Abstract
Background
In insects, including Anopheles mosquitoes, Dscam (Down syndrome cell adhesion molecule) appears to be involved in phagocytosis of pathogens, and shows pathogen-specific splice-form expression between divergent pathogen (or parasite) types (e.g. between bacteria and Plasmodium or between Plasmodium berghei and Plasmodium falciparum). Here, data are presented from the first study of Dscam expression in response to genetic diversity within a parasite species.
Methods
In independent field and laboratory studies, a measure of Dscam splice-form diversity was compared between mosquitoes fed on blood that was free of P. falciparum to mosquitoes exposed to either single or mixed genotype infections of P. falciparum.
Results
Significant increases in Anopheles gambiae Dscam (AgDscam) receptor diversity were observed in parasite-exposed mosquitoes, but only weak evidence that AgDscam diversity rises further upon exposure to mixed genotype parasite infections was found. Finally, a cluster of AgDscam exon 4 variants that become especially common during Plasmodium invasion was identified.
Conclusions
While the data clearly indicate that AgDscam diversity increases with P. falciparum exposure, they do not suggest that AgDscam diversity rises further in response to increased parasite diversity.
Background
The innate immune system is common to both invertebrates and vertebrates, and although less well studied than the adaptive immune response, is probably responsible for eliminating the majority of infectious organisms. This is achieved through engulfing cells (e.g. phagocytes), antimicrobial compounds (e.g. defensins) and non-specific reactive intermediates such as nitric oxide [1-5]. The vertebrate adaptive immune system appears to have more complex features: functional antibodies assembled by V-(D)-J joining of gene segments and diversified by somatic hypermutation accommodate an unrivalled resolution in terms of pathogen recognition for the vertebrate adaptive immune response [6]. Invertebrates, lacking antibodies and having only an innate immune system, rely on germline encoded pattern recognition receptors (PRRs) to detect pattern associated molecular patterns and initiate a response [2,7-9].
The absence of an equivalent of the vertebrate adaptive immune system has long fostered doubts that the invertebrate immune system could incorporate specificity and/or memory [10-12]. However, the absence of the cellular and genetic components of a vertebrate-like anticipatory immune system does not preclude a functional equivalent in invertebrates [13], and there exists evidence of enhanced secondary responses to homologous infectious challenges [14-22]. Moreover, in invertebrates, the genetic background of both hosts and parasites plays a critical role in determining the probability of infection, a phenomenon called genetic specificity [13,23,24]. Thus invertebrate defences do not lack sophistication, but the genetic and cellular mechanisms that underlie either invertebrate genetic specificity or enhanced secondary responses remain obscure (but see [25]).
Alternative splicing could permit a single gene to mediate alternative immune responses via the production of multiple proteins, and the flexibility of such a mechanism could have important implications for the spread of resistance alleles [26]. The Down syndrome cell adhesion molecule (Dscam), which can take some tens of thousands of different forms through alternative splicing, is commonly associated with its function in the vertebrate and invertebrate nervous systems, but seems to also play a role in invertebrate immunity [27-30]. In the fruit fly Drosophila, Dscam is expressed in cell types that play major roles in the fly's immune system, and RNA interference-mediated depletion of Dscam was shown to impair the insect's capacity to engulf bacteria by phagocytosis [29]. Similarly, the silencing of Anopheles gambiae Dscam (AgDscam) compromises the mosquito's ability to resist Plasmodium [30]. Moreover, AgDscam produces pathogen-specific splice form repertoires upon immune challenge [30]. In particular, the Dscam repertoire in response to parasite exposure differs between bacteria and Plasmodium and between Plasmodium berghei and Plasmodium falciparum [30]. However, such specificity has so far only been observed in studies comparing these divergent Plasmodium parasites, which probably last shared a common ancestor around 55 million years ago [31].
The response of AgDscam transcription to P. falciparum diversity (i.e. within-species rather than between-species parasite exposure) may shed light on the resolution of the innate immune system's specificity and dynamics, as well as its limitations. In theory, 31,920 unique splice forms of Dscam can be generated through the alternative splicing of 84 variable exons contained within three variable exon cassettes (these are exon 4, exon 6, and exon 10) [30], and which could potentially contribute to a capability to distinguish between different genotypes of Plasmodium. This capability would imply a more specific innate immune response than previously supposed. Here, research is described that relates P. falciparum genetic diversity to the expression characteristics of the alternatively spliced Dscam receptor in the An. gambiae mosquito.
Two independent experiments were performed. The first was a field study that utilized freshly harvested blood from human subjects for which the genetic diversity of naturally acquired P. falciparum infections was characterized. The second experiment was based in the laboratory, where mosquitoes were exposed to either single parasite clones or mixtures of clones contained within human red blood cells in culture following an established protocol [32]. AgDscam receptor diversity was studied in two ways. First, a diversity index was calculated based on exon 4 and exon 6 frequencies to assess whether overall AgDscam expression diversity increased under exposure to P. falciparum parasites, and if it increased further with greater parasite infection diversity. Second, it was assessed whether particular Dscam exon transcripts were associated with infection diversities (in the field study) or particular parasite genotypes (in the laboratory study).
Methods
Mosquito infection in Kenya
Blood was obtained from primary school students in Iguhu (34°45'E, 0°10'N) in Kakamega district, western Kenya. The predominant malaria vector species in the area is An. gambiae s.s. [33,34]. During and shortly after the rainy season, children (5-14 years of age) were screened for gametocytes by thick blood-films stained in Giemsa's stain.
Gametocyte carriers who had >40 gametocytes/μL of blood and who consented to participate in the study were asked to donate 10 mL of blood, which was obtained intravenously by a clinician, and drawn into heparinized tubes. A total of six gametocyte donors were used in this study (two donors per gametocyte-positive infection group). Most of this blood was used for mosquito infections through membrane feeders, with around 50 μL also spotted onto Whatman paper for later DNA extraction using a Chelex-100 isolation technique [35]. Methods for infecting mosquitoes are described in [36]. Drawn blood was immediately centrifuged at 700 × g, and the serum discarded and replaced with human AB serum (Cambrex Bio Science, Walkersville, MD, USA). Blood was then placed in warmed membrane feeders. Five to seven-day old Anopheles gambiae Kisumu strain mosquitoes were placed in paper cups at a density of 60/cup and allowed to feed on the infected blood for 30 minutes. Mosquitoes used in this experiment were originally obtained near the Kenya Medical Research Institute in Kisumu [37], but had been bred in an insectary and adapted to feed from a membrane feeder for many years (thus the mosquitoes were unlikely to be highly polymorphic).
A total of twenty four mosquitoes were used in the field study (three mosquitoes per treatment × four infection groups × two independent replicates). Mosquitoes were transferred to cages post-blood meal, and then placed into RNAlater (Ambion) at 24-hours post-blood meal. Mosquitoes were harvested 24-hours post-feeding because this is the peak time that Plasmodium ookinetes penetrate the mosquito midgut [30]. Total RNA extraction was carried out using a Qiagen RNeasy Mini kit. On day 7 after they had been exposed to infected blood, the remaining fed mosquitoes from each cage were dissected in 2% mercurochrome and examined for oocysts to confirm the presence of Plasmodium infections.
Plasmodium microsatellite typing
To estimate P. falciparum diversity, microsatellite loci were chosen based on their strength in terms of percentage PCR positives, frequency distributions of allele length important for sizing amplicons [38], size in base-pairs [39], and allele frequencies [40]. A hemi-nested PCR reaction was carried out to amplify six select microsatellite loci from each DNA extraction sample [38]. Applied Biosystems Genemapper v4.0 software was used to automate the measurement of allele length and to quantify peaks in samples containing multiple alleles per locus. Only peaks from samples that amplified >200 fluorescent units were included in the deduction of infection diversity. Multiple alleles per locus were scored if the minor microsatellite peaks were >33% the height of the predominant allele.
Mosquito infection in the laboratory
Five to seven-day old female An. gambiae (Keele strain) mosquitoes were offered blood meals containing in vitro grown gametocytes of two different genotypes of P. falciparum (clone 3D7 [41] and clone HB3 [42]) through membrane feeders, and three whole mosquitoes per treatment were harvested after 24-hours. Three independent experiments on three different dates were performed. A total of thirty six mosquitoes were used in this study (three mosquitoes per treatment × four treatment groups × three independent replicates). The gametocyte culture and membrane feeding protocols followed those previously described [32]. The isolation of total RNA was carried out using a Qiagen RNeasy Mini technology kit.
Quantifying Dscam diversity
RNA extracted from three individual mosquitoes per treatment was reverse transcribed using random hexamers and primers were designed to amplify a fragment of AgDscam spanning from exon 3 to exon 7 (primer sequences were: Forward; 5' - GTATACGCCTGCATGGCTAAGA - 3', Reverse; 5' - GCCCTTATCCTCCTTCTTG - 3'). Thus, the amplicons comprised variable exons 4 and 6, and the conserved exon 5. PCR products were cloned using a TOPO TA cloning kit with pCR4-TOPO vector and transformed in chemically competent Escherichia coli. Up to 48 clones per mosquito were sequenced in a 96-capillary ABI 3730xl DNA Analyzer (provided by the Gene Pool Sequencing facility, University of Edinburgh). Sequences were aligned using BioEdit version 7.0.9.0 and identified by cross-referencing with the known An. gambiae genome sequence available from the Ensembl genome browser.
Statistical analyses
Diversity was measured with Simpson's Index (1-D) [43], which quantified the combination of expressed exon 4 and exon 6 variants in each transcript as determined by sequencing. The presence and abundance of each individual exon, and exon 4:6 combination was identified. Statistical analysis of data using general linear modelling and one-way analysis of variance was carried out using Minitab 15.1.1.0 software. For the laboratory study, 'clone' was the single fixed effect with three levels, 3D7, HB3 or mixture of both, and for the field study, the number of genotypes detected (single, double or triple infection) was a fixed effect and donor was added as a random effect to account for variation. Data were normalized with a square root transformation of Simpson's Index (D) before analyses. The Neighbour-Joining trees (additional file 1) were produced using PHYLIP and variation in counts of exon groupings were carried out with chi-square contingency table analysis.
Ethical statement
The ethical review boards of the Kenya Medical Research Institute, Kenya reviewed and approved our protocol for screening of P. falciparum gametocyte carriers and subsequent intravenous blood drawing. Written, informed consent to participate in the study was provided by all study subjects and/or their parents or guardians (see also [36]). All gametocyte donors were subsequently treated with amodiaquine by the clinicians at the Iguhu Health Centre, per the guideline of the Ministry of Health of Kenya. Feeding of mosquitoes was conducted in a secure, insect-proof room at the Iguhu Health Centre.
Results and Discussion
For the field study, An. gambiae were membrane-fed on blood samples taken from gametocyte-carrying children, and RNA was harvested from the insects 24-hours post-exposure, a time when parasites are traversing the midgut epithelium [30]. cDNAs were then cloned and sequenced to identify specific AgDscam gene variants (at exons 4 and 6) expressed within insects fed from different blood samples. Control blood was taken from children carrying no Plasmodium (as detected with microscopy). A set of P. falciparum microsatellite markers [38] were used to identify parasite genotypes in gametocyte carriers. As blood samples contain whole populations of parasites, it is not possible to precisely estimate the number of genotypes present. However, by simply counting alleles at each locus, it is possible to identify the minimum number of genotypes present in a sample, and thus the methods used yielded a lower-bound of parasite genetic diversity. Single, double and triple infections were subsequently identified.
AgDscam expression, as characterized by a diversity index [43] and averaged over both studied exons, was affected by the blood that the mosquitoes fed upon in the field (F3,20 = 3.22, P = 0.045; Figure 1). It appears that AgDscam is more diverse when parasites are present (diversity was significantly lower in uninfected blood controls than in all other samples; Figure 1). It is the combined diversity at exons 4 and 6 that drive this pattern, as a relationship between expression diversity and parasite diversity was not apparent when the exons were studied separately (exon 4: F1,22 = 3.33, P = 0.081; exon 6: F1,22 = 0.02, P = 0.901). It was interesting to note that there was a non-statistically significant trend for an association between exon 4 (but not exon 6) diversity and parasite diversity. This may imply that exon 4 has a bigger role than exon 6 in responding to Plasmodium in the field. Additionally, AgDscam diversity was also higher when mosquitoes fed upon blood with double infections when compared to single infections, but diversity did not rise further with triple infections (Figure 1). Thus, these field data provided only limited evidence of a link between host response recognition capacity and parasite intraspecific diversity. As the blood stage diversity as measured by microsatellites could not be certain to be representative of gametocyte diversity, i.e. it is conceivable that not all parasite types were producing gametocytes (see [44]), a laboratory-based study was carried out using gametocyte-producing lines of P. falciparum. In this way, the diversity of gametocytes entering the experimental mosquitoes could be certain.
Figure 1.
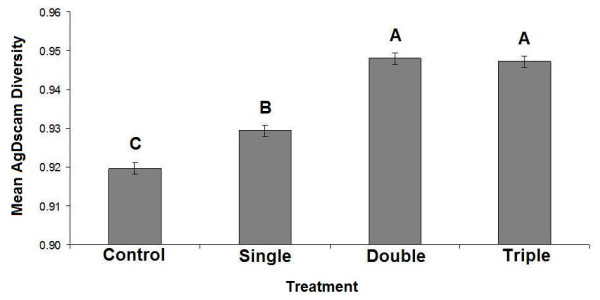
Anopheles gambiae Dscam expression diversity versus increasing parasite diversity in the field. AgDscam diversity (as 1- Simpson's Index) increased between controls and exposed treatments and between single and double genotype-exposures only. Levels not connected by same letter are significantly different. Error bars represent standard error (SE). Key: Control; blood meal with no parasites, Single; mosquitoes exposed to a minimum of one P. falciparum genotype, Double; mosquitoes exposed to a minimum of two P. falciparum genotypes, Triple; mosquitoes exposed to a minimum of three P. falciparum genotypes.
For the laboratory study, mosquitoes were membrane-fed on blood infected with gametocytes of either P. falciparum clone 3D7 [41] or HB3 [42], or a mixture of the two, and RNA was harvested from the insects 24-hours post-exposure. cDNAs were cloned and sequenced and AgDscam exon 4 and exon 6 variants expressed were identified within mosquitoes exposed to different treatments (control blood with no Plasmodium, clone 3D7, clone HB3, and clones 3D7 and HB3 mixed). It was found that AgDscam expression was affected by the blood that the mosquitoes fed upon (F3,32 = 5.29, P = 0.004; Figure 2). Thus, as with the field study, it appears that AgDscam is more diverse when parasites are present (diversity was significantly lower in uninfected blood controls than in all other samples; Figure 2). Although diversity increased under exposure to either laboratory parasite clone, it did not increase further in response to a mixture of the two clones (Figure 2). As with the field data, it was the combined diversity at exons 4 and 6 that drove this pattern, as variation in Dscam diversity was not apparent when the exons were studied separately (exon 4: F1,34 = 3.10, P = 0.087; exon 6: F1,34 = 0.16, P = 0.690). Again, there was some evidence of a trend for exon 4 but not exon 6, as previously seen in the field study, implying that the variability rendered by exon 4 in responding to Plasmodium may be relatively more important than the variability offered by exon 6 expression.
Figure 2.
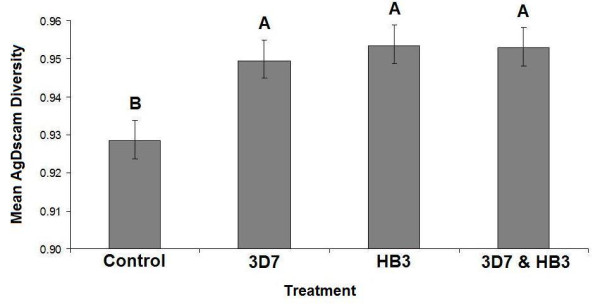
Anopheles gambiae Dscam expression diversity versus increasing parasite diversity in the lab. AgDscam diversity (as 1- Simpson's Index) increased between controls and exposed treatments, but did not increase between the single genotype-exposure and the mixed genotype-exposure. Levels not connected by same letter are significantly different. Error bars represent standard error (SE). Key: Control; blood meal with no parasites, 3D7; mosquitoes exposed to Plasmodium falciparum clone 3D7, HB3; mosquitoes exposed to Plasmodium falciparum clone HB3, 3D7 & HB3; mosquitoes exposed to both clones in the same blood meal.
A χ2 analysis was used to identify whether particular splice variants were over- or under-represented in any of the field (control, single, double or triple) or laboratory (control, clone 3D7, clone HB3, mixed) infections. Because the extreme splice variation leads to very low counts for individual exons, exon variants were grouped into categories based on their genetic distance. Although this grouping may not reflect any relationship between biological function and antigen recognition similarity, varying the cut-off points did not change the qualitative results. Based on a Neighbour-Joining (NJ) tree (exon 4, see additional file 1; exon 6, see additional file 1), genetic groupings of exon variants were defined such that exon 4 variants were clustered into 3 groups (see additional file 1), and exon 6 into 3 groups also (see additional file 1).
χ2 contingency tests were used to determine whether the number of observations (counts) of each group differed between the infection categories (see additional file 2 for raw data). In general, no exons were over- or under-represented in any particular infection grouping in either the field or laboratory experiments. The only exceptions to this were exon 4 variants 4.11, 4.12 and 4.13 which are clearly a distinct genetic grouping (see additional file 1), and were under-represented in control mosquitoes compared to exposed treatments in the field (Pearson's Chi-Square = 6.318, DF = 2, P = 0.042), and exon 6 variants 6.1 and 6.9 which are also a distinct genetic grouping (see additional file 1), and were under-represented in control mosquitoes, but also over-represented in mosquitoes exposed to a single genotype of P. falciparum in the field (Pearson Chi-Square = 19.975, DF = 6, P-Value = 0.003). These data suggests that exon 4 variants 4.11, 4.12 and 4.13, and exon 6 variants 6.1 and 6.9, may be particularly important for the mosquito's immune response to Plasmodium in the field.
Conclusion
The results show an increase in AgDscam splice-form diversity at 24-hours post-exposure, when the parasites are crossing the insect's midgut epithelium [30,45,46]. This observation, confirmed both in the field and the laboratory, reinforce that the AgDscam receptor responds to Plasmodium at a vital stage for the parasite's development. The limited association between AgDscam diversity and P. falciparum genotype-diversity in the field raises the possibility that the alternatively-spliced receptor could be responding to P. falciparum diversity. This observation, however, was not seen between double- and triple-exposed treatments. Although it cannot be assumed that every parasite genotype detected in the blood samples in the field is represented in the sexual stage, there is an apparent consistency in the results showing a lack of genotype-specific Dscam expression diversity following two different experimental approaches (field and laboratory). Naturally, the field data may also be affected by unmeasured factors, for example some of the blood samples could conceivably have harboured other parasite species. This possibility was investigated using PCR detection for other Plasmodium parasites (specifically Plasmodium malariae, Plasmodium ovale and Plasmodium vivax), and the presence of P. malariae in both of the blood samples containing a single genotype of P. falciparum was recorded (see additional file 3). Consequently, the single infections were confounded with multi-species infections, and yet AgDscam diversity of these multi-species exposures was lower than the diversity levels of the double and triple P. falciparum exposures. Although even higher Dscam expression diversity could be expected in this instance, this was not observed, and thus other interpretations are possible depending on whether one species has a stronger influence on Dscam expression within a particular multiple-infection than another. It was also interesting to note that the over-representation of the group of exon 6 variants (variants 6.1 and 6.9) found in these multi-species infections was not found in the single-species infections, implying that these variants could be influenced by the presence of P. malariae. The significance of these observations remains to be investigated. It was also determined whether P. falciparum infection intensity in the blood samples, i.e. abundance of parasite stages (as determined by quantitative PCR), could have affected our results, but we found no relationship between parasite abundance and Dscam diversity (see additional file 3). Finally, future studies may benefit from the use of multiple mosquito genotypes. It is possible that different genotypes may respond differently to the same parasite challenge in terms of AgDscam expression. This may be a logical direction for future work as the relative absence of related studies on AgDscam allows little speculation on whether the colonies used in this study would be a good proxy for what can be expected in natural populations. Thus, in summary, the data clearly indicate that AgDscam diversity increases with parasite exposure, but they do not suggest that AgDscam diversity rises further in response to increased parasite diversity.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
TJL, YAA and GY conceived the experiment. TJL, GY and LRC designed the experiment. PHS, JMM and YAA carried out the experiment. PHS, DJO and TJL analysed the data. PHS and TJL wrote the paper. All authors read, commented on, and approved the final manuscript.
Supplementary Material
Additional information containing phylogenetic trees and contingency tests.
Additional file containing exon 4 and exon 6 raw data.
Details of additional experiments.
Contributor Information
Paul H Smith, Email: paul.smith@ed.ac.uk.
Jonathan M Mwangi, Email: Jonathan.Mwangi@glasgow.ac.uk.
Yaw A Afrane, Email: yaw_afrane@yahoo.com.
Guiyun Yan, Email: guiyuny@uci.edu.
Darren J Obbard, Email: darren.obbard@ed.ac.uk.
Lisa C Ranford-Cartwright, Email: lisa.ranford-cartwright@glasgow.ac.uk.
Tom J Little, Email: tom.little@ed.ac.uk.
Acknowledgements
We would like to thank Marian Thompson, Dario Beraldi, Desiree Allen, Seanna McTaggart, Ben Longdon, and Lydia Chambers for technical advice and practical assistance. Paul Smith is supported by a BBSRC doctoral training grant. Research in the Little lab is supported by a Wellcome Trust UK Senior Fellowship in Basic Biomedical Sciences. Darren J. Obbard is funded by Wellcome Trust Research Career Development Fellowship 085064/Z/08/Z. Yaw Afrane and Guiyun Yan are supported by NIH grant R01AI050243. Research in the Ranford-Cartwright laboratory is supported by the Wellcome Trust (grant numbers 078749, 091791) and the European Union FP7 (MALSIG).
References
- Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40:845–859. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway C. Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065X.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Hoffmann JA. Innate Immunity of Insects. Curr Opin Immunol. 1995;7:4–10. doi: 10.1016/0952-7915(95)80022-0. [DOI] [PubMed] [Google Scholar]
- Chang TL, Klotman ME. Defensins: Natural anti-HIV peptides. Aids Reviews. 2004;6:161–168. [PubMed] [Google Scholar]
- Murphy KM, Travers P, Walport M. Janeway's Immunobiology. Seventh. New York: Garland Science; 2007. [Google Scholar]
- Christophides GK, Vlachou D, Kafatos FC. Comparative and functional genomics of the innate immune system in the malaria vector Anopheles gambiae. Immunol Rev. 2004;198:127–148. doi: 10.1111/j.0105-2896.2004.0127.x. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Christensen BM, Li JY, Chen CC, Nappi AJ. Melanization immune responses in mosquito vectors. Trends Parasitol. 2005;21:192–199. doi: 10.1016/j.pt.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Hauton C, Smith VJ. Adaptive immunity in invertebrates: a straw house without a mechanistic foundation. Bioessays. 2007;29:1138–1146. doi: 10.1002/bies.20650. [DOI] [PubMed] [Google Scholar]
- Rowley AF, Powell A. Invertebrate immune systems-specific, quasi-specific, or nonspecific? J Immunol. 2007;179:7209–7214. doi: 10.4049/jimmunol.179.11.7209. [DOI] [PubMed] [Google Scholar]
- Klein J. Homology between immune responses in vertebrates and invertebrates: Does it exist? Scand J Immunol. 1997;46:558–564. doi: 10.1046/j.1365-3083.1997.d01-164.x. [DOI] [PubMed] [Google Scholar]
- Little TJ, Hultmark D, Read AF. Invertebrate immunity and the limits of mechanistic immunology. Nat Immunol. 2005;6:651–654. doi: 10.1038/ni1219. [DOI] [PubMed] [Google Scholar]
- Kurtz J, Franz K. Evidence for memory in invertebrate immunity. Nature. 2003;425:37–38. doi: 10.1038/425037a. [DOI] [PubMed] [Google Scholar]
- Little TJ, O'Connor B, Colegrave N, Watt K, Read AF. Maternal transfer of strain-specific immunity in an invertebrate. Curr Biol. 2003;13:489–492. doi: 10.1016/S0960-9822(03)00163-5. [DOI] [PubMed] [Google Scholar]
- Sadd BM, Schmid-Hempel P. Insect immunity shows specificity in protection upon secondary pathogen exposure. Curr Biol. 2006;16:1206–1210. doi: 10.1016/j.cub.2006.04.047. [DOI] [PubMed] [Google Scholar]
- Roth O, Sadd BM, Schmid-Hempel P, Kurtz J. Strain-specific priming of resistance in the red flour beetle, Tribolium castaneum. Proc R Soc B-Biol Sci. 2009;276:145–151. doi: 10.1098/rspb.2008.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadd BM, Kleinlogel Y, Schmid-Hempel R, Schmid-Hempel P. Trans-generational immune priming in a social insect. Biology Letters. 2005;1:386–388. doi: 10.1098/rsbl.2005.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadd BM, Schmid-Hempel P. Facultative but persistent transgenerational immunity via the mother's eggs in bumblebees. Curr Biol. 2007;17:R1046–R1047. doi: 10.1016/j.cub.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Johnson KN, van Hulten MCW, Barnes AC. "Vaccination" of shrimp against viral pathogens: Phenomenology and underlying mechanisms. Vaccine. 2008;26:4885–4892. doi: 10.1016/j.vaccine.2008.07.019. [DOI] [PubMed] [Google Scholar]
- Cooper EL, Roch P. 2nd-Set allograft responses in the earthworm Lubricus terrestris - kinetics and characteristics. Transplantation. 1986;41:514–520. doi: 10.1097/00007890-198604000-00019. [DOI] [PubMed] [Google Scholar]
- Hartman RS, Karp RD. Short-term immunological memory in the allograft response of the American cockroach, Periplaneta americana. Transplantation. 1989;47:920–922. doi: 10.1097/00007890-198905000-00042. [DOI] [PubMed] [Google Scholar]
- Carius HJ, Little TJ, Ebert D. Genetic variation in a host-parasite association: Potential for coevolution and frequency-dependent selection. Evolution. 2001;55:1136–1145. doi: 10.1111/j.0014-3820.2001.tb00633.x. [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P Ebert D On the evolutionary ecology of specific immune defence Trends Ecology & Evolution 20031827–32. 10.1016/S0169-5347(02)00013-721651916 [DOI] [Google Scholar]
- Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS. A specific primed immune response in Drosophila is dependent on phagocytes. Plos Pathogens. 2007;3 doi: 10.1371/journal.ppat.0030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding KC, Hansen BJL, Goodman SJ. Acquired immunity and stochasticity in epidemic intervals impede the evolution of host disease resistance. American Naturalist. 2005;166:722–730. doi: 10.1086/497580. [DOI] [PubMed] [Google Scholar]
- Brites D, McTaggart S, Morris K, Anderson J, Thomas K, Colson I, Fabbro T, Little TJ, Ebert D, Du Pasquier L. The Dscam homologue of the crustacean Daphnia is diversified by alternative splicing like in insects. Mol Biol Evol. 2008;25:1429–1439. doi: 10.1093/molbev/msn087. [DOI] [PubMed] [Google Scholar]
- Du Pasquier L. Insects diversify one molecule to serve two systems. Science. 2005;309:1826–1827. doi: 10.1126/science.1118828. [DOI] [PubMed] [Google Scholar]
- Watson FL, Puttmann-Holgado R, Thomas F, Lamar DL, Hughes M, Kondo M, Rebel VI, Schmucker D. Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science. 2005;309:1874–1878. doi: 10.1126/science.1116887. [DOI] [PubMed] [Google Scholar]
- Dong YM, Taylor HE, Dimopoulos G. AgDscam, a hypervariable immunoglobulin domain-containing receptor of the Anopheles gambiae innate immune system. Plos Biology. 2006;4:1137–1146. doi: 10.1371/journal.pbio.0040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman SK, Barry AE, Lyons EJ, Nielsen KM, Thomas SM, Choi M, Thakore SS, Day KP, Wirth DF, Hartl DL. Recent origin of Plasmodium falciparum from a single progenitor. Science. 2001;293:482–484. doi: 10.1126/science.1059878. [DOI] [PubMed] [Google Scholar]
- Carter R, Ranford-Cartwright L, Alano P. The culture and preparation of gametocytes of Plasmodium falciparum for immunochemical, molecular, and mosquito infectivity studies. Methods Mol Biol. 1993;21:67–88. doi: 10.1385/0-89603-239-6:67. [DOI] [PubMed] [Google Scholar]
- Minakawa N, Sonye G, Mogi M, Yan G. Habitat characteristics of Anopheles gambiae s.s. larvae in a Kenyan highland. Med Vet Entomol. 2004;18:301–305. doi: 10.1111/j.0269-283X.2004.00503.x. [DOI] [PubMed] [Google Scholar]
- Githeko A Ndegwa W Predicting malaria epidemics in the Kenyan highlands using climate data: a tool for decision makers Global Change & Human Health 2001254–63. 10.1023/A:101194313164321656455 [DOI] [Google Scholar]
- Wooden J, Kyes S, Sibley CH. PCR and Strain Identification in Plasmodium falciparum. Parasitol Today. 1993;9:303–305. doi: 10.1016/0169-4758(93)90131-X. [DOI] [PubMed] [Google Scholar]
- Afrane YA, Little TJ, Lawson BW, Githeko AK, Yan GY. Deforestation and vectorial capacity of Anopheles gambiae giles mosquitoes in malaria transmission, Kenya. Emerg Infect Dis. 2008;14:1533–1538. doi: 10.3201/eid1410.070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulule JM, Beach RF, Atieli FK, Roberts JM, Mount DL, Mwangi RW. Reduced susceptibility of Anopheles gambiae to permethrin associated with the use of permethrin-impregnated bednets and curtains in Kenya. Med Vet Entomol. 1994;8:71–75. doi: 10.1111/j.1365-2915.1994.tb00389.x. [DOI] [PubMed] [Google Scholar]
- Anderson TJC, Su XZ, Bockarie M, Lagog M, Day KP. Twelve microsatellite markers for characterization of Plasmodium falciparum from finger-prick blood samples. Parasitology. 1999;119:113–125. doi: 10.1017/S0031182099004552. [DOI] [PubMed] [Google Scholar]
- Su XZ, Wellems TE. Toward a high-resolution Plasmodium falciparum linkage map: Polymorphic markers from hundreds of simple sequence repeats. Genomics. 1996;33:430–444. doi: 10.1006/geno.1996.0218. [DOI] [PubMed] [Google Scholar]
- Anderson TJC, Haubold B, Williams JT, Estrada-Franco JG, Richardson L, Mollinedo R, Bockarie M, Mokili J, Mharakurwa S, French N, Whitworth J, Velez ID, Brockman AH, Nosten F, Ferreira MU, Day KP. Microsatellite markers reveal a spectrum of population structures in the malaria parasite Plasmodium falciparum. Mol Biol Evol. 2000;17:1467–1482. doi: 10.1093/oxfordjournals.molbev.a026247. [DOI] [PubMed] [Google Scholar]
- Walliker D, Quakyi IA, Wellems TE, McCutchan TF, Szarfman A, London WT, Corcoran LM, Burkot TR, Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987;236:1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]
- Bhasin VK, Trager W. Gametocyte-forming and non-gametocyte-forming clones of Plasmodium falciparum. Am J Trop Med Hyg. 1984;33(4):534–537. doi: 10.4269/ajtmh.1984.33.534. [DOI] [PubMed] [Google Scholar]
- Simpson EH. Measurement of diversity. Nature. 1949;163:688–688. doi: 10.1038/163688a0. [DOI] [Google Scholar]
- Nwakanma D, Kheir A, Sowa M, Dunyo S, Jawara M, Pinder M, Milligan P, Walliker D, Babiker HA. High gametocyte complexity and mosquito infectivity of Plasmodium falciparum in the Gambia. Int J Parasitol. 2008;38:219–227. doi: 10.1016/j.ijpara.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Dong Y, Dimopoulos G. Pattern recognition diversity in the Anopheles gambiae innate immune system. Am J Trop Med Hyg. 2006;75:291–292. [Google Scholar]
- Dimopoulos G, Seeley D, Wolf A, Kafatos FC. Malaria infection of the mosquito Anopheles gambiae activates immune-responsive genes during critical transition stages of the parasite life cycle. Embo Journal. 1998;17:6115–6123. doi: 10.1093/emboj/17.21.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information containing phylogenetic trees and contingency tests.
Additional file containing exon 4 and exon 6 raw data.
Details of additional experiments.


