Abstract
We have isolated, cloned and analyzed small polydisperse circular (spc) DNA from mouse 3T6 cells. The representation of highly repeated mouse genome sequence families in spcDNA has been examined, and the B1 repeat appears overrepresented in spcDNA by two criteria. The majority of spcDNA clones, however, is made out by as yet uncharacterized middle repetitive sequences. We have investigated the increase in the spcDNA population upon cycloheximide treatment of individual sequences, which are found to amplify differentially.
Full text
PDF





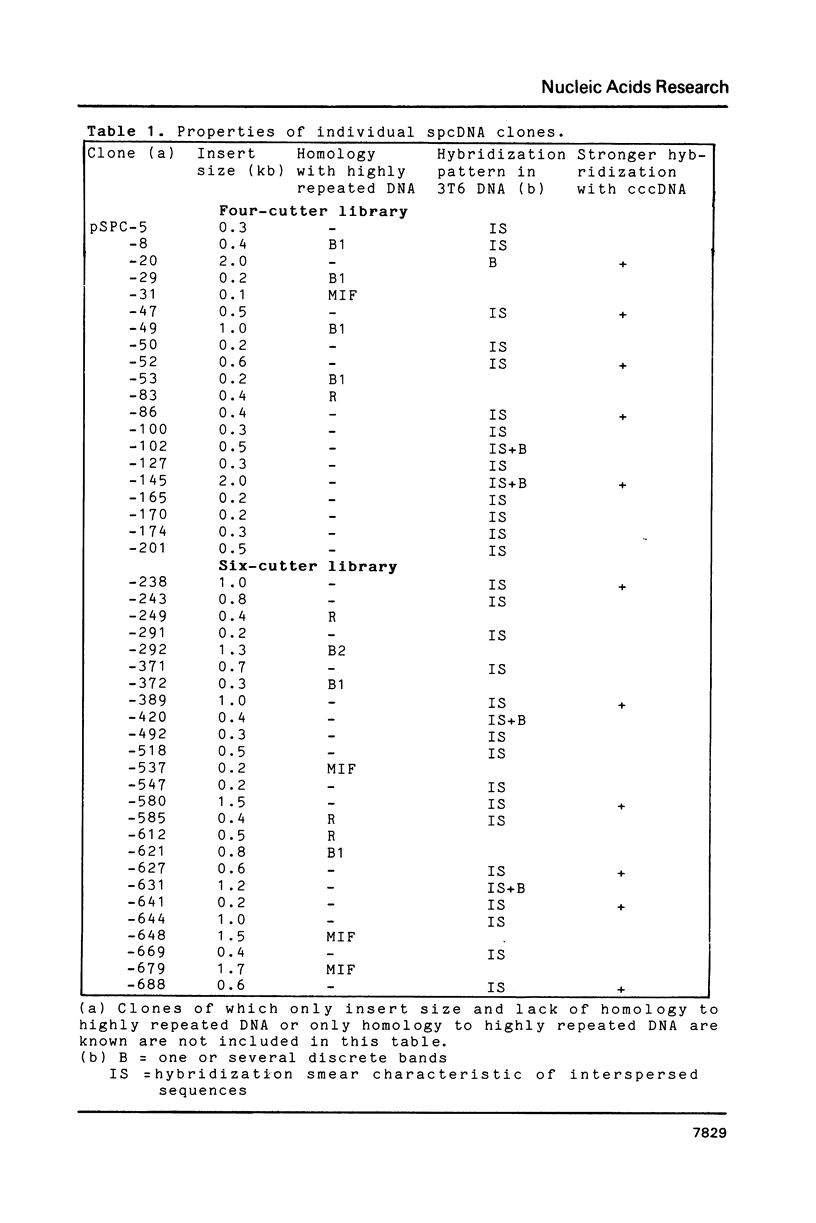


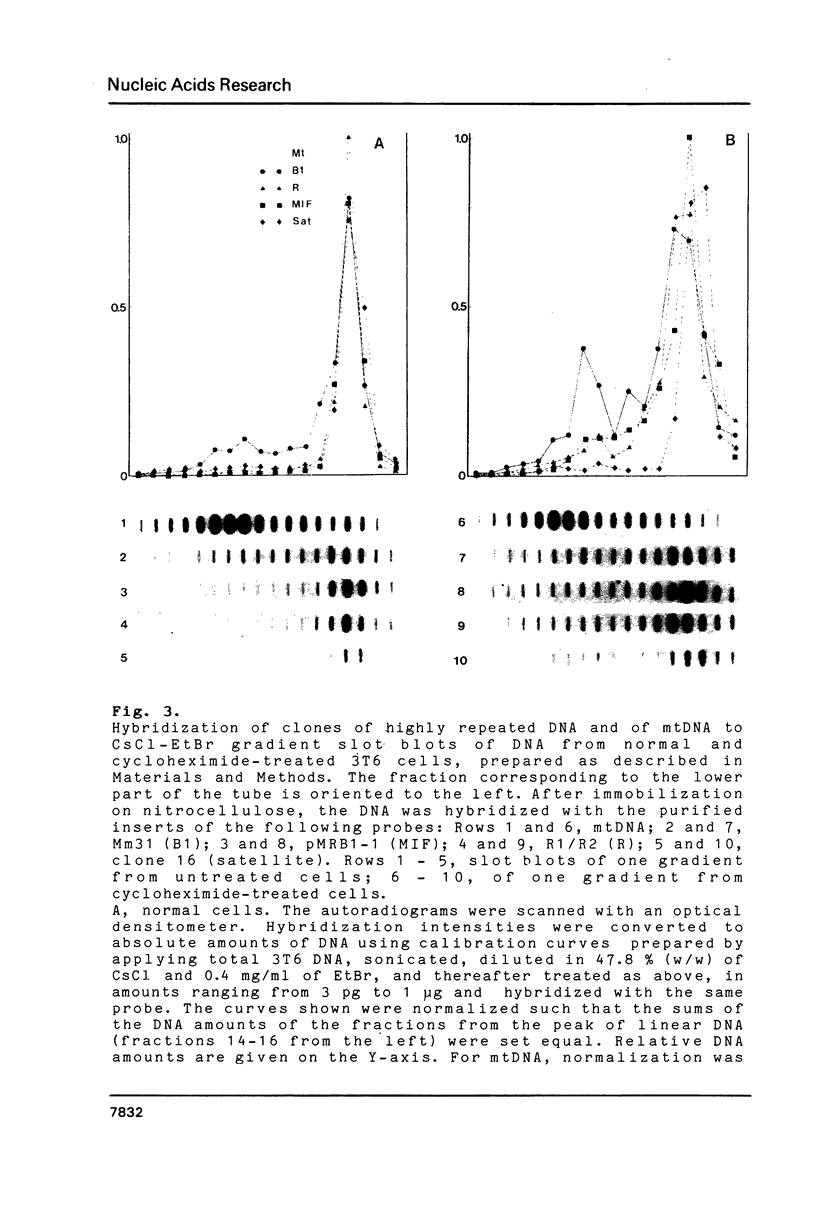






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariga H. Replication of cloned DNA containing the Alu family sequence during cell extract-promoting simian virus 40 DNA synthesis. Mol Cell Biol. 1984 Aug;4(8):1476–1482. doi: 10.1128/mcb.4.8.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett K. L., Hill R. E., Pietras D. F., Woodworth-Gutai M., Kane-Haas C., Houston J. M., Heath J. K., Hastie N. D. Most highly repeated dispersed DNA families in the mouse genome. Mol Cell Biol. 1984 Aug;4(8):1561–1571. doi: 10.1128/mcb.4.8.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelsen A. H., Humayun M. Z., Karfopoulos S. G., Rush M. G. Molecular characterization of small polydisperse circular deoxyribonucleic acid from an African green monkey cell line. Biochemistry. 1982 Apr 27;21(9):2076–2085. doi: 10.1021/bi00538a015. [DOI] [PubMed] [Google Scholar]
- Bertelsen A. H., Humayun M. Z., Lippman A., Rush M. G. Identification of extrachromosomal DNA in hematolymphoid cells of chickens and mice. Biochem Biophys Res Commun. 1982 Apr 14;105(3):977–984. doi: 10.1016/0006-291x(82)91066-x. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLap R. J., Rush M. G. Change in quantity and size distribution of small circular DNAs during development of chicken bursa. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5855–5859. doi: 10.1073/pnas.75.12.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deugau K. V., Mitchel R. E., Birnboim H. C. Nucleotide sequence of polypyrimidines from cloned mouse DNA as determined by base-specific blockage of exonuclease action. Anal Biochem. 1983 Feb 15;129(1):88–97. doi: 10.1016/0003-2697(83)90056-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fujimoto S., Tsuda T., Toda M., Yamagishi H. Transposon-like sequences in extrachromosomal circular DNA from mouse thymocytes. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2072–2076. doi: 10.1073/pnas.82.7.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhard W., Meitinger T., Höchtl J., Zachau H. G. A new family of interspersed repetitive DNA sequences in the mouse genome. J Mol Biol. 1982 May 25;157(3):453–471. doi: 10.1016/0022-2836(82)90471-5. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Harr R., Fällman P., Häggström M., Wahlström L., Gustafsson P. GENEUS, a computer system for DNA and protein sequence analysis containing an information retrieval system for the EMBL data library. Nucleic Acids Res. 1986 Jan 10;14(1):273–284. doi: 10.1093/nar/14.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörz W., Altenburger W. Nucleotide sequence of mouse satellite DNA. Nucleic Acids Res. 1981 Feb 11;9(3):683–696. doi: 10.1093/nar/9.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krayev A. S., Kramerov D. A., Skryabin K. G., Ryskov A. P., Bayev A. A., Georgiev G. P. The nucleotide sequence of the ubiquitous repetitive DNA sequence B1 complementary to the most abundant class of mouse fold-back RNA. Nucleic Acids Res. 1980 Mar 25;8(6):1201–1215. doi: 10.1093/nar/8.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krayev A. S., Markusheva T. V., Kramerov D. A., Ryskov A. P., Skryabin K. G., Bayev A. A., Georgiev G. P. Ubiquitous transposon-like repeats B1 and B2 of the mouse genome: B2 sequencing. Nucleic Acids Res. 1982 Dec 11;10(23):7461–7475. doi: 10.1093/nar/10.23.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolewski J. J., Rush M. G. Some extrachromosomal circular DNAs containing the Alu family of dispersed repetitive sequences may be reverse transcripts. J Mol Biol. 1984 Mar 25;174(1):31–40. doi: 10.1016/0022-2836(84)90363-2. [DOI] [PubMed] [Google Scholar]
- Kunisada T., Yamagishi H. Sequence repetition and genomic distribution of small polydisperse circular DNA purified from HeLa cells. Gene. 1984 Nov;31(1-3):213–223. doi: 10.1016/0378-1119(84)90212-9. [DOI] [PubMed] [Google Scholar]
- Meunier-Rotival M., Soriano P., Cuny G., Strauss F., Bernardi G. Sequence organization and genomic distribution of the major family of interspersed repeats of mouse DNA. Proc Natl Acad Sci U S A. 1982 Jan;79(2):355–359. doi: 10.1073/pnas.79.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson K. E., Deka N., Schmid C. W., Misra R., Schindler C. W., Rush M. G., Kadyk L., Leinwand L. A transposon-like element in human DNA. Nature. 1985 Jul 25;316(6026):359–361. doi: 10.1038/316359a0. [DOI] [PubMed] [Google Scholar]
- Riabowol K., Shmookler Reis R. J., Goldstein S. Interspersed repetitive and tandemly repetitive sequences are differentially represented in extrachromosomal covalently closed circular DNA of human diploid fibroblasts. Nucleic Acids Res. 1985 Aug 12;13(15):5563–5584. doi: 10.1093/nar/13.15.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Schindler C. W., Rush M. G. The KpnI family of long interspersed nucleotide sequences is present on discrete sizes of circular DNA in monkey (BSC-1) cells. J Mol Biol. 1985 Jan 20;181(2):161–173. doi: 10.1016/0022-2836(85)90082-8. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Vinograd J. Small polydisperse circular DNA of HeLa cells. J Mol Biol. 1972 Aug 21;69(2):163–178. doi: 10.1016/0022-2836(72)90222-7. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanfield S. W., Helinski D. R. Cloning and characterization of small circular DNA from Chinese hamster ovary cells. Mol Cell Biol. 1984 Jan;4(1):173–180. doi: 10.1128/mcb.4.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield S., Helinski D. R. Small circular DNA in Drosophila melanogaster. Cell. 1976 Oct;9(2):333–345. doi: 10.1016/0092-8674(76)90123-9. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. On the possibility of metabolic control of replicon "misfiring": relationship to emergence of malignant phenotypes in mammalian cell lineages. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3673–3677. doi: 10.1073/pnas.78.6.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wiberg F. C., Sunnerhagen P., Bjursell G. New, small circular DNA in transfected mammalian cells. Mol Cell Biol. 1986 Feb;6(2):653–662. doi: 10.1128/mcb.6.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg F. C., Sunnerhagen P., Kaltoft K., Zeuthen J., Bjursell G. Replication and expression in mammalian cells of transfected DNA; description of an improved erythrocyte ghost fusion technique. Nucleic Acids Res. 1983 Nov 11;11(21):7287–7302. doi: 10.1093/nar/11.21.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]




