Abstract
Stimulation of endothelial cells by various inflammatory mediators leads to release of Weibel–Palade bodies and therefore to exocytosis of both P-selectin (adhesion receptor for leukocytes) and von Willebrand factor (vWf) (platelet ligand). The potential role of vWf in leukocyte recruitment was investigated with the use of vWf-deficient mice. We report a strong reduction of leukocyte rolling in venules of vWf-deficient mice. Similarly, vWf deficiency led to a decrease in neutrophil recruitment in a cytokine-induced meningitis model as well as in early skin wounds. In all instances with an antibody that preferentially recognizes plasma membrane P-selectin, we observed a dramatic reduction in P-selectin expression at the cell surface of vWf-deficient endothelium. With confocal microscopy, we found that the typical rodlike shape of the Weibel–Palade body is missing in vWf −/− endothelial cells and that part of the P-selectin content in the vWf −/− cells colocalized with LAMP-1, a lysosomal marker. However, intracellular P-selectin levels were similar in tumor necrosis factor α- and lipopolysaccharide-activated cells of both genotypes. We conclude that the absence of vWf, as found in severe von Willebrand disease, leads to a defect in Weibel–Palade body formation. This defect results in decreased P-selectin translocation to the cell surface and reduced leukocyte recruitment in early phases of inflammation.
von Willebrand factor (vWf) is a multimeric glycoprotein (GP) that plays an essential role in primary hemostasis by mediating platelet adhesion to the injured vessel wall. vWf forms a bridge between platelet membrane glycoproteins GPIbα and GPIIb/IIIa and some exposed components of the subendothelium (1). vWf is synthesized by only two cell types, endothelial cells and megakaryocytes. Whereas megakaryocyte-derived vWf is found in the platelet α-granules, endothelial cell-derived vWf can either be secreted constitutively or targeted to specialized storage granules, the Weibel–Palade bodies (2).
Interestingly, the transmembrane protein P-selectin, which is involved in leukocyte rolling and extravasation (3), is stored in the same locations as vWf (4, 5). Therefore, the same stimuli (vascular injury or inflammatory mediators) that induce Weibel–Palade body release lead simultaneously to both vWf secretion and P-selectin translocation to the plasma membrane, which suggests the possibility of overlapping functions between these two proteins. Indeed, a role for P-selectin in hemostasis has already been described (6). Furthermore, it has recently been shown that P-selectin can interact with platelet GPIbα, an important receptor for vWf (7). Our laboratory has also shown that, depending on the hemodynamic conditions, platelets can interact in vivo with activated endothelium via either component of the Weibel–Palade bodies (8, 9). In view of these similarities between vWf and P-selectin, we decided to investigate a potential role for vWf in inflammatory responses with the use of mice deficient in vWf that represent a model of severe von Willebrand disease (10).
Materials and Methods
Animals.
All mice used in this study were on a mixed background C57BL/6J/129Sv, so that comparison with previously published inflammatory models with P-selectin (P −/−) mice (3) would be possible (11, 12). The experimental procedures were approved by the Animal Care and Use Committee of the Center for Blood Research.
Intravital Microscopy.
Male mice (14–19 g) were anesthetized with 2.5% tribromoethanol (0.15 ml/10 g). An incision was made through the abdominal wall to expose the mesentery, and mesenteric venules of 100- to 200-μm diameter were studied. The shear rate (95–100 s−1) was calculated with the use of an optical Doppler velocimeter and the venules were visualized as described (13). One venule per animal was filmed for 4 min before the A23187 superfusion (30 μl of a 10 μM solution) and for 8 min thereafter. For the study with histamine, mice were treated intraperitoneally with 200 μl of a 1 mM solution 15 min before the surgical procedure. Then four to six venules were sequentially observed for 4 min during the hour after the surgical procedure.
Video Analysis.
Rolling leukocytes were quantitated by counting the number of cells passing a given plane perpendicular to the vessel axis in 1 min. For A23187, this determination was made before and at different times after treatment, as indicated on the figure. For the histamine study, rolling after activation was determined by averaging several 1-min periods because no difference was found during the 4 min of observation of each venule. The velocity was determined by the time required for a leukocyte to travel a defined distance (μm) along the vessel. A minimum of 100 measurements were taken per min.
Immunohistochemistry.
Mouse mesentery was collected before and 15 min after histamine treatment, and brain tissue was collected 4 h after meningitis induction. Both tissues were fixed for 40 min in PBS containing 2% paraformaldehyde. The biopsies were embedded in OCT compound, frozen in a methanol/dry ice mixture, and stored at −80°C. Six-micrometer-thick cryostat sections were cut and transferred to poly-l-lysine-coated slides (Sigma). Endogenous peroxidase activity was quenched by treating tissue sections with 3% hydrogen peroxide in PBS for 10 min. P-selectin was detected with the use of a polyclonal antibody to human P-selectin (1:50 dilution) that cross-reacts with the mouse protein (a kind gift of M. C. Berndt, Baker Medical Research Institute, Prahran Victoria, Australia). An immunohistological kit (Zymed) was used for revelation. Negative controls were obtained by omission of the primary antibody.
Cytokine-Induced Meningitis and Blood–Brain Barrier (BBB) Permeability.
Meningitis was induced as described (11). Four hours after induction of the meningitis with lumbar injection of human recombinant IL-1β (25 pg/g) and recombinant human tumor necrosis factor α (TNFα) (80 pg/g) (Genzyme) the cerebrospinal fluid (CSF) was collected by aspiration with a glass capillary pipette. One microliter of the CSF was fixed with 2% formaldehyde and used for quantification of the leukocyte counts with a hemocytometer. To assess BBB permeability, FITC-labeled BSA (50 μg/g) was injected i.v. 15 min after cytokine inoculation. Both CSF and plasma samples, prepared from centrifugation of the blood at 2,500 × g for 15 min, were diluted in PBS, and the fluorescence intensity was measured with a fluorometer (Perkin–Elmer) with an excitation wavelength of 497 nm and an emission wavelength of 519 nm. The BBB permeability index was expressed as the ratio of the fluorescence intensity of the CSF sample divided by the fluorescence intensity of the plasma (11, 14).
Wound Healing Experiment.
Mice were anesthetized with methoxyflurane, and hair was removed with electric clippers. Full-thickness skin excisional wounds were made as described (12). One hour after wounding, the mice were killed and wounds were harvested with 1–2 mm of normal skin around them. The wounds were cut in half, fixed in 4% paraformaldehyde, and embedded in paraffin. Sections (7 μm thick) were stained with hematoxylin and eosin. Extravascular neutrophils were counted in the entire section with the use of a light microscope (Olympus BX40 at ×50 magnification) by an investigator without knowledge of the genotype.
Mouse Lung Endothelial Cell Culture.
For each preparation, lung tissues were collected from three vWf +/+ or vWf −/− mice as described (15). The digested tissue was filtered through a 100-μm nylon mesh and centrifuged at 150 × g at 4°C for 5 min. Pelleted cells were resuspended in 9 ml PBS Ca2+/Mg2+. A negative selection was performed as recommended by the manufacturer to remove most of the fibroblasts; Dynabeads coated with sheep anti-rat IgG (Dynal, Great Neck, NY) were incubated with a rat anti-mouse FcγRIII/II (PharMingen) at 4°C overnight, washed three times in PBS/2% FBS, resuspended in medium (Ham's F-12/DME-low glucose supplemented with 20% heat-inactivated FBS/1% penicillin/streptomycin/2 mM l-glutamine/50 μg/ml endothelial mitogen/100 μg/ml heparin), and then added to the cell suspension. After 45 min of incubation at 4°C, cells were placed in a magnetic field. Cells remaining in suspension were collected, centrifuged, washed, resuspended in medium, and plated on coverslips coated with 1% gelatin. In some experiments, 3-day-cultured endothelial cells were treated for 4 h with 0.5 μg/ml murine recombinant TNFα (Genzyme).
Laser Scanning Confocal Microscopy.
Primary lung endothelial cells grown on microscope slides were washed twice in PBS, fixed with 3.7% formaldehyde, washed, permeabilized with 0.5% Triton X-100, washed again, and blocked with normal goat serum at room temperature for 20 min. After being washed, the cells were sequentially treated for 30 min at 37°C with a 1:40 dilution rabbit anti-human P-selectin antibody (5), rhodamine goat anti-rabbit antibody (1:500 dilution; Cappel), sheep anti-rat vWf (1:200 dilution; Cedarlane Laboratories), and a FITC-conjugated donkey anti-sheep antibody (1:1,000 dilution; Jackson ImmunoResearch). Cells were washed three times between antibodies. For the P-selectin/LAMP-1 double staining, cells were washed twice, fixed in PBS containing 3.7% paraformaldehyde and 30 mM sucrose for 15 min at 4°C, and incubated for 15 min at room temperature in 50 mM NH4Cl/PBS. Cells were sequentially incubated for 45 min at 4°C in permeabilizing buffer (PBS/BSA containing 0.05% saponin) with rabbit anti-human P-selectin antibody (1:40 dilution) (5), rhodamine goat anti-rabbit antibody (1:500 dilution; Cappel), and FITC-conjugated rat anti-mouse lysosome-associated protein LAMP-1 (1:15; PharMingen). After washing in PBS/BSA containing 0.05% saponin, coverslips were mounted in GVA solution (Zymed) and examined under a radiancem 2000-multiphoton confocal microscope (Bio-Rad).
ELISA for Soluble P-Selectin.
Mice were anesthetized, and blood was obtained by retro-orbital venous plexus sampling in polypropylene Eppendorf tubes containing 0.1 volume of 38 mM citric acid, 75 mM trisodium citrate, and 100 mM dextrose. Plasma was prepared by centrifugation of the blood at 2,500 × g for 15 min at room temperature. ELISA was done as described (15).
Western Blot Analysis of Homogenates from Whole Lung.
Mice were injected i.p. with lipopolysaccharide (LPS) (20 μg/g body weight). After 4 h, lungs were collected, sliced into small pieces, and homogenized in 2% Triton X-100 in PBS supplemented with 2 mM phenylmethylsulfonyl fluoride. Samples were centrifuged at 3,400 rpm for 15 min, and the pellet was discarded. A protein assay (Bio-Rad) was carried out to determine the protein concentration in the supernatant, and 100 μg of protein was loaded on a SDS/7.5% PAGE gel under reducing conditions. The gel was transferred to poly(vinylidene difluoride) membrane (Millipore). The blot was blocked overnight at 4°C in 5% nonfat dry milk in Tris-buffered saline (TBS) containing 0.2% Tween-20 (TBS-T). The primary reagent, a polyclonal antibody to human P-selectin that recognizes mouse P-selectin (PharMingen), was added at a dilution of 1:1,000 in TBS-T for 2 h at room temperature. The blot was washed three times for 20 min in TBS-T and incubated for 45 min with the secondary antibody, a goat anti-rabbit antibody coupled to horseradish peroxidase (Zymed) diluted by 1:10,000 in TBS-T. After three washes, the blot was covered for 1 min with enhanced chemiluminescence detection reagents (Amersham Pharmacia) and exposed for 1 h with Hyperfilm (Amersham Pharmacia).
Statistical Analysis.
Data are presented as means ± SEM. Statistical significance was calculated with Student's t test for all analyses.
Results
Effect of vWf Deficiency on Leukocyte Rolling in Stimulated Venules.
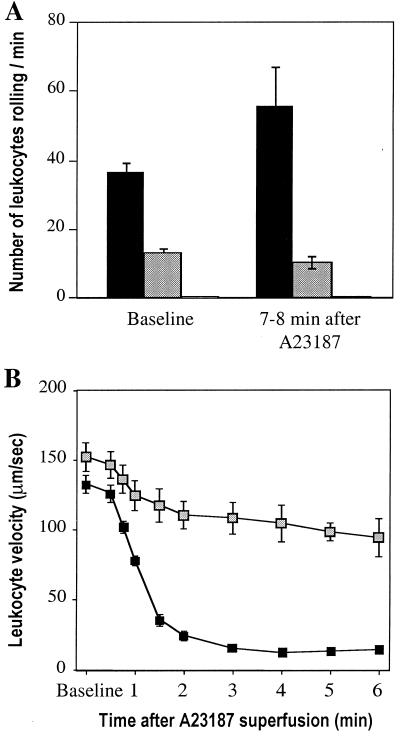
We compared leukocyte rolling by intravital microscopy of mesenteric venules (100–200 μm in diameter) in vWf +/+, vWf −/−, and P −/− mice. As described, rolling in P −/− mice was absent (3, 16). Surprisingly, baseline rolling was also reduced by almost 70% in vWf −/− mice compared with vWf +/+ mice (Fig. 1A). To determine whether leukocyte rolling could be induced in the vWf −/− mice by Weibel–Palade secretagogues (17), we added 10 μM calcium ionophore A23187 to the exposed mesentery. In vWf +/+ mice, A23187 treatment led to a marked decrease in leukocyte rolling velocity (Fig. 1B) as a result of the increased concentration of P-selectin at the cell surface. As a consequence of the decrease in velocity, there was only a slight increase in the number of rolling leukocytes per minute, but this increase was significant (P < 0.05) (Fig. 1A). In P −/− mice, A23187 did not promote any leukocyte rolling (3) (Fig. 1A). In vWf −/− mice, A23187 treatment did not affect the number of rolling leukocytes, which remained severalfold below the vWf +/+ levels (Fig. 1A). In addition, the leukocyte velocity was only slightly reduced, suggesting that P-selectin translocation to the cell surface was significantly impaired.
Figure 1.
Characteristics of leukocyte rolling in vWf −/− venules stimulated by A23187. (A) The number of leukocytes rolling per min was observed by intravital microscopy in mesenteric venules of vWf +/+ (black bars), vWf −/− (gray bars), or P-selectin-deficient (white bars, too small to see) mice before and after superfusion with the calcium ionophore A23187 (n ≥ 6). There were fewer leukocytes rolling in the absence of vWf both before (P < 0.0001) and after (P < 0.0025) A23187. In P-selectin-deficient mice, rolling was undetectable. (B) Leukocyte velocity was measured in mesenteric veins of vWf +/+ (■) or vWf −/− (░⃞) mice before and after A23187 treatment. Leukocytes roll faster in vWf −/− mice after A23187 treatment (P < 0.0001 for all time points).
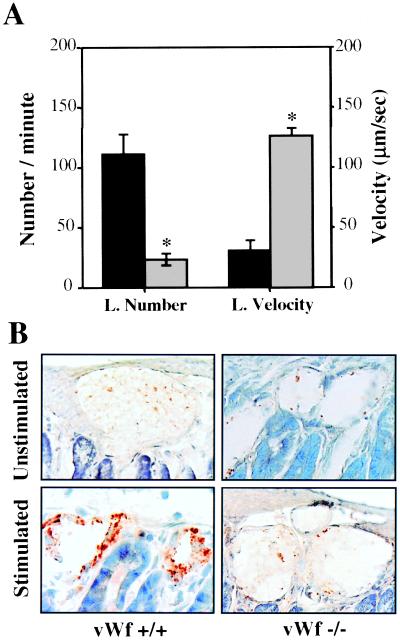
To determine whether leukocyte rolling was also impaired in inflamed venules we injected 1 mM histamine, another secretagogue for Weibel–Palade bodies (18), intraperitoneally into vWf +/+, vWf −/−, and P −/− mice and observed leukocyte-vessel wall interactions 15 min later through intravital microscopy. As expected, there was no rolling in P −/− mice (not shown). In vWf −/− mice, the number of rolling leukocytes was only 20% of that of vWf +/+ mice, and again the rolling velocity was much higher in vWf −/− mice compared with vWf +/+ (Fig. 2A). We examined P-selectin expression in the mesenteric venules treated with histamine, with the use of an antibody recognizing preferentially plasma membrane P-selectin. Histamine treatment induces strong P-selectin surface expression in vWf +/+ mice, whereas resting venules stain poorly for P-selectin (Fig. 2B). In contrast, almost no surface staining for P-selectin was observed in vWf −/− venules before or after histamine treatment (Fig. 2B).
Figure 2.
Effect of histamine on leukocyte rolling and P-selectin expression in vWf-deficient mice. (A) Mice were injected intraperitoneally with histamine, and 15 min later mesenteric venules were observed through intravital microscopy. Numbers of rolling leukocytes (L. Number) and their velocity (L. Velocity) were measured in vWf +/+ (black bars) and vWf −/− (gray bars) mice (n = 7). *, P < 0.0001 compared with vWf +/+. (B) Mouse mesentery from wild-type (vWf +/+) or vWf-deficient (vWf −/−) mice were collected before (unstimulated) and 15 min after (stimulated) treatment, fixed, sectioned, and stained for P-selectin. No staining can be seen in the resting mesenteries of either genotype. Staining becomes bright brown in vWf +/+ but not in vWf −/− mesentery after histamine treatment.
Cytokine-Induced Meningitis.
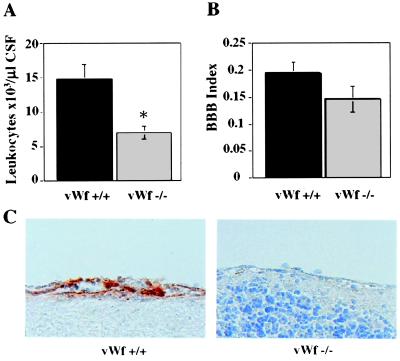
To determine whether the diminution in leukocyte rolling in vWf −/− mice would result in a reduced inflammatory reaction in a complex pathology, we tested a model of meningitis induced by lumbar injection of cytokines (11). Leukocyte recruitment was measured in the CSF 4 h after cytokine inoculation. In vWf −/− mice, a significant reduction (50%) of leukocyte recruitment in the CSF was observed (Fig. 3A), which is similar to the phenotype observed in the P −/− mice (11). Immunohistochemical staining of the brain after meningitis to visualize released P-selectin showed strong P-selectin reactivity in vWf +/+ mice but almost no staining in vWf −/− mice (Fig. 3C). The same model allowed us to calculate an index of BBB permeability by measuring the CSF traversal of systemically injected FITC-conjugated BSA (11). The extent of BSA penetration into the CSF was not significantly different between vWf +/+ and vWf −/− mice (Fig. 3B), a result distinctly different from that obtained with P −/− mice (11), indicating that in vWf −/− mice P-selectin still plays some role.
Figure 3.
Cytokine-induced meningitis in vWf −/− mice. vWf +/+ (black bars) and vWf −/− (gray bars) mice were inoculated with cytokines via lumbar puncture and with FITC-BSA intravenously 15 min later. Four hours later, cerebrospinal fluid was collected (n ≥ 10). (A) Total leukocyte counts in the CSF were determined. VWf −/− mice had significantly fewer CSF leukocytes. (*, P = 0.005.) (B) Fluorescence in the CSF and the plasma was measured and used to calculate a BBB permeability index. No significant differences were found between vWf +/+ and vWf −/−. (P = 0.12.) (C) Brain tissue was collected and fixed 4 h after meningitis induction. Sections were stained with an antibody to P-selectin. Very strong positive staining was seen on the endothelium of vWf +/+ brains. Very faint staining was present in vWf −/− brain endothelium.
Neutrophil Recruitment in Skin Excisional Wounds.
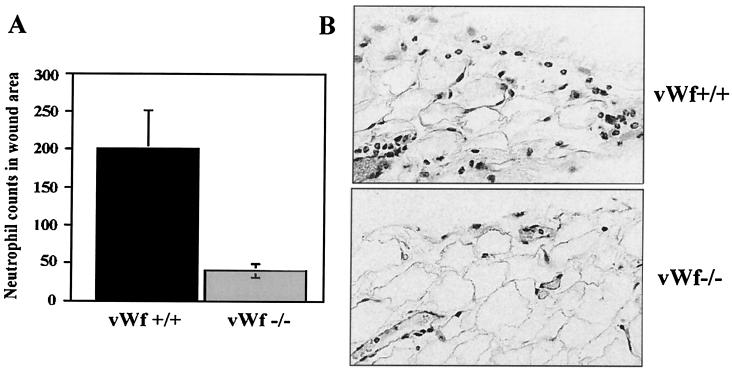
We next evaluated the role of vWf in the initial inflammatory phase of wound healing in a full thickness skin excisional wound model (12). The number of neutrophils that had migrated outside the blood vessels in the tissues 1 h after wounding were counted microscopically in hematoxylin and eosin-stained sections. In the vWf −/− mice, significantly less neutrophil infiltration was present in the tissue sections of 1-h wounds compared with vWf +/+ mice (40 ± 8 vs. 200 ± 50, respectively, P = 0.02) (Fig. 4). As in P −/− mice (12), the time needed for wound closure in vWf −/− mice was as in vWf +/+ (not shown).
Figure 4.
Effect of vWf deficiency on early neutrophil recruitment in skin excisional wounds. (A) The number of neutrophils in the wound area was counted in hematoxylin and eosin-stained sections of vWf +/+ (black bars) or vWf −/− (gray bars) mice. A strong reduction in neutrophil recruitment was observed in vWf −/− mice 1 h after wounding (n = 5–7; P = 0.025). (B) Histology sections from the same experiment. Numerous neutrophils emigrated in the tissue outside the blood vessels in the vWf +/+ mice, but very few were seen in the vWf −/− sections.
Localization and Biosynthesis of P-Selectin in Endothelial Cells.
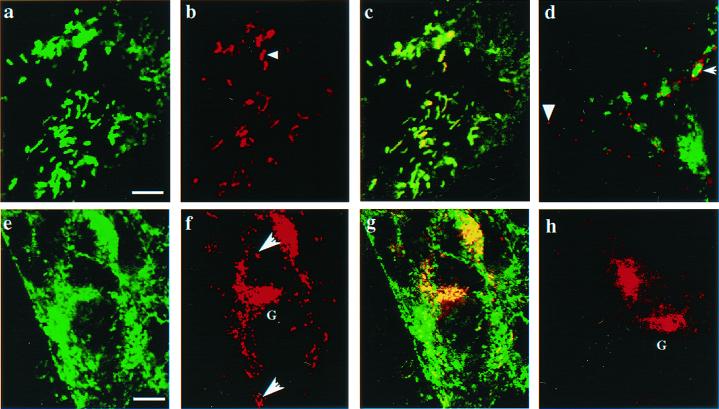
To determine the intracellular localization of P-selectin in endothelial cells of vWf +/+ and vWf −/− mice, lung microvascular endothelial cells were isolated and cultured in vitro. In nonactivated vWf +/+ endothelial cells, vWf and P-selectin colocalized in rodlike organelles characteristic of the Weibel–Palade bodies (Fig. 5 a–c). In contrast, in vWf −/− endothelial cells, P-selectin was found in small round organelles, some of which contained the lysosomal protein LAMP-1 (Fig. 5d). The difference in the P-selectin localization in vWf +/+ and vWf −/− cells was emphasized upon stimulation with TNFα. Indeed, typical surface patches of Weibel–Palade body release were observed in vWf +/+ cells (Fig. 5f), whereas a weak diffuse staining was found in vWf −/− cells. Interestingly, in cells of both genotypes, P-selectin showed strong perinuclear staining characteristic of the Golgi complex (Fig. 5 f and h). This staining was not seen in untreated cells (Fig. 5 b and d), suggesting that the biosynthesis of P-selectin was similarly increased by TNFα (19) in both vWf +/+ and vWf −/− endothelial cells.
Figure 5.
Intracellular localization of P-selectin in cultured mouse lung endothelial cells. (Upper) Nonactivated endothelial cells. A vWf +/+ cell (a–c) was stained for vWf (green) and P-selectin (red). Overlapping distribution of the two antigens is shown in yellow (c). Note the typical rod-shaped Weibel–Palade bodies (arrowhead). (d) A vWf −/− endothelial cell stained for P-selectin (red) and LAMP-1 (green). In a vWf −/− cell, small round organelles were stained for P-selectin (arrowhead). Some of these colocalized with green lysosomes (white arrow). (Lower) Endothelial cells treated for 4 h with TNFα. (e–g) vWf +/+ endothelial cells stained for vWf (e), P-selectin (f), and both (g). (h) vWf −/− endothelial cells were double-stained for both vWf and P-selectin. Similarly, intense staining for newly synthesized P-selectin is seen in the Golgi complex (G) of vWf +/+ (f) and vWf −/− (h) cells. Typical patches of Weibel–Palade body release (white arrow) are found in vWf +/+ cells (f), whereas only a weak surface staining for P-selectin is observed in vWf −/− cells (h). [Bar = 3 μm (a–d) or 12 μm (e–h).]
Because P-selectin expression at the surface of activated vessels was impaired in vWf −/− mice, despite what appeared to be normal de novo synthesis upon TNFα stimulation, we decided to examine protein levels of P-selectin more rigorously. This examination of protein levels was made by Western blot analysis of lung homogenates of LPS-treated vWf +/+ and vWf −/− mice. No significant differences in the protein levels were found (Fig. 6). In addition, similar amounts of P-selectin mRNA were present in lungs of vWf +/+ and vWf −/− mice (not shown). This finding indicates that the biosynthesis of P-selectin was not affected by the absence of vWf.
Figure 6.
Western blot analysis for P-selectin in whole lung homogenates. Wild-type (vWf +/+) and vWf-deficient (vWf −/−) mice were injected intraperitoneally with LPS, and 4 h later lungs were collected and homogenized. One hundred micrograms of protein was loaded onto each lane of a 5% polyacrylamide gel. The blot was probed with a polyclonal antibody against P-selectin. Numbers indicate the migration of molecular mass markers (in kDa).
To determine whether the cleaved form of P-selectin (20) was constitutively released into plasma in the absence of vWf, we measured the amount of soluble P-selectin in vWf +/+ and vWf −/− plasma. The plasma of mice expressing P-selectin without the cytoplasmic tail (ΔCT mice), which have increased levels of soluble P-selectin (15), was used as a positive control. No increase in soluble P-selectin was detected in the plasma of vWf −/− mice compared with vWf +/+ mice (OD at 1:20 dilution was 0.077 ± 0.02 for vWf +/+ and 0.082 ± 0.02 for vWf −/−; P = 0.8) showing that, in the absence of vWf, P-selectin is not constitutively transported to the plasma membrane and shed in blood.
Platelet P-Selectin.
In contrast to the endothelial cells, platelet storage of P-selectin was not affected by vWf deficiency. Alpha-granules of vWf −/− platelets still contained P-selectin, which redistributed normally to the plasma membrane after treatment with calcium ionophore A23187, as determined by FACS analysis (not shown). Thus vWf is not required for platelet P-selectin storage and surface expression.
Discussion
In endothelial cells, vWf and P-selectin are located in the same storage granules, the Weibel–Palade bodies (21). Until recently, this common localization was believed to be the only similarity shared by these two proteins. Indeed, the role of P-selectin in leukocyte rolling and that of vWf in platelet adhesion to the subendothelium did not seem closely related. An indication of overlapping functions between vWf and P-selectin came from a study by Subramaniam et al., which showed that mice deficient in P-selectin exhibited a prolonged bleeding time and a defective hemostasis in a local Shwartzman reaction (6). Soluble P-selectin was documented to promote fibrin deposition in this model (22). In the present study, we further demonstrate that the roles of vWf and P-selectin can be intricately linked because mice deficient in vWf present a strong reduction in leukocyte recruitment in several different inflammatory models. These findings emphasize the importance of Weibel–Palade bodies at the interface between the inflammatory and the hemostatic response. In addition to their role in exposing P-selectin to the cell surface after endothelial cell activation, Weibel–Palade bodies may play a role in inflammation through IL-8, which has been reported as being stored in these granules in microvascular endothelial cells (23, 24). Furthermore, in another inflammatory setting, atherosclerosis, Weibel–Palade bodies were found in great numbers at sites of lesions (25). We can therefore imagine that interfering with the behavior of Weibel–Palade bodies is likely to lead to defects in inflammation. With the deletion of the gene for vWf, it appears that the normal biology of the Weibel–Palade bodies was disrupted. The physical structure of these organelles, with the observed longitudinal tubules, seems to be due to the multimeric vWf molecule itself (21). Indeed, transfection of heterologous cells with pro-vWf cDNA leads to formation of vWf-containing organelles that are morphologically similar to Weibel–Palade bodies and different from the endogenous granules of the transfected cells (26, 27). This result indicates that vWf by itself is capable of directing the formation of its own storage granules. In contrast, transfection of P-selectin cDNA leads to its storage in the endogenous granules of the transfected cell lines (28, 29). Even though this result indicates that P-selectin contains an independent sorting signal now identified in the cytoplasmic tail (30), it also indicates that P-selectin needs some pre-existing storage granules to be directed to, as recently confirmed in transfected cells (31). Immunolocalization of P-selectin with the use of confocal microscopy showed that in resting vWf −/− endothelial cells, P-selectin is found in a reduced number of small vesicles, compared with vWf +/+ cells (Fig. 5 b and d). Activation of the cells with TNFα revealed the presence of intense patches of P-selectin released from Weibel–Palade bodies in vWf +/+ cells and only a low diffuse staining in vWf −/− cells (Fig. 5 f and h). On the other hand, we showed that the amount of P-selectin synthesized after TNFα or LPS stimulation appeared normal in the vWf-deficient mice. Therefore, our results suggest that it is the storage and regulated secretion of P-selectin to the cell surface that are affected in vWf −/− cells. The newly synthesized P-selectin is either missorted to the lysosomal compartment, where it could be degraded, or remains in small, as yet unidentified, vesicles (Fig. 5 d and h) that undergo inefficient secretion.
In two models of inflammation, cytokine-induced meningitis and wound healing, the defect observed in vWf-deficient mice closely resembles the results obtained in P-selectin-deficient mice (11, 12). However, the phenotype of vWf-deficient mice is not totally identical to that of P-selectin-deficient mice. Although much reduced, rolling of leukocytes in the mesentery is not completely absent, as is the case in P-selectin-deficient mice (3). Furthermore, in a long-term inflammatory model such as atherosclerosis, preliminary results show P-selectin staining on the endothelium surface of the aortic sinus of low-density lipoprotein receptor and vWf double-deficient mice after 4 weeks on an atherogenic diet (N. Methia and D.D.W., unpublished observations). Inflammatory mediators inducing a sustained up-regulation of P-selectin synthesis might therefore be the key in expressing enough P-selectin at the cell surface in the absence of Weibel–Palade bodies.
In conclusion, we have shown that vWf plays an important role in the biology of the endothelium, inasmuch as its absence leads to a defect in P-selectin plasma membrane expression, which results in impaired early inflammatory responses. This finding suggests that patients suffering from severe von Willebrand disease, in addition to their hemostatic problems, may be more susceptible to infections.
Acknowledgments
We thank Drs. Tanya Mayadas and Angela Coxon for teaching us the meningitis procedure and Dr. Ann Dvorak for helpful discussions. We also thank Jessie Papalia for mouse husbandry and Lesley Cowan for help with preparation of the manuscript. This work was supported by National Institutes of Health Grant R37 HL41002 to D.D.W.
Abbreviations
- vWf
von Willebrand factor
- GP
glycoprotein
- CSF
cerebrospinal fluid
- BBB
blood–brain barrier
- TNFα
tumor necrosis factor α
- LPS
lipopolysaccharide
- TBS
Tris-buffered saline
- TBST
TBS containing 0.2% Tween-20
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ruggeri Z M. Thromb Haemostasis. 1999;82:576–584. [PubMed] [Google Scholar]
- 2.Wagner D D. Annu Rev Cell Biol. 1990;6:217–246. doi: 10.1146/annurev.cb.06.110190.001245. [DOI] [PubMed] [Google Scholar]
- 3.Mayadas T N, Johnson R C, Rayburn H, Hynes R O, Wagner D D. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 4.Stenberg P E, McEver R P, Shuman M A, Jacques Y V, Bainton D F. J Cell Biol. 1985;101:880–886. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonfanti R, Furie B C, Furie B, Wagner DD. Blood. 1989;73:1109–1112. [PubMed] [Google Scholar]
- 6.Subramaniam M, Frenette P S, Saffaripour S, Johnson R C, Hynes R O, Wagner D D. Blood. 1996;87:1238–1242. [PubMed] [Google Scholar]
- 7.Romo G M, Dong J F, Schade A J, Gardiner E E, Kansas G S, Li C Q, McIntire L V, Berndt M C, Lopez J A. J Exp Med. 1999;190:803–814. doi: 10.1084/jem.190.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frenette P S, Johnson R C, Hynes R O, Wagner D D. Proc Natl Acad Sci USA. 1995;92:7450–7454. doi: 10.1073/pnas.92.16.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.André P, Denis C V, Ware J, Saffaripour S, Hynes R O, Ruggeri Z M, Wagner D D. Blood. 2000;96:3322–3328. [PubMed] [Google Scholar]
- 10.Denis C, Methia N, Frenette P S, Rayburn H, Ullman-Cullere M, Hynes R O, Wagner D D. Proc Natl Acad Sci USA. 1998;95:9524–9529. doi: 10.1073/pnas.95.16.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang T, Frenette P S, Hynes R O, Wagner D D, Mayadas T N. J Clin Invest. 1996;97:2485–2490. doi: 10.1172/JCI118695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subramaniam M, Saffaripour S, Van De Water L, Frenette P S, Mayadas T N, Hynes R O, Wagner D D. Am J Pathol. 1997;150:1701–1709. [PMC free article] [PubMed] [Google Scholar]
- 13.Frenette P S, Moyna C, Hartwell D W, Lowe J B, Hynes R O, Wagner D D. Blood. 1998;91:1318–1324. [PubMed] [Google Scholar]
- 14.Kurose I, Yamada T, Wolf R, Granger D N. J Leukocyte Biol. 1994;55:771–777. doi: 10.1002/jlb.55.6.771. [DOI] [PubMed] [Google Scholar]
- 15.Hartwell D W, Mayadas T N, Berger G, Frenette P S, Rayburn H, Hynes R O, Wagner D D. J Cell Biol. 1998;143:1129–1141. doi: 10.1083/jcb.143.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ley K. J Reconstr Microsurg. 1992;8:495–503. doi: 10.1055/s-2007-1006736. [DOI] [PubMed] [Google Scholar]
- 17.Sporn L A, Marder V J, Wagner D D. Cell. 1986;46:185–190. doi: 10.1016/0092-8674(86)90735-x. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton K K, Sims P J. J Clin Invest. 1987;79:600–608. doi: 10.1172/JCI112853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weller A, Isenmann S, Vestweber D. J Biol Chem. 1992;267:15176–15183. [PubMed] [Google Scholar]
- 20.Berger G, Hartwell D W, Wagner D D. Blood. 1998;92:4446–4452. [PubMed] [Google Scholar]
- 21.Wagner D D. Thromb Haemostasis. 1993;70:105–110. [PubMed] [Google Scholar]
- 22.André P, Hartwell D, Hrachovinová I, Saffaripour S, Wagner D D. Proc Natl Acad Sci USA. 2000;97:13835–13840. doi: 10.1073/pnas.250475997. . (First Published November 28, 2000; 10.1073/pnas.250475997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Utgaard J O, Jahnsen F L, Bakka A, Brandtzaeg P, Haraldsen G. J Exp Med. 1998;188:1751–1756. doi: 10.1084/jem.188.9.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolff B, Burns A R, Middleton J, Rot A. J Exp Med. 1998;188:1757–1762. doi: 10.1084/jem.188.9.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trillo A A, Prichard R W. Lab Invest. 1979;41:294–302. [PubMed] [Google Scholar]
- 26.Wagner D D, Saffaripour S, Bonfanti R, Sadler J E, Cramer E M, Chapman B, Mayadas T N. Cell. 1991;64:403–413. doi: 10.1016/0092-8674(91)90648-i. [DOI] [PubMed] [Google Scholar]
- 27.Voorberg J, Fontijn R, Calafat J, Janssen H, van Mourik J A, Pannekoek H. EMBO J. 1993;12:749–758. doi: 10.1002/j.1460-2075.1993.tb05709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koedam J A, Cramer E M, Briend E, Furie B, Furie B C, Wagner D D. J Cell Biol. 1992;116:617–625. doi: 10.1083/jcb.116.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleming J C, Berger G, Guichard J, Cramer E M, Wagner D D. Eur J Cell Biol. 1998;75:331–343. doi: 10.1016/s0171-9335(98)80066-6. [DOI] [PubMed] [Google Scholar]
- 30.Disdier M, Morrissey J H, Fugate R D, Bainton D F, McEver R P. Mol Biol Cell. 1992;3:309–321. doi: 10.1091/mbc.3.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hop C, Guilliatt A, Daly M, de Leeuw H P, Brinkman H J, Peake I R, van Mourik J A, Pannekoek H. Arterioscler Thromb Vasc Biol. 2000;20:1763–1768. doi: 10.1161/01.atv.20.7.1763. [DOI] [PubMed] [Google Scholar]