Abstract
Background
Cystic fibrosis (CF) lung disease has a unique profile of pathogens predominated by Pseudomonas aeruginosa (PsA) and Staphylococcus aureus (SA). These microorganisms must overcome host immune defense to colonize the CF lungs. Polymorphonuclear neutrophils are a major component of the host defense against bacterial infection. A crucial microbicidal mechanism is the production of oxidants including hydrogen peroxide (H2O2) and hypochlorous acid (HOCl) by neutrophils to achieve efficient bacterial killing. To determine to what degrees various CF pathogens resist the oxidants relative to non-CF pathogens, we compared the susceptibility of PsA, SA, Burkholderia cepacia (BC), Klebsiella pneumoniae (KP), and Escherichia coli (EC) to various concentrations of H2O2 or HOCl, in vitro. The comparative oxidant-resistant profiles were established. Oxidant-induced damage to ATP production and cell membrane integrity of the microbes were quantitatively assessed. Correlation of membrane permeability and ATP levels with bacterial viability was statistically evaluated.
Results
PsA was relatively resistant to both H2O2 (LD50 = 1.5 mM) and HOCl (LD50 = 0.035 mM). SA was susceptible to H2O2 (LD50 = 0.1 mM) but resistant to HOCl (LD50 = 0.035 mM). Interestingly, KP was extremely resistant to high doses of H2O2 (LD50 = 2.5-5.0 mM) but was very sensitive to low doses of HOCl (LD50 = 0.015 mM). BC was intermediate to resist both oxidants: H2O2 (LD50 = 0.3-0.4 mM) and HOCl (LD50 = 0.025 mM). EC displayed the least resistance to H2O2 (LD50 = 0.2-0.3 mM) and HOCl (LD50 = 0.015 mM). The identified profile of H2O2-resistance was KP > PsA > BC > EC > SA and the profile of HOCl-resistance PsA > SA > BC > EC > KP. Moreover, both oxidants affected ATP production and membrane integrity of the cells. However, the effects varied among the tested organisms and, the oxidant-mediated damage correlated differentially with the bacterial viability.
Conclusions
The order of HOCl-resistance identified herein best fits the clinical profile of CF infections. Even though oxidants are able to disrupt ATP production and cell membrane integrity, the degrees of damage vary among the organisms and correlate differentially with their viability.
Keywords: Hydrogen Peroxide, Hypochlorous Acid, Microbicidal, Cystic Fibrosis, Neutrophils, Oxidants
Background
Cystic fibrosis (CF) is the most common fatal genetic disease in Caucasians and is caused by mutations of the CF transmembrane conductance regulator (CFTR), a cAMP-stimulated chloride (Cl-) channel [1]. The most devastating anomaly of CF is the lung disease which is characterized by chronic bacterial infection, abnormal airway inflammation, extensive neutrophil infiltration and small airway obstruction [2,3]. CF lung infection has a unique pathogen profile which is distinct from other lung infections. Pseudomonas aeruginosa, Staphylococcus aureus, Haemophilus influenzae, Stenotrophomonas maltophilia, Achromobacter xylosoxidans, Burkholderia cepacia are the most prevalent, among which P. aeruginosa predominates [4-6]. Strikingly, all the CF organisms except S. aureus are opportunistic pathogens, which do not cause infections in healthy hosts [6]. It is not fully understood why CF patients are particularly susceptible to these organisms and how the organisms manage to escape the host defense at the early infection stage when there is little antibiotic selection and environmental pressure. Apparently, it is the early microbe-host interaction that determines the early pathogen colonization and subsequently persistent infection in CF lungs.
The first line of host defense against invading bacteria is the recruitment of polymorphonuclear neutrophils (PMNs) to sites of infection. Normally, PMNs effectively contain the microbes by phagocytosis and then mount multi-tiered chemical attacks with pre-fabricated and de novo-produced agents to kill the phagocytosed organisms [7-9]. The NADPH oxidase-myeloperoxidase (MPO) system constitutes a major antimicrobial mechanism employed by PMNs to fight infections and accounts for ~90% of the oxygen consumed during the phagocyte respiratory burst [10]. This system generates a number of microbicidal oxidants including superoxide (O2-), hydrogen peroxide (H2O2), and hypochlorous acid (HOCl) [11], among which HOCl is most potent. HOCl biosynthesis is catalyzed by MPO by using H2O2, H+ and Cl- as its substrates. As shown in the reaction 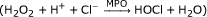 , the availability of chloride anion in the neutrophil phagosomes limits the production of HOCl. Consequently, any decreased HOCl production reduces H2O2 consumption, thus affecting the level of H2O2 in the organelle. Our recent publications demonstrated that the PMNs from CF patients are deficient in chloride transport to phagosomes and deficient in intraphagosomal chlorination and killing of PsA [12-14], suggesting an altered oxidant production in CF neutrophils. Such a defect in phagocytic innate immunity may preferentially allow certain bacterial strains to evade the compromised host defense.
, the availability of chloride anion in the neutrophil phagosomes limits the production of HOCl. Consequently, any decreased HOCl production reduces H2O2 consumption, thus affecting the level of H2O2 in the organelle. Our recent publications demonstrated that the PMNs from CF patients are deficient in chloride transport to phagosomes and deficient in intraphagosomal chlorination and killing of PsA [12-14], suggesting an altered oxidant production in CF neutrophils. Such a defect in phagocytic innate immunity may preferentially allow certain bacterial strains to evade the compromised host defense.
In the current study, we hypothesized that if the HOCl production abnormality in CF neutrophils plays a major role in the disease pathogenesis, then the HOCl-resistant bacteria should be the most clinically prevalent. To test the hypothesis, we sought to investigate the intrinsic resistance of CF and non-CF organisms to H2O2 and HOCl in a cell-free system. Responses of PsA, SA, BC, KP and EC to the chemical oxidants were determined and the resistance profiles of the tested organisms established. Moreover, effects of the oxidants on cell membrane permeability and ATP production were compared among the CF and non-CF pathogens to assess whether the oxidant-induced damages correlate with bacterial viability.
Methods
Reagents and cultures
PsA, SA and BC were CF clinical isolates which were characterized by conventional microbiological methods including colony morphology, pigment production, Gram staining and standard biochemical tests [15]. KP (Strain 43816, serotype 2) was obtained from American Type Culture Collection (Manassas, VA). EC (Strain DH5α) was from Invitrogen (Carlsbad, CA). Percoll, 30% reagent-grade H2O2, and NaOCl (5% chlorine) were purchased from Fisher Scientific (Pittsburgh, PA). All cell and microbial culture media were purchased from Invitrogen.
Microbial growth and storage
Luria-Bertani (LB) broth media (10 ml) were inoculated with PsA, SA, BC, KP or EC and cultured overnight at 37°C and 220 rpm. The following day, the cultures were streaked onto LB agar plates without antibiotics for colony isolation. New cultures were inoculated from single colonies of each organism and grown overnight at 37°C and 220 rpm. The pure cultures were cryogenically preserved by freezing a mixture of 0.5 ml of each culture with 0.5 ml of 30% glycerol in water at -80°C. Freshly streaked agar plate cultures for each organism were prepared from cryo stock bi-weekly.
In vitro microbial killing with reagent H2O2 and HOCl
Bacterial cultures from isolation plates were grown overnight in LB broth media at 37°C with vigorous agitation at 230 rpm. On the day of experiments, the cultures were diluted 1:100 in LB broth media and subcultured to late-log phase. The subcultures were pelleted at 5000 × g and washed with Delbecco's Phosphate Buffered Saline (DPBS, pH 7.4, no Ca2+ or Mg2+). The cell density was determined by the formula 1.0 OD600 = 1 × 109 cells/ml where OD600 is the optical density read at 600 nm in Beckman Coulter DU 640 spectrophotometer.
Oxidant-mediated killing by H2O2 and HOCl was carried out by modification of the methods described by McKenna and Davies [16]. For H2O2-mediated killing, microbes were suspended to 5 × 105 cells/ml in DPBS. Killing reactions were carried out in duplicate wells of 96-well microtiter plates containing 5 × 103 microbial cells in a final volume of 200 μl of DPBS containing various concentrations of H2O2. The mixtures were incubated at 37°C for 1 hour and were then transferred to ice to halt any additional growth. The samples were mixed by repeated pipetting just before plating 20 μl to LB agar plates. The plates were then incubated overnight at 37°C and the number of viable microbial cells for each H2O2 concentration was determined by colony forming unit (CFU) counting.
For HOCl-mediated killing, 5 × 108 bacterial cells were aliquotted, in duplicate, to 15 ml conical tubes at a final volume of 1 ml of DPBS containing various concentrations of HOCl as indicated. The tubes were incubated at 37°C for 1 hour with agitation and were then placed on ice. The samples were then passed through 25 gauge needles. Bacterial samples were then diluted 1:105 in DPBS. Fifty microliters of each diluted sample was plated to LB agar and cultured at 37°C. Microbial viability was assessed by CFU counting.
Assessing HOCl- and H2O2-induced bacterial membrane permeability
Permeability of bacterial membranes after exposure of the organisms to reagent HOCl or H2O2 was measured using the LIVE/DEAD BacLight Bacterial Viability and Counting Kit (Molecular Probes, Carlsbad, CA). For HOCl-mediated membrane permeability studies, PsA, SA, KP, BC, and EC were grown in LB broth medium at 37°C overnight and subsequently subcultured (1:100) in fresh LB media until the culture reached late-log phase. The cells were then pelleted and washed with DPBS, quantified, and resuspended to 6.67 × 109 cells per milliliter. Cells (5 × 108) were aliquotted to 15 ml conical tubes, and reagent NaOCl was added to the final concentrations indicated. The bacterial suspensions were incubated with the oxidant for 1 hour at 37°C and 220 rpm. The samples were placed on ice. Finally, the bacteria were pelleted in a table-top centrifuge at full speed for 2 minutes, and pellets were washed with ice-cold DPBS. The samples were stained according to manufacturer protocol with the vital dye Syto 9 as well as with propidium iodide (PI) which stains permeabilized cells. The percentages of fluorescently stained intact and permeable cells were assessed by flow cytometry, and the data were normalized to the oxidant-free controls. Controls for intact and permeable bacteria were produced by 1 hour incubation with either 0.85% NaCl or 70% ethanol, respectively, followed by washing and resuspension in 0.85% NaCl.
For H2O2-mediated membrane permeability studies, 1.25 × 106 cells were used per sample, each in a volume of 50 ml of DPBS to preserve the same cell density as was used in the above described CFU viability assay. Incubation times were the same as for the HOCl membrane permeability experiments. After incubation, the 50 ml samples were concentrated to 1 ml by centrifugation at 3000 × g for 15 minutes followed by washing, staining, and analysis as described above for HOCl assays. The controls were similarly set as well.
Quantifying the effect of H2O2 and HOCl on bacterial ATP production
The indicated organisms were exposed to H2O2 or HOCl as indicated above in the membrane permeability studies. ATP production was quantified following oxidant exposure using the BacTiter-Glo Microbial Cell Viability Assay from Promega according to manufacturer protocol. 5 × 106 cells were used in each assay sample to yield a signal-to-noise ratio of approximately 104-105:1. ATP-specific luminescence was measured using a BioTek (Winooski, VT) Synergy HT microplate reader, and ATP concentration was determined by fitting the luminescence values to a standard curve generated using 10-fold dilutions of Na-ATP from 1 μM to 10 pM. Data are represented as percent ATP recovery relative to oxidant-free controls.
Statistical analysis
Two-way ANOVA with replication was used when analyzing organism viability. Differences in the single parameter of membrane integrity or ATP level were analyzed by One-way ANOVA. Linear regression was performed for correlating membrane permeability and ATP production with bacterial CFU viability.
Results
Oxidant resistance of CF and non-CF pathogens to H2O2 and HOCl
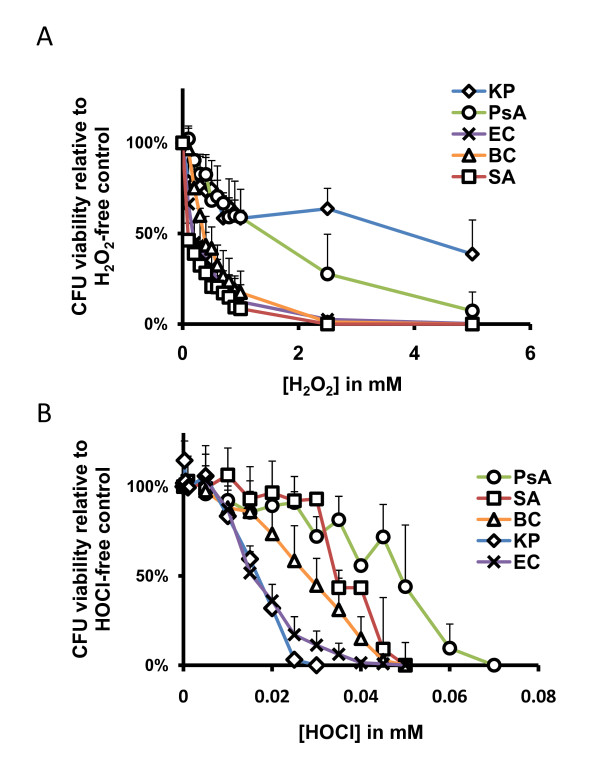
We exposed PsA, SA, KP, BC, and EC to reagent-grade H2O2 or HOCl, in vitro, to compare their intrinsic susceptibility or resistance as described in Materials and Methods. The results (Figure 1A) demonstrated that KP and PsA were the most resistant organisms to H2O2. Unexpectedly, KP, a non-CF pathogen, showed almost an equal, if not greater, resistance to H2O2 than PsA by two-way ANOVA test (p = 0.79; Figure 1A and Table 1). Both PsA and KP were vastly more resistant to H2O2 than any of the other organisms tested (p < 0.0001 for all comparisons). BC, SA, and EC were the most susceptible to H2O2 with approximately 90% eradication at approximately 1 mM of the oxidant. Statistically, the profile of greatest to least H2O2-resistant organisms is as follows: KP > PsA > BC > EC > SA.
Figure 1.
Bacterial killing by reagent H2O2 and HOCl in vitro. Microbes were exposed to various concentrations of H2O2 or HOCl, as indicated, for 1 hour at 37°C. At the end of the exposure, the samples were plated to LB agar plates for overnight culture. Bacterial killing by oxidants was measured as percent of viable bacteria relative to the number of colonies from the oxidant-free controls. A) Organisms indicated were exposed to 0 mM to 5.0 mM H2O2 or (B) 0 mM to 0.1 mM HOCl. PsA = P. aeruginosa, SA = S. aureus, BC = B. cepacia, KP = K. pneumoniae, and EC = DH5α-E. coli. Error bars represent standard deviation of at least n = 3 experiments.
Table 1.
Comparisons of H2O2 in vitro killing of various species of bacteria (P-value from two-way ANOVA with replication)
| PsA | SA | BC | KP | EC | |
|---|---|---|---|---|---|
| PsA | - | <0.0001 | <0.0001 | 0.79 | <0.0001 |
| SA | <0.0001 | - | <0.0001 | <0.0001 | 0.0006 |
| BC | <0.0001 | <0.0001 | - | <0.0001 | 0.0002 |
| KP | 0.79 | <0.0001 | <0.0001 | - | <0.0001 |
| EC | <0.0001 | 0.0006 | 0.0002 | <0.0001 | - |
When exposed to HOCl, the organisms tested displayed a different profile of oxidant-mediated killing. Other than the fact that HOCl is vastly more microbicidal to all the organisms tested at lower concentrations than H2O2, the most noticeable difference was the sharp decline in viability of KP with increasing HOCl concentration (Figure 1B). Where we previously observed strong resistance of KP to H2O2, here it appeared to be among the most susceptible to HOCl assault. PsA and SA emerged as the most resistant organisms to HOCl-mediated killing, and the difference between the two organisms was not statistically significant (p = 0.39; Table 2). However, the killing curves of PsA and SA did terminate at slightly different values; that is, complete abolition of CFU formation occurred at 0.05 mM HOCl for SA while PsA was not completely eradicated until the HOCl concentration reached 0.07 mM. Both PsA and SA killing curves were significantly different from that of BC (p < 0.0001), and BC survived HOCl-mediated assault at significantly higher concentrations than did KP or EC (p = 0.006 and p < 0.0001, respectively). Under these conditions, the profile of greatest to least HOCl-resistant organisms is as follows: PsA > SA > BC > EC > KP.
Table 2.
Comparisons of HOCl in vitro killing of various species of bacteria (P-value from two-way ANOVA with replication)
| PsA | SA | BC | KP | EC | |
|---|---|---|---|---|---|
| PsA | - | 0.39 | <0.0001 | 0.0007 | <0.0001 |
| SA | 0.39 | - | <0.0001 | 0.004 | <0.0001 |
| BC | <0.0001 | <0.0001 | - | 0.006 | <0.0001 |
| KP | 0.0007 | 0.004 | 0.006 | - | 0.02 |
| EC | <0.0001 | <0.0001 | <0.0001 | 0.02 | - |
Based on the above oxidant-resistance data, we recognized that the HOCl bacterial killing profile remarkably fit the infection profile observed in CF patients clinically. Among the CF and non-CF pathogens tested, PsA was the strongest organism resistant to both oxidants.
Oxidant-induced membrane injury of CF and non-CF pathogens
The bacterial membrane is the first contact point for oxidants to act on these cells. To examine effects of the oxidants on bacterial membrane integrity, we measured the cell permeability before and after oxidant exposure. The uptake of fluorescent Syto9, a cell vital dye, and propidium iodide (PI), a permeable cell dye, were analyzed by flow cytometry. The percent of cells with intact cytoplasmic membranes were compared and normalized to the percent of bacteria with the intact membranes in the oxidant-free controls.
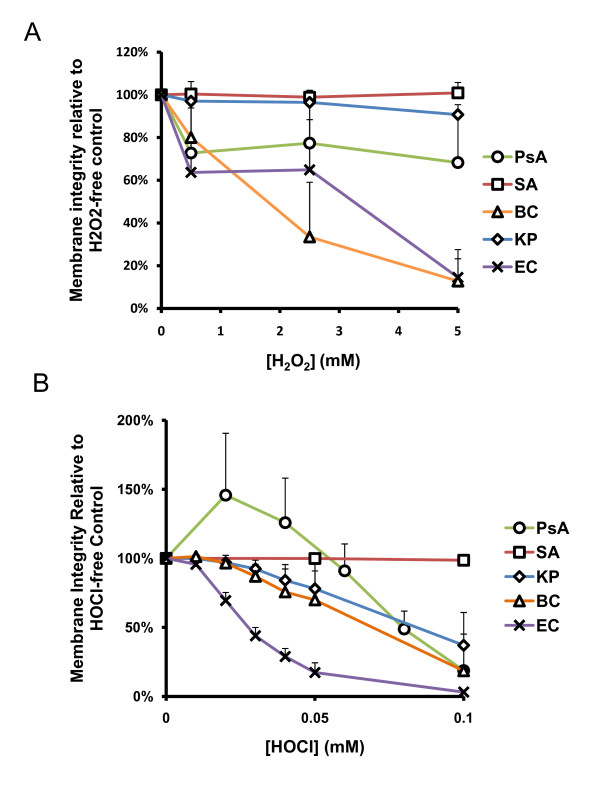
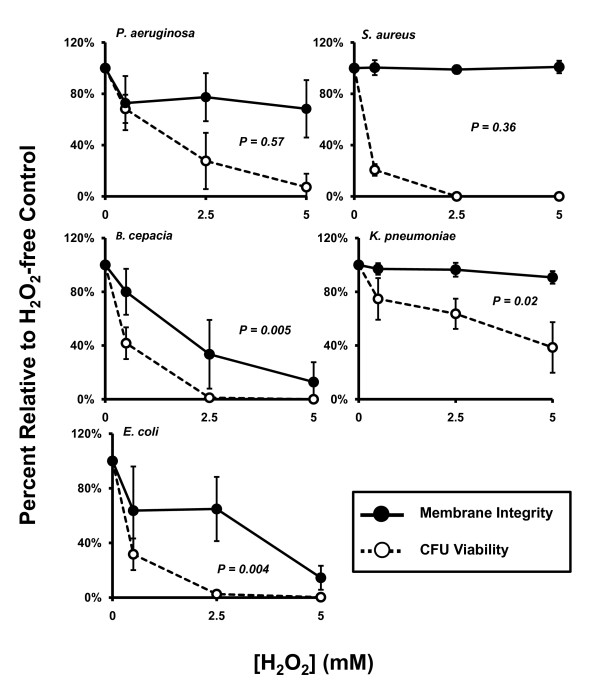
The membrane integrity of PsA, SA, and KP were not significantly affected by H2O2 up to 5 mM, the maximum concentration measured herein, as compared to each corresponding buffer controls. Single factor (One-way) ANOVA analyses revealed a p value of 0.22, 0.94 or 0.12 for PsA, SA or KP, respectively (Figure 2A). BC and EC displayed increasing percentages of permeable cells after exposure to H2O2 from 0 mM to 5 mM (p = 0.0008 and 0.006, respectively) with 50% permeability occurring at approximately 2.5 mM for each. To relate the membrane damage to cell viability, we performed linear regression test for each organism. As shown (Figure 3), the percent of PsA and SA with intact membranes after exposure to a range of H2O2 concentrations relative to the oxidant-free control was statistically independent from the measure of CFU viability at the same peroxide concentrations (p = 0.57 and 0.36, respectively), suggesting the two phenomena were not related. Declining CFU viability from exposure to increased peroxide concentration did correlate statistically with the loss of membrane integrity after exposure of BC and EC to H2O2 (p = 0.005 and 0.004, respectively). Though membrane integrity of KP was statistically unaffected by H2O2 exposure while CFU viability did significantly decline with increasing H2O2 concentration, the two parameters are not statistically independent of each other (p = 0.02).
Figure 2.
H2O2 and HOCl-induced membrane permeability. Bacteria were exposed to reagent A) H2O2 or B) HOCl as indicated, and the effect of the oxidant on membrane integrity was measured by the BacLight Bacterial Viability and Counting Kit (Molecular Probes). Membrane integrity of PsA, SA, and KP were not significantly affected by H2O2 up to 5 mM by single-factor ANOVA analyses. All organisms tested demonstrated HOCl dose-dependent membrane permeability except SA which remained unaffected up to 0.1 mM. Error bars represent standard deviations of at least n = 3 experiments.
Figure 3.
Correlating H2O2-mediated membrane permeabilization and CFU viability. For BC, KP, and EC, loss of membrane integrity correlated statistically with decline in CFU viability while these two parameters were statistically independent of each other for PsA and SA. Solid circles and lines: membrane integrity. Open circles and dotted lines: bacterial viability. Both parameters were expressed as percent relative to oxidant-free controls. P-values represent linear regression of the raw data values from membrane permeability versus bacterial viability. Values less than 0.05 were considered significant and denote correlation between the parameters; values greater than 0.05 indicate independence of the parameters. Error bars represent standard deviation of at least n = 3 experiments.
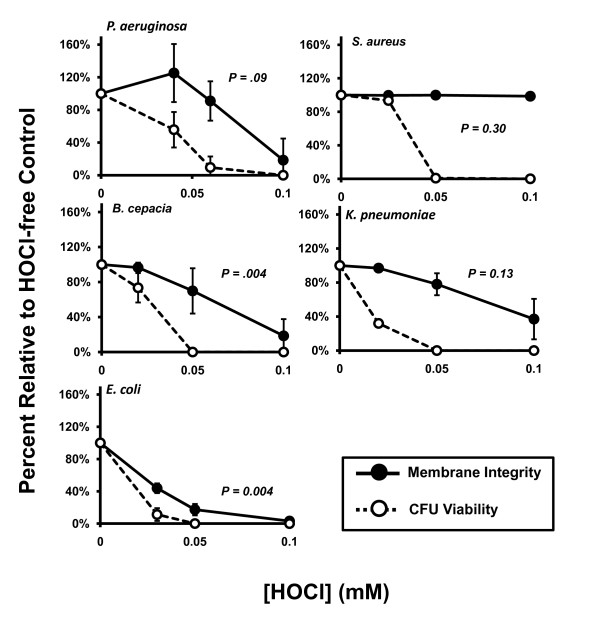
Membrane integrity of PsA, BC, KP, and EC was affected significantly by exposure to up to 0.1 mM HOCl, in a dose-dependent manner, as compared to each corresponding buffer controls (p = 0.007, 0.003, 0.002, and <0.0001, respectively, by One-way ANOVA test; Figure 2B), while SA membrane integrity was unaffected by these concentrations. Furthermore, linear regression tests, shown in Figure 4, revealed that CFU viability was abolished at lower concentrations than those required to produce the same degree of membrane permeabilization in PsA, SA, and KP; that is, no correlation was detected between these two parameters for each organism (p = 0.09, 0.30, and 0.13, respectively). BC and EC demonstrated HOCl-induced membrane permeabilization that correlated significantly with CFU viability of these organisms after oxidant exposure (p = 0.004, and 0.004, respectively). EC membrane integrity was most affected by HOCl exposure with 50% permeabilization occurring between 0 mM and 0.03 mM while all other organisms with affected membrane integrity lost 50% integrity between 0.05 mM and 0.1 mM.
Figure 4.
Correlating HOCl-induced membrane permeability and CFU viability. Bacteria were exposed to reagent HOCl in vitro to determine the effect of the oxidant on membrane integrity as measured by the BacLight Bacterial Viability and Counting Kit (Molecular Probes). Concentrations of HOCl used were based on the amounts necessary to eradicate CFU viability as assessed in the previous experiments. In general, bacterial membranes remain intact at concentrations beyond that required to inhibit CFU formation and kill the organism. Under these conditions, PsA, SA, and KP were killed at statistically lower concentrations of HOCl than were required to produce the same degree of membrane permeabilization. Membrane permeabilization by HOCl in BC and EC correlated with loss of CFU viability. Solid circles and lines: membrane integrity. Open circles and dotted lines: bacterial viability. Both parameters were expressed as percent relative to oxidant-free controls. P-values represent linear regression of the raw data values from membrane permeability versus CFU viability. Values less than 0.05 were considered significant and denote correlation among the parameters; values greater than 0.05 indicate independence of the parameters. Error bars represent standard deviation of at least n = 3 experiments.
Effect of oxidants on bacterial ATP production
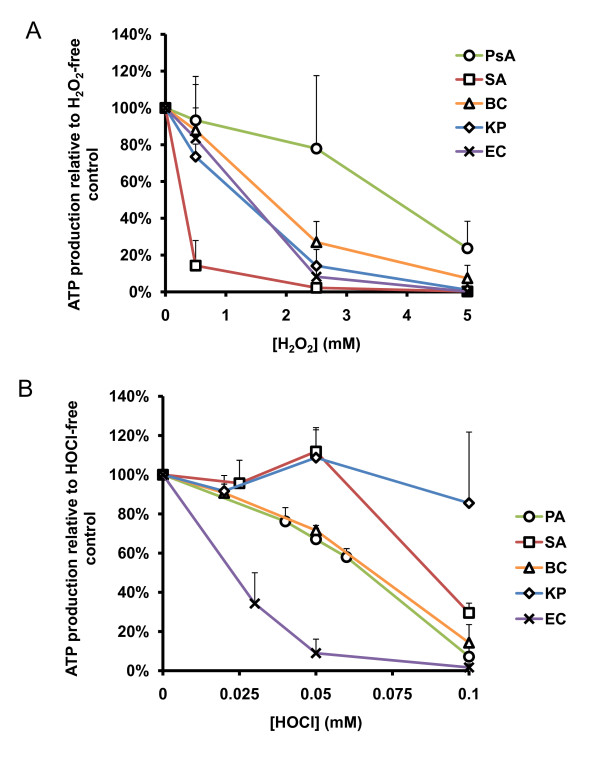
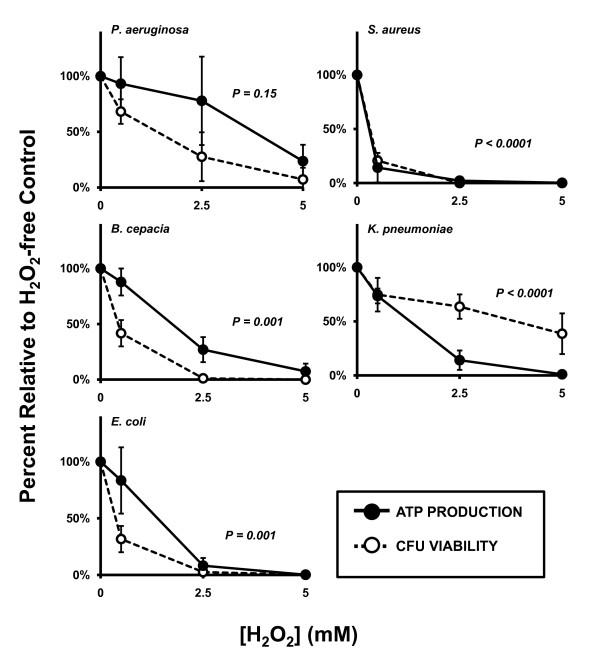
Energy supply is another house-keeping factor vital to bacterial viability. Because the F1F0 ATP synthase is a cell membrane-bound protein which is exposed to outside, oxidants applied may preferentially target the energy production system. A previous publication has reported that ATP production is a major target of oxidants [17]. Here, we treated the CF and non-CF pathogens with H2O2 from 0 mM to 5.0 mM or with HOCl from 0 mM to 0.1 mM for 1 hour at 37°C. After oxidant exposure, the bacteria were analyzed for cellular ATP levels. All organisms tested displayed significant reduction in ATP content with increasing doses of H2O2 by One-way ANOVA analyses (PsA, p = 0.02; SA, p < 0.0001; BC, p < 0.0001; KP, p < 0.0001 and EC, p < 0.0001; Figure 5A). This reduction correlated statistically with CFU viability under the same conditions for all organisms except PsA which failed to reach statistical correlation by linear regression analysis (Figure 6) (SA: p < 0.0001; BC: p = 0.001; KP: p < 0.0001; EC: p = 0.001 and PsA: p = 0.15). Interestingly, the relative H2O2 dose-dependent decline in ATP content in KP was more dramatic than the loss of CFU viability under the same conditions.
Figure 5.
H2O2- and HOCl-induced ATP changes in bacterial pathogens. Bacteria were exposed to reagent H2O2 or HOCl, in vitro, to determine the effect of the oxidant on ATP production as measured by the BacTiter-Glo Microbial Cell Viability Assay (Promega). Concentrations of oxidants used were based on the amounts necessary to eradicate CFU viability as assessed in the previous experiments. A) All organisms displayed significant reduction in ATP production (One-way ANOVA) in an H2O2 dose-dependent manner up to 5 mM. B) ATP production by KP was statistically unaffected by HOCl exposure up to 0.1 mM according to one-way ANOVA (p = 0.53) while all other organisms tested displayed significant HOCl dose-dependent reduction in ATP production in this concentration range. Error bars represent standard deviation of at least n = 3 experiments.
Figure 6.
Correlating H2O2-induced loss of ATP production with bacterial viability. H2O2-induced disruption of ATP production correlated statistically with abolishment of CFU viability for all organisms tested except PsA (p = 0.15) at concentrations up to 5 mM. Though the decline of ATP production in PsA for this oxidant was statistically significant in this range, the percent change remains independent of the percent reduction in CFU viability. Solid circles and lines: ATP recovery after oxidant exposure. Open circles and dotted lines: CFU viability. Both parameters are measured as percent relative to oxidant-free controls. P-values represent linear regression of the raw data values from percent ATP recovery versus CFU viability. Values less than 0.05 were considered significant and denote correlation between the parameters; values greater than 0.05 indicate independence of the parameters. Error bars represent standard deviation of at least n = 3 experiments.
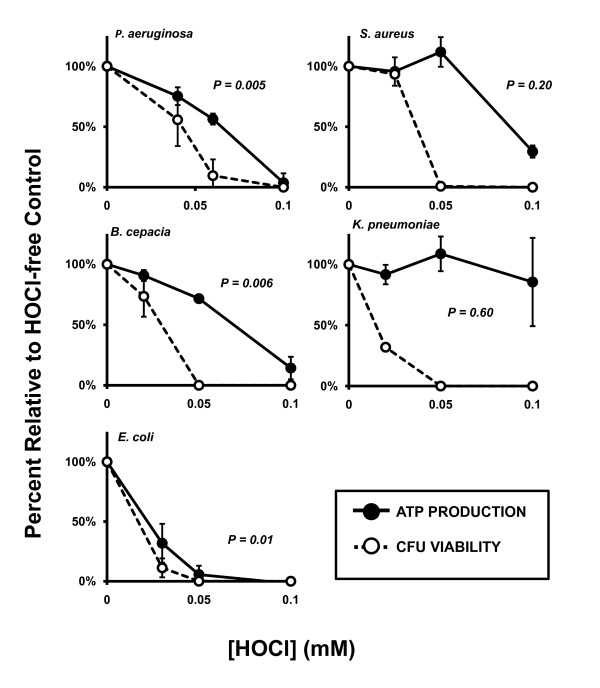
ATP production was dose-dependently abolished in PsA, SA, BC, and EC while KP remained statistically unaffected even at HOCl doses up to 0.1 mM (PsA, p < 0.0001; SA, p < 0.0001; BC, p < 0.0001; EC, p < 0.0001 and KP, p = 0.53; Figure 5B). The decline in ATP production correlated with HOCl-induced loss of CFU viability in PsA, BC, and EC (p = 0.005, 0.006, and 0.01, respectively, Figure 7) but was independent of diminished CFU viability in SA and KP (p = 0.20 and 0.60, respectively).
Figure 7.
Correlating HOCl-induced ATP changes with bacterial viability. ATP production is affected by HOCl exposure and correlates statistically with CFU viability in PsA, BC, and EC (p = 0.005, 0.006, and 0.01, respectively); however, SA and KP lose CFU viability after exposure to lower concentrations of HOCl than are required to abolish ATP production during the assay time. Solid circles and lines: ATP recovery after oxidant exposure. Open circles and dotted lines: CFU viability. Both parameters are measured as percent relative to oxidant-free controls. P-values represent linear regression of the raw data values from percent ATP recovery versus CFU viability. Values less than 0.05 were considered significant and denote correlation among the parameters; values greater than 0.05 indicate independence of the parameters. Error bars represent standard deviation of at least n = 3 experiments.
Discussion
In this report, we used a cell-free system with reagent H2O2 and HOCl to examine the intrinsic resistance of prevalent CF and Non-CF respiratory pathogens to the oxidants. We found that the in vitro HOCl-resistance profile (PsA > SA > BC > EC > KP) best fits the infection profile observed clinically in CF lungs; that is, the most HOCl-resistant bacteria such as PsA and SA are the most frequent pathogens in CF patients. This finding implies that differential HOCl resistance across microbial species may allow for persistence of some infections over others by subversion of the host innate immunity and supports our previous finding that CF neutrophils with a compromised HOCl production may not be able to clear the most resistant organisms effectively [12,13]. From a microbiological point of view, PsA and SA, the relatively more resistant strains to HOCl, would be more likely to survive and be selected for, if the host neutrophils were deficient in their ability to make HOCl. Burns and coworkers did a longitudinal study on young children with CF and found that 97% of the children are colonized with PsA [18]. The early isolates tend to be nonmucoid and antibiotic-sensitive. However, if the initial infection is not effectively eradicated by the host defense, which could happen, for example, if HOCl or other oxidant production was suboptimal, then the bacteria which escape the initial host defenses will grow and spread within the lung, establishing a long-term chronic colonization. Subsequently, environmental pressure in the lung such as antibiotic application selects for the mucoid PsA phenotype. Increased PsA density in the lower respiratory tract and development of antibiotic-resistant mucoid biofilms causes chronic airway inflammation and deteriorating lung function [19-22]. SA has long been recognized to be among the first organisms to colonize the airways of CF patients [23]. Colonization with SA occurs within the first few months of life, and persistent variants of this organism may arise due to a selective pressure from long-term antibiotic treatment in CF patients [24]. However, SA infection does not usually persist or progress to chronic disease. We would like to point out that our current study only tested bacteria in log-phase growth. Such an experimental design was intended to study the nonmucoid form which is assumed by the bacteria during the early CF infections. It is important to recognize that only after initial bacterial colonization is established, can chronic persistent infections ensue in CF lungs.
Neutrophils are highly specialized for bacterial killing especially in the case of extracellular infections. The cells employ at least two microbicidal mechanisms to execute this function: one is oxidant-mediated and another is non-oxidant-mediated. Pseudomonas bacteria possess tough polysaccharide capsules, which are resistant to nonoxidant killing mechanisms, such as protease and hydrolase digestion [25]. This feature determines the requirement of oxidant-killing mechanisms for complete eradication. The importance of neutrophils in defending Pseudomonas infection is reflected by significant increase in infection rate in neutropenic patients [4]. Winterbourn and colleagues modeled the reactions of oxidant production in neutrophil phagosomes. They calculated that superoxide is produced at a rate of ~312 mM/min and HOCl 134 mM per minute [10]. In this current study, the maximal concentration of H2O2 used was 5 mM and HOCl 0.07 mM. A recent report documented that bleaching of GFP expressed in SA is seen at concentrations of 0.05-0.1 mM HOCl which correlated well with killing of SA by this oxidant [26], suggesting that similar concentrations of HOCl were likely achieved in vivo. The mathematical model proposed by Winterbourn and colleagues predicts that such levels can be reached within seconds after activation of the NADPH oxidase [10]. Thus, we believe that the selected concentrations of H2O2 and HOCl in our studies are well within the scope of the achievable oxidant levels in neutrophils.
Precise mechanisms of oxidant-mediated bacterial killing are not fully defined. Early studies using EC as a model organism indicated a correlation between EC envelope permeabilization and bacterial inactivation by HOCl; however, only low-molecular weight compounds became freely permeable while the cell maintained its barrier function to proteins [27]. Albrich et al. (1986) tested the small-molecule permeability theory in EC by measuring the transport of H+ ion and glycerol and reported that the intercellular movements of these molecules were only marginally affected [28]. Their conclusion was that HOCI inactivation of bacteria does not occur by loss of membrane structural integrity, which contradicts the previous report. In the current study, we demonstrated that membrane integrity is affected by H2O2 and HOCl, but the effect is organism-specific (Figures 2 and 3). Statistically, permeability of BC and EC caused by H2O2 and HOCl did correlate with loss of viability while permeability of KP with only H2O2 exposure correlated with loss of viability. It is notable that permeability and CFU viability were statistically independent of each other for PsA and SA, the two most prevalent CF pathogens, in both H2O2 and HOCl exposures.
EC and PsA have been shown to recover from reduced adenylate energy charge, when subsequently supplied with nutrients which facilitate ATP hydrolase activity of the F1F0 complex of the bacterial ATP synthase [29]. After treatment with bactericidal doses of HOCl, however, adenylate energy charge is unrecoverable and further ATP production is abolished [17]. These findings suggest that a potent oxidant-induced killing mechanism may cause destruction of ATP production by specific oxidation of the F1F0 ATP synthase [30]. We measured the ATP concentration in bacterial suspensions after exposure to H2O2 and HOCl exposure and compared the degree of ATP reduction to the degree of loss of CFU viability at various oxidant concentrations (Figure 4). Of the organisms tested, all except PsA demonstrated significant decline in ATP production which correlated with loss of CFU viability; ATP production in PsA declined significantly up to 5 mM but did not correlated with decline in CFU viability. These data present evidence that H2O2 affects ATP production in bacteria suggesting that there are H2O2-sensitive sites in the bacterial ATP production machinery or that H2O2 assault disrupts pathways of energy production.
The profile of abolished ATP production with HOCl treatment was different from that of H2O2 in that HOCl-induced loss of ATP production correlated significantly with the loss of CFU viability in PsA, BC, and EC, while these two parameters were statistically independent in SA and KP (Figure 5). Interestingly, ATP production in KP was unaffected by HOCl concentrations up to 0.1 mM, a dose exceeding that required for complete eradication of the entire samples at the cellular densities used herein. Given the results obtained in SA and KP, it can be inferred that loss of CFU viability is not completely dependent on disruption of ATP production. In light of these results, further studies are required to elucidate the specific mechanisms of oxidant-induced bactericidal activity against different bacterial species.
Conclusions
We have demonstrated that the HOCl-resistance profile of microorganisms relates to its clinical pathogenicity in CF lung disease. Therefore, defective oxidant-mediated phagocytic host defense in CF may predispose the patient to chronic infections, especially those caused by PsA. Furthermore, oxidants affect bacterial membrane permeability and ATP energy production. But the effects are organism-specific, indicating that varied survival advantages exist among the bacteria when they are phagocytosed and encounter phagocyte-produced oxidants.
Authors' contributions
RWB performed experiments, data analyses and manuscript writing; RGP provided technical assistance and experimental design; EML contributed to statistical analysis; GW did experimental design, data interpretation and manuscript writing. All authors read and approved the final manuscript.
Contributor Information
Ryan W Bonvillain, Email: rbonvill@tulane.edu.
Richard G Painter, Email: rpaint@lsuhsc.edu.
Elisa M Ledet, Email: eledet@lsuhsc.edu.
Guoshun Wang, Email: gwang@lsuhsc.edu.
Acknowledgements
The work was supported by the grant from the National Institutes of Health to G. Wang (R01 AI72327).
References
- Collins FS. Cystic fibrosis: molecular biology and therapeutic implications. Science. 1992;256(5058):774–779. doi: 10.1126/science.1375392. [DOI] [PubMed] [Google Scholar]
- Welsh MJ, Ramsey BW, Accurso F, Cutting G. In: Metabolic and Molecular Basis of Interited Disease. 8. Scriver CR, editor. New York: McGraw-Hill; 2001. Cystic Fibrosis; pp. 5121–5188. [Google Scholar]
- Davis PB, Drumm M, Konstan MW. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154(5):1229–1256. doi: 10.1164/ajrccm.154.5.8912731. [DOI] [PubMed] [Google Scholar]
- Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2005;171(11):1209–1223. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundation CF. Cystic Fibrosis Foundation Patient Rigestry: 2009 Annual Data Report. http://www.cff.org/UploadedFiles/research/ClinicalResearch/Patient-Registry-Report-2009.pdf
- Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168(8):918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77(5):598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- Palazzolo-Ballance AM, Reniere ML, Braughton KR, Sturdevant DE, Otto M, Kreiswirth BN, Skaar EP, DeLeo FR. Neutrophil microbicides induce a pathogen survival response in community-associated methicillin-resistant Staphylococcus aureus. J Immunol. 2008;180(1):500–509. doi: 10.4049/jimmunol.180.1.500. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC, Hampton MB, Livesey JH, Kettle AJ. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J Biol Chem. 2006;281(52):39860–39869. doi: 10.1074/jbc.M605898200. [DOI] [PubMed] [Google Scholar]
- Hampton MB, Kettle AJ, Winterbourn CC. Involvement of superoxide and myeloperoxidase in oxygen-dependent killing of Staphylococcus aureus by neutrophils. Infect Immun. 1996;64(9):3512–3517. doi: 10.1128/iai.64.9.3512-3517.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter RG, Valentine VG, Lanson NA Jr, Leidal K, Zhang Q, Lombard G, Thompson C, Viswanathan A, Nauseef WM, Wang G. CFTR Expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry. 2006;45(34):10260–10269. doi: 10.1021/bi060490t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter RG, Bonvillain RW, Valentine VG, Lombard GA, LaPlace SG, Nauseef WM, Wang G. The role of chloride anion and CFTR in killing of Pseudomonas aeruginosa by normal and CF neutrophils. J Leukoc Biol. 2008;83(6):1345–1353. doi: 10.1189/jlb.0907658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter RG, Marrero L, Lombard GA, Valentine VG, Nauseef WM, Wang G. CFTR-mediated halide transport in phagosomes of human neutrophils. J Leukoc Biol. 2010;87:933–942. doi: 10.1189/jlb.1009655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA. Manual of Clinical Microbiology. 9. Vol. 1. Washington, DC: ASM Press; 2007. [Google Scholar]
- McKenna SM, Davies KJ. The inhibition of bacterial growth by hypochlorous acid. Possible role in the bactericidal activity of phagocytes. Biochem J. 1988;254(3):685–692. doi: 10.1042/bj2540685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrette WC Jr, Hannum DM, Wheeler WD, Hurst JK. General mechanism for the bacterial toxicity of hypochlorous acid: abolition of ATP production. Biochemistry. 1989;28(23):9172–9178. doi: 10.1021/bi00449a032. [DOI] [PubMed] [Google Scholar]
- Burns JL, Gibson RL, McNamara S, Yim D, Emerson J, Rosenfeld M, Hiatt P, McCoy K, Castile R, Smith AL, Ramsey BW. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J Infect Dis. 2001;183(3):444–452. doi: 10.1086/318075. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M, Gibson RL, McNamara S, Emerson J, Burns JL, Castile R, Hiatt P, McCoy K, Wilson CB, Inglis A, Smith A, Martin TR, Ramsey BW. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr Pulmonol. 2001;32(5):356–366. doi: 10.1002/ppul.1144. [DOI] [PubMed] [Google Scholar]
- Muhlebach MS, Stewart PW, Leigh MW, Noah TL. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am J Respir Crit Care Med. 1999;160(1):186–191. doi: 10.1164/ajrccm.160.1.9808096. [DOI] [PubMed] [Google Scholar]
- Armstrong DS, Grimwood K, Carlin JB, Carzino R, Olinsky A, Phelan PD. Bronchoalveolar lavage or oropharyngeal cultures to identify lower respiratory pathogens in infants with cystic fibrosis. Pediatr Pulmonol. 1996;21(5):267–275. doi: 10.1002/(SICI)1099-0496(199605)21:5<267::AID-PPUL1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- Burns JL, Emerson J, Stapp JR, Yim DL, Krzewinski J, Louden L, Ramsey BW, Clausen CR. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis. 1998;27(1):158–163. doi: 10.1086/514631. [DOI] [PubMed] [Google Scholar]
- Kahl BC, Duebbers A, Lubritz G, Haeberle J, Koch HG, Ritzerfeld B, Reilly M, Harms E, Proctor RA, Herrmann M, Peters G. Population dynamics of persistent Staphylococcus aureus isolated from the airways of cystic fibrosis patients during a 6-year prospective study. J Clin Microbiol. 2003;41(9):4424–4427. doi: 10.1128/JCM.41.9.4424-4427.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathy-Hartert M, Deby-Dupont G, Melin P, Lamy M, Deby C. Bactericidal activity against Pseudomonas aeruginosa is acquired by cultured human monocyte-derived macrophages after uptake of myeloperoxidase. Experientia. 1996;52(2):167–174. doi: 10.1007/BF01923364. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Leidal KG, Femling JK, Weiss JP, Nauseef WM. Neutrophil bleaching of GFP-expressing staphylococci: probing the intraphagosomal fate of individual bacteria. J Immunol. 2009;183(4):2632–2641. doi: 10.4049/jimmunol.0804110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sips HJ, Hamers MN. Mechanism of the bactericidal action of myeloperoxidase: increased permeability of the Escherichia coli cell envelope. Infect Immun. 1981;31(1):11–16. doi: 10.1128/iai.31.1.11-16.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrich JM, Gilbaugh JH, Callahan KB, Hurst JK. Effects of the putative neutrophil-generated toxin, hypochlorous acid, on membrane permeability and transport systems of Escherichia coli. J Clin Invest. 1986;78(1):177–184. doi: 10.1172/JCI112548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrette WC Jr, Hannum DM, Wheeler WD, Hurst JK. Viability and metabolic capability are maintained by Escherichia coli, Pseudomonas aeruginosa, and Streptococcus lactis at very low adenylate energy charge. J Bacteriol. 1988;170(8):3655–3659. doi: 10.1128/jb.170.8.3655-3659.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum DM, Barrette WC Jr, Hurst JK. Subunit sites of oxidative inactivation of Escherichia coli F1-ATPase by HOCl. Biochem Biophys Res Commun. 1995;212(3):868–874. doi: 10.1006/bbrc.1995.2049. [DOI] [PubMed] [Google Scholar]