion in 2007, an increase of 25% when adjusted for inflation. The largest contributor to biomedical research remained the pharmaceutical sector, followed by biotechnology firms and then medical device companies. Conversely, medical device firms demonstrated the largest rate of growth (59%, adjusted for inflation), followed by biotechnology firms (41%) and pharmaceutical companies (15%).
When compared to our previous study, growth in biomedical research expenditures by industry has decreased from a compound annual growth rate (adjusted for inflation) of 8.1% from 1994–2003 compared to 5.8% for 2003–2007 (P=0.05).
From 2003–2008, the number of new and novel drug and device approvals did not increase (Table 2).
Table 2.
New Drug and Device Approvals by US Food and Drug Administration, 2003–2008
| Category | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 |
|---|---|---|---|---|---|---|
| New Molecular Entities | 21 | 31 | 18 | 18 | 16 | 17 |
| Biologic License Applicationsa | -- | 5 | 2 | 4 | 2 | 3 |
| Device Premarket Application Approvalsb | 33 | 46 | 32 | 38 | 25 | 25 |
The Food and Drug Administration reported biologic license approvals beginning in 2004.
Figures include instruments, implantables, patient monitoring, diagnostic devices, and in vitro tests.
Expenditures at Colleges and Universities
Total biomedical research expenditures at universities were $27.7 billion in 2007, up from $22.0 billion in 2003. When adjusted for inflation, this represents an increase of 7.8% from 2003 to 2007. Federal sources remain the largest contributor to academic biomedical research expenditures, accounting for 65% of expenditures, followed by institutional funds (18% of expenditures).
The distribution of NIH funding among the most heavily funded institutions has remained almost unchanged from 1994 to 2007. In 2007, the ten most heavily funded institutions received 20% of NIH funding (compared to 19% in 1994) and the top fifty institutions received 58% of NIH funding (compared to 55% in 1994).
Funding for Health Policy and Health Services Research
The federal government and foundations spent $1.8 billion in 2003 and $2.2 billion in 2008 (nominal figures) on health services research. The primary sources of federal funding for health services research were the NIH ($1.0 billion in 2008 and the Agency for Healthcare Research and Quality ($335 million in 2008). The Robert Wood Johnson Foundation contributed $317 million to health services research in 2003 and $523 million in 2008.
COMMENT
Since our report in 2005, the rate of increase of research spending has slowed, with a compounded annualized growth rate of 3.4% (2003–2007) versus 7.8% (1994–2003). Total spending on biomedical research accounted for approximately 4.5% of total US health expenditures in 2007. While the decline has occurred at a time of intense economic instability and financial upheaval in the world’s financial markets, the rate diminished even before the events of 2007–2008. Funding from the NIH and industry, which includes pharmaceutical, biotechnology, and medical device firms slowed from 2003 to 2007 and, after adjusting for inflation, has decreased in 2008. Therefore, research investment appears to have returned to its previous cyclical pattern of increases noted since the 1940s.20 The rate and cyclic nature of sponsorship affects researchers and institutions, since it influences career choice, selection of projects, building of laboratories, and establishment of new programs. It makes them cautious and may portend a trend to favor incremental research rather than high risk/high reward avenues, which have particular value to refractory diseases and those of great clinical or public health impact.
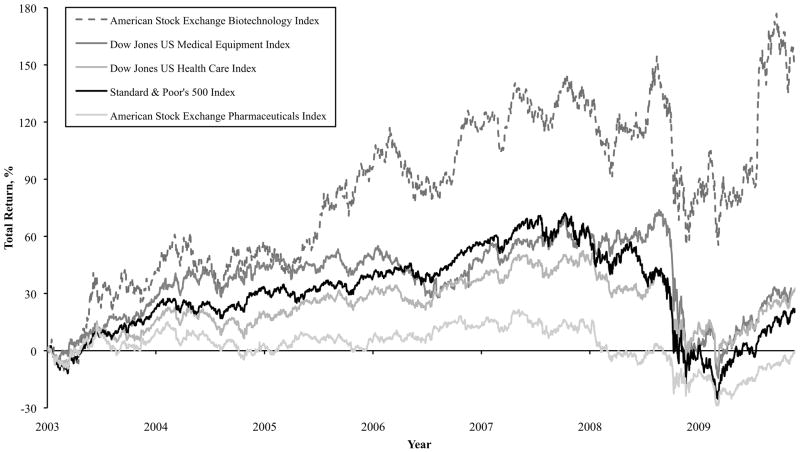
Companies continue to supply the largest proportion of total research spending (58%). However, the rates of growth vary by type of company with medical device firms eclipsing biotechnology companies, and both surpassing conventional pharmaceutical firms. Over this same time period, the return as measured by stock price appreciation was greatest for the medical device sector (Figure 2). In turn, stock performance influences companies’ overall ability to invest and the choice between low-risk, near-term projects versus high-risk, long-term undertakings. The findings are consistent with our 2005 analysis, though biotechnology companies replaced device companies as leaders of total shareholder return. These companies’ stock performance vis-à-vis conventional pharmaceuticals likely reflects several factors that influence the investor’s perception of value: an easier route to FDA approval; lower cost of trials, favorable pricing and eventual profitability of the commercial product; and predictable demand and direct marketing channels through a limited number of practitioners. Thus, both devices and bioengineered drugs are judged to be better company investments, a trend since 1994 that we can confirm. Furthermore, a higher proportion of companies’ spending is in late-stage clinical trials rather than drug discovery.1 Their preference for investments of lower risk is also reflected in accelerated purchasing of small biotech firms by large pharmaceutical companies. Because of these trends, some observers have predicted that the very model of the industry is changing, with large companies buying small ones, rather than investing in (and conducting) early-stage, discovery research themselves.21 This is clearly problematic in the absence of increased research investment pools for the small company, since this is the most common route for academic research to enter clinical use.22 It highlights the important role of foundations and other private research sponsors, as they fill the gap between government-sponsored and commercial research.
Figure 2.
Stock Market Performance of Publicly Traded Life Science and Health Care Companies, 2003 – 2009*
*Total return is calculated from January 2, 2003 to November 23, 2009 by the difference in index price from the base price (January 2, 2003) as a percentage of the base price.
Funding by foundations and charities also slowed from 2003–2007 compared to a decade earlier. These institutions were especially affected by the 2007–2009 recession, a time when their ability to fund speculative, high-risk research is particularly needed. With the intent to enhance the effectiveness and productivity of grants, foundations are exploring alternative research models, such as joint funding with industry and the NIH, , freestanding institutes outside of academia, off-shore (low-overhead) contracting, and pay-for-performance contracts in lieu of conventional grants.23
Ours is a US perspective. However, previous analyses indicate that about 70% to 80% of total global biomedical research and development is sponsored by the US public sector, US-based foundations, and US-headquartered corporations.24 This is in contrast to other research and development, where the US accounts for only about one third.25 As a proportion of total healthcare spending, the US invests 4.5%, an amount higher than any other country.24 An exception is the US support for health services research, which accounts for only 2% of total research or 0.1% of total US health care spending, and is more handsomely funded in Europe.26
Our estimates for 2003 and 2004 have been revised downward from our previous study due to improved data and analyses that reduce double counting of research and development support from industry. However, though the absolute amounts have been revised limiting the ability to compare the magnitude of the totals from 1994–2003 to 2003–2007, the comparison of relative trends remains valid. The selection of 2003 as the point for comparing the change in the trends in funding is arbitrary and reflects the time at which the analyses were conducted. Because of differences in the data sources between the two time periods, we cannot identify the exact year where the rate of funding changed nor was that the study’s objective. The objective was to quantify the funding for biomedical research from 2003–2007 and to compare changes in this period to those observed from 1994–2003. In this analysis, our data are improved but still have limitations. To quantify total funding across all major sponsors, we had to rely on disparate sources that may not be directly comparable. In addition, our estimates of biomedical research funding are likely conservative as the data due not capture all sources (e.g., private philanthropy) and are not exhaustive (e.g., excludes small device firms).
Our analysis suggest that market value of different industry sectors move in parallel with research investment, which has driven the strikingly favorable performance of the medical device sector from 1994 to 2003 and medical biotechnology (chiefly large-molecule drugs) sector since 2003. Conventional pharmaceuticals (predominantly small-molecule drugs) have suffered in comparison. Many reasons can explain their lag, including higher regulatory hurdles, longer and more expensive clinical trials, higher failure rates in pre-clinical studies, and less flexibility in pricing. When conventional small-molecule drugs are compared with large-molecule agents that have known biological actions, or with devices that rely on minor engineering changes, the incentives for drugs are less attractive. For this reason, some have suggested new incentives for new drugs that are effective against diseases of high societal burden or gravity for the individual. These include incentives for novelty and comparative effectiveness, extended patent life, and pricing enhancements for drugs in particular need. Such incentives have been used successfully for vaccines and low prevalence “orphan” diseases.27
The number of new drug or device approvals is an incomplete measure of research productivity. Broader health status measures do favorably reflect the result of biomedical research investments. These include: lower death rates for cancer, stroke, and heart disease; longer life expectancy and improved quality of life for those over 65; more effective and earlier disease detection; substitution of non-invasive for invasive interventions; and improved pain avoidance and control. Notably, few of these rely on the model of one drug for one disease; many reflect public health advances not solely new technology.28
We have entered a period when the cost of care will be an even greater influence over research investment than it has been in the era just concluded. The United States and other developed countries all have aging populations, greater burdens of chronic diseases, increased sense of obligation toward disease in the developing world, and new or refractory infections that have public health import (e.g., H1N1 influenza). Economic limitations are also palpable as the US considers alternatives to private insurance, limits to public funding, and more realistic actuarial assumptions. Moreover, all countries are facing nearly identical rates of increase of total spending (albeit from very different base amounts as a percentage of GDP). Therefore, the cost and value of technology will likely receive additional scrutiny. Hence, it will be even more important to examine research productivity critically.
What might improve researchers’ productivity and their effectiveness? Changes recently recommended include: routine interchange of ideas and people between laboratories having complementary capabilities; less onerous patents for basic discoveries to promote access and research use; recruitment of scientists from other fields, especially from informatics and information sciences, materials, and physics; and the systematic search for unconventional approaches to refractory research problems. Such remedies have motivated the NIH’s Roadmap programs, industry’s reorganization of their internal research and development efforts, and foundations’ experimentation with unconventional alliances and grants. Many of these changes are aimed at creating “semi-permeable membranes” between laboratories within and outside of different institutions, as well as between companies and universities.29
New technology can be viewed either as an undesirable cost or as a source of value. In the countries within the Organisation for Economic Co-operation and Development (comprising the 8 largest economies), between 30% and 45% of the yearly increase in medical spending is attributed to new technology.26 Some in the US and Europe consider that rate of increase to be unsustainable (as the population ages) and as an undesirable drain on overall economic activity (each 1% increase in medical spending lowers GDP by 0.3%).30 Others counter that view and see investment in health care and technology as sources of a country’s competitive advantage, and not a financial drain.31 Therefore, it is inevitable that incentives for development of new medical technology will be scrutinized. Also, since the amount spent on health services research, effectiveness, and clinical epidemiology is so much less than that on new technology, pressure will likely mount to direct funds toward new tools to evaluate technology’s clinical value.32
Biomedical research captures the public’s imagination. It serves many masters. It is highly valued as a source of new and more effective treatments for common or devastating diseases. Conducting research itself and the products and services it spawns are sources of economic development, which is recognized in the developed and developing world alike. Therefore, we will see in the coming years a growing debate between those who view technology as a source of additional cost and others as a source of value. The research community should be mindful of how others view it, and take aggressive steps to enhance its own productivity.
Acknowledgments
Funding/Support: This publication was made possible by Grant Number KL2 RR024136 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Role of the Sponsor: The funding sources had no role in the design and conduct of the study; the analysis or interpretation of the data; or the preparation or final approval of the manuscript.
We thank Aaron Catlin, MS, of the Center for Medicare and Medicaid Services for his assistance understanding changes in the National Health Expenditure data.
Footnotes
Conflicts of Interest: Potential conflicts of interest are indicated under financial disclosures. The authors report no additional conflicts of interest.
Author contributions: Dr. Dorsey had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Dorsey, Moses III
Acquisition of data: de Roulet, Thompson, Reminick, Thai, White-Stellato, George
Analysis and interpretation of data: Dorsey, de Roulet, Thompson, Reminick, Thai, White-Stellato, Beck, George, Moses III
Drafting of the manuscript: Dorsey, de Roulet, George, Moses III
Critical revision of the manuscript for important intellectual content: Dorsey, de Roulet, Thompson, Reminick, Thai, White-Stellato, Beck, George, Moses III
Statistical analysis: Thompson, Beck
Obtained funding: Dorsey
Administrative, technical, or other material support: Dorsey
Study Supervision: Dorsey, Moses III
Financial Disclosures: Dr. Dorsey has received research support from industry (Amarin and Medivation, Inc.), the federal government (NIH), foundations (American Parkinson Disease Association, CHDI Foundation, Inc., Michael J. Fox Foundation for Parkinson’s Research, National Parkinson Foundation, and the Robert Wood Johnson Foundation), and the American Academy of Neurology. Dr. Dorsey has consulted for Avid Radiopharmaceuticals, Inc., Lundbeck Inc., and Merck & Co., Inc. and has stock options in Avid Radiopharmaceuticals, Inc. Dr. Beck has received research support from industry (Amarin and Guidant Corporation), the federal government (NIH), and foundations (CHDI Foundation, Inc., and National Parkinson Foundation). Dr. Moses is chairman of The Alerion Institute and its associated Alerion Advisors, which conducts studies for academic institutions, foundations, and industry on research policy. He is a former partner of The Boston Consulting Group, which advises corporations internationally.
References
- 1.Moses H, III, Dorsey ER, Matheson DH, Thier SO. Financial Anatomy of Biomedical Research. JAMA. 2005;294:1333–1342. doi: 10.1001/jama.294.11.1333. [DOI] [PubMed] [Google Scholar]
- 2.Biomedical Research and Development Price Index (BRDPI) Bethesda, Md: Office of Science and Policy Planning; 2009. [Accessed April 13, 2009]. Available at: http://officeofbudget.od.nih.gov/gbiPriceIndexes.html. [Google Scholar]
- 3.BRDPI: FY 2008 Update and Projections for FY 2009–2014. National Institute of Health, Office of Budget; [Accessed November 23, 2009.]. Available at: http://officeofbudget.od.nih.gov/pdfs/FY09/BRDPI_Proj_Feb_2009_final.pdf. [Google Scholar]
- 4.National Science Foundation, Division of Science Resource Statistics. [Accessed April 13, 2009.];Federal Funds for Research and Development: Fiscal Years 2005–07. Publication No. 09–300. Available at: http://www.nsf.gov/statistics/nsf09300/pdf/nsf09300.pdf.
- 5.Funding: Glossary & Acronym List. US Department of Health & Human Services; [Accessed November 23, 2009.]. Available at: http://grants.nih.gov/Grants/glossary.htm. [Google Scholar]
- 6.National Health Expenditure Accounts. Baltimore, Md: Centers for Medicare & Medicaid Services; [Accessed April 13, 2009.]. Available at: http://www.cms.hhs.gov/NationalHealthExpendData/ [Google Scholar]
- 7.Summary of Benchmark Changes (NHEA) Baltimore Md: Centers for Medicare & Medicaid Services; 2004. [Accessed April 13, 2009.]. Available at: http://www.cms.hhs.gov/NationalHealthExpendData/downloads/benchmark.pdf. [Google Scholar]
- 8.Pharmaceutical Research and Manufacturers of America. Pharmaceutical Industry Profile 2009. Washington DC: PhRMA; 2009. [Google Scholar]
- 9.Biopharmaceutical Industry Research & Development Tops $49 Billion. [Accessed April 13, 2009.];Burrill & Company News Report. 2004 Available at: http://www.burrillandco.com/news-227.html.
- 10.Biopharmaceutical Industry Research & Development Tops $51 Billion. [Accessed April 13, 2009.];Burrill & Company News Report. 2005 Available at: http://www.burrillandco.com/news-213.html.
- 11.Biopharmaceutical Industry Research & Development Tops $55 Billion. [Accessed April 13, 2009.];Burrill & Company News Report. 2006 Available at: http://www.burrillandco.com/news-197.html.
- 12.R&D Spending by US Biopharmaceutical Companies increases 3 percent in 2008. [Accessed April 13, 2009.];Burrill & Company News Report. Available at: http://www.burrillandco.com/news-359.html.
- 13.SEC filings and forms. US Securities and Exchange Commission; [Accessed April 13, 2009.]. Available at: http://www.sec.gov/edgar.shtml. [Google Scholar]
- 14.Drugs: NME Drug and New Biologic Approvals. US Food and Drug Administration; [Accessed April 13, 2009.]. Available at: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/DrugandBiologicApprovalReports/ucm121136.htm. [Google Scholar]
- 15.Medical Devices: PMA Approvals. US Food and Drug Administration; [Accessed April 13, 2009.]. Available at: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/PMAApprovals/default.htm. [Google Scholar]
- 16.Academic Research & Development Expenditures: Fiscal Year 2007. National Science Foundation, Division of Science Resources Statistics; [Accessed April 13, 2009.]. Publication No. 09–303. Available at: http://www.nsf.gov/statistics/nsf09303/pdf/nsf09303.pdf. [Google Scholar]
- 17.Reports on Federal Funding For Health Services Research: 2004 – 2008. Coalition for Health Services Research; [Accessed August 18, 2009.]. Available at: http://www.chsr.org/reports.htm. [Google Scholar]
- 18.Annual Reports: 2003 – 2008. Robert Wood Johnson Foundation; [Accessed August 24, 2009.]. Available at: http://www.rwjf.org/about/annualreportlist.jsp. [Google Scholar]
- 19.National Economic Accounts. US Department of Commerce, Bureau of Economic Analysis; [Accessed April 13, 2009.]. Available at: http://www.bea.gov/national/index.htm. [Google Scholar]
- 20.Goldston D. A Delicate Balance. Nature. 2008;453:838. doi: 10.1038/453838a. [DOI] [PubMed] [Google Scholar]
- 21.Danzon PM. National Bureau of Economic Research; [Accessed September 9, 2009.]. Economics of the Pharmaceutical Industry. Available at: http://www.nber.org/cgi-bin/printit?uri=/reporter/fall06/danzon.html. [Google Scholar]
- 22.Pisano GP. Science Business. Harvard Business School Press; 2006. pp. 122–124. [PubMed] [Google Scholar]
- 23.Butler D. Transitional Research: crossing the valley of death. Nature. 2008;453:840–842. doi: 10.1038/453840a. [DOI] [PubMed] [Google Scholar]
- 24.Schweitzer SO. Pharmaceutical Economics and Policy. New York: Oxford University Press; 2007. [Google Scholar]
- 25.Guide to R&D Funding Data-International Comparisons. American Association for the Advancement of Science; [Accessed August 18, 2009.]. Available at: http://www.aaas.org/spp/rd/guiintl.htm. [Google Scholar]
- 26.Wilensky GR. Developing a center for comparative effectiveness information. Health Affairs. 2006;25:w572–w585. doi: 10.1377/hlthaff.25.w572. [DOI] [PubMed] [Google Scholar]
- 27.Wood AJ. A proposal for radical changes in the drug-approval process. New England Journal of Medicine. 2006;355:618–623. doi: 10.1056/NEJMsb055203. [DOI] [PubMed] [Google Scholar]
- 28.Drews J. Case histories, magic bullets and the state of drug discovery. Nat Rev Drug Discov. 2009;5:635–640. doi: 10.1038/nrd2084. [DOI] [PubMed] [Google Scholar]
- 29.Fitzgerald GA. Drugs, Industry, and Academia. Science. 2008;320:1563. doi: 10.1126/science.1161006. [DOI] [PubMed] [Google Scholar]
- 30.Anderson GF, Reinhardt UE, Hussey PS, Petrosyan V. It’s the price, stupid: why the United States is so different from other countries. Health Affairs. 2003;22:89–105. doi: 10.1377/hlthaff.22.3.89. [DOI] [PubMed] [Google Scholar]
- 31.Newhouse JP. Why is there a quality chasm? Health Affairs. 2009;21:13–25. doi: 10.1377/hlthaff.21.4.13. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman A, Pearson SD. ‘Marginal Medicine’: Targeting Comparative Effectiveness Research To Reduce Waste. Health Affairs. 2009;28:w710–w718. doi: 10.1377/hlthaff.28.4.w710. [DOI] [PubMed] [Google Scholar]