Abstract
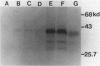
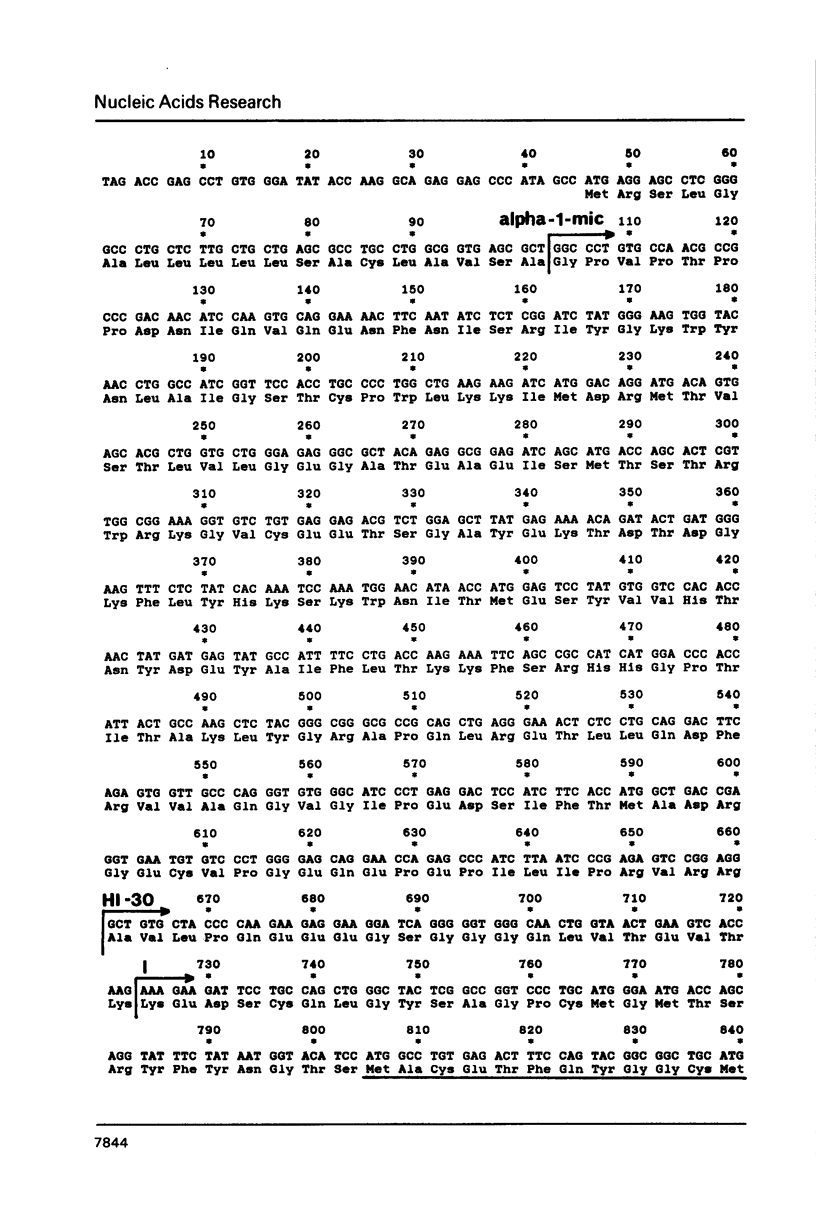
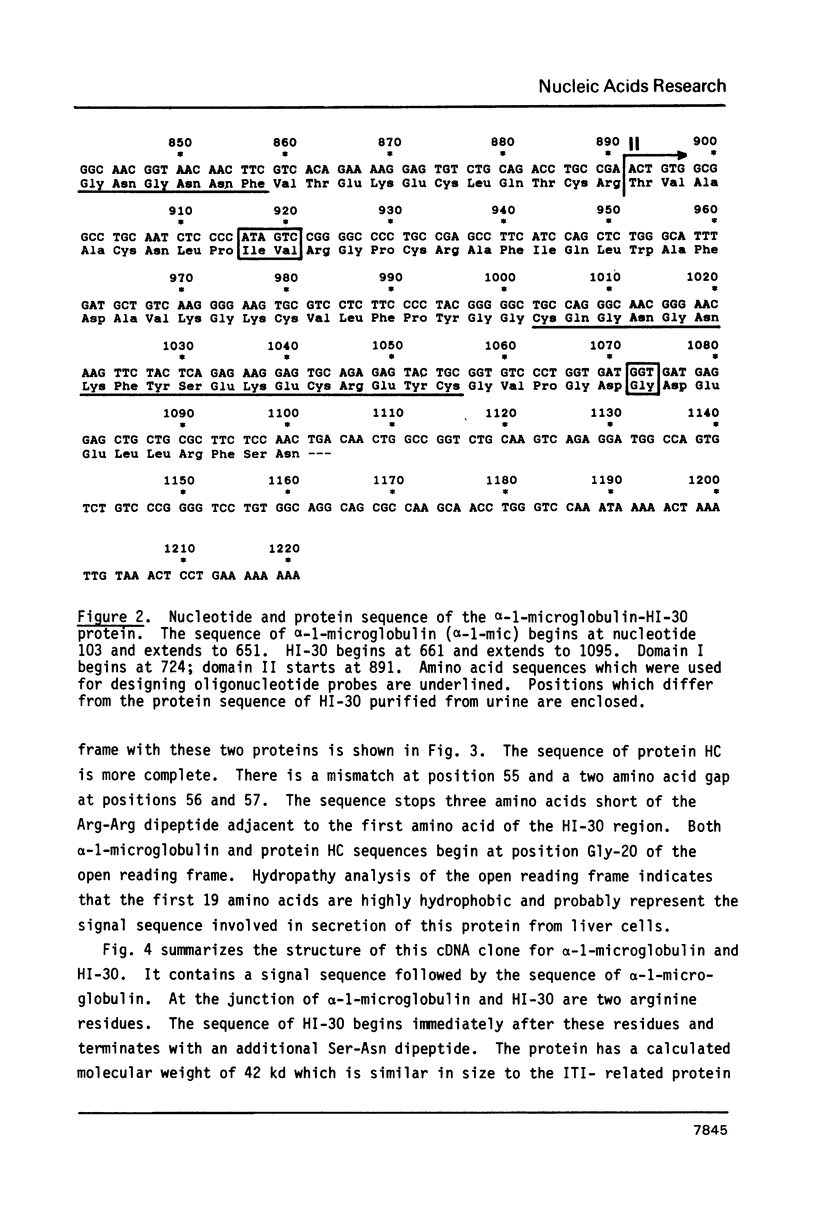
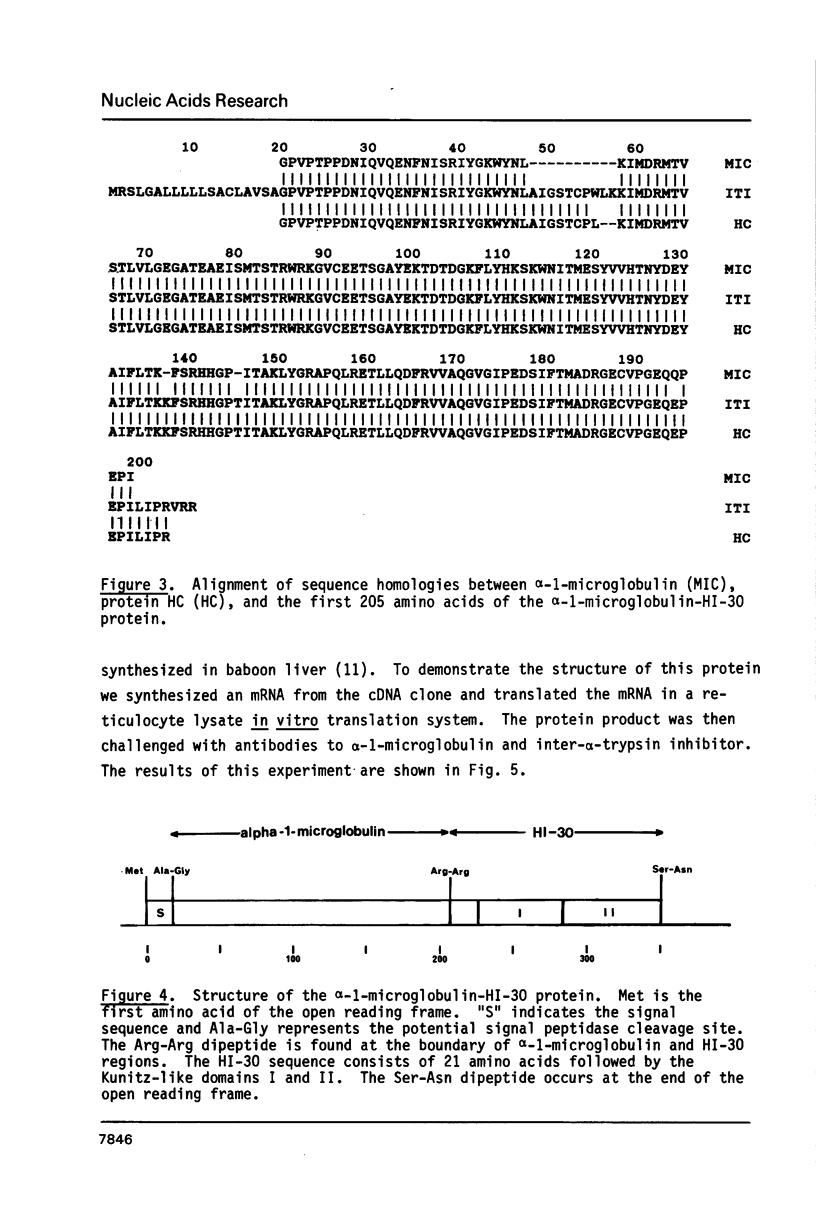
Inter-alpha-trypsin inhibitor (ITI) is a 180 kd serine proteinase inhibitor found in human serum. Treatment of 180 kd ITI with trypsin releases a 30 kd fragment (HI-30) which contains the anti-proteolytic activity of the high molecular weight form. We have isolated a cDNA clone from a human liver library which codes for HI-30, and have determined its DNA sequence. The mRNA not only codes for HI-30 but also another serum protein, alpha-1-microglobulin, which has not been previously associated with ITI or HI-30. The alpha-1-microglobulin sequence is found in the amino-terminus of the protein and is preceded by a signal sequence. HI-30 is found at the carboxy-terminus. The two protein sequences are separated by two arginine residues.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht G. J., Hochstrasser K., Salier J. P. Elastase inhibition by the inter-alpha-trypsin inhibitor and derived inhibitors of man and cattle. Hoppe Seylers Z Physiol Chem. 1983 Dec;364(12):1703–1708. doi: 10.1515/bchm2.1983.364.2.1703. [DOI] [PubMed] [Google Scholar]
- Anderson S., Kingston I. B. Isolation of a genomic clone for bovine pancreatic trypsin inhibitor by using a unique-sequence synthetic DNA probe. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6838–6842. doi: 10.1073/pnas.80.22.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bourguignon J., Diarra-Mehrpour M., Sesboü R., Frain M., Sala-Trepat J. M., Martin J. P., Salier J. P. Human inter-alpha-trypsin-inhibitor: characterization and partial nucleotide sequencing of a light chain-encoding cDNA. Biochem Biophys Res Commun. 1985 Sep 30;131(3):1146–1153. doi: 10.1016/0006-291x(85)90210-4. [DOI] [PubMed] [Google Scholar]
- Bourguignon J., Vercaigne D., Sesboü R., Martin J. P., Salier J. P. Inter-alpha-trypsin-inhibitor (ITI): two distinct mRNAs in baboon liver argue for a discrete synthesis of ITI and ITI derivatives. FEBS Lett. 1983 Oct 17;162(2):379–383. doi: 10.1016/0014-5793(83)80791-1. [DOI] [PubMed] [Google Scholar]
- Bromke B. J., Kueppers F. The major urinary protease inhibitor: simplified purification and characterization. Biochem Med. 1982 Feb;27(1):56–67. doi: 10.1016/0006-2944(82)90008-4. [DOI] [PubMed] [Google Scholar]
- Deininger P. L. Random subcloning of sonicated DNA: application to shotgun DNA sequence analysis. Anal Biochem. 1983 Feb 15;129(1):216–223. doi: 10.1016/0003-2697(83)90072-6. [DOI] [PubMed] [Google Scholar]
- Dobberstein B., Garoff H., Warren G., Robinson P. J. Cell-free synthesis and membrane insertion of mouse H-2Dd histocompatibility antigen and beta 2-microglobulin. Cell. 1979 Aug;17(4):759–769. doi: 10.1016/0092-8674(79)90316-7. [DOI] [PubMed] [Google Scholar]
- Ekström B., Berggård I. Human alpha1-microglobulin. Purification procedure, chemical and physiochemical properties. J Biol Chem. 1977 Nov 25;252(22):8048–8057. [PubMed] [Google Scholar]
- Ekström B., Peterson P. A., Berggård I. A urinary and plasma alpha1-glycoprotein of low molecular weight: isolation and some properties. Biochem Biophys Res Commun. 1975 Aug 18;65(4):1427–1433. doi: 10.1016/s0006-291x(75)80388-3. [DOI] [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M., Jacobzone M., Mercier R. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 1981 Jan 10;9(1):r43–r74. doi: 10.1093/nar/9.1.213-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb A. O., López C., Tejler L., Mendez E. Isolation of human complex-forming glycoprotein, heterogeneous in charge (protein HC), and its IgA complex from plasma. Physiochemical and immunochemical properties, normal plasma concentration. J Biol Chem. 1983 Dec 10;258(23):14698–14707. [PubMed] [Google Scholar]
- Hochstrasser K., Bretzel G., Feuth H., Hilla W., Lempart K. [The inter-alpha-trypsin inhibitor as precursor of the acid-stable proteinase inhibitors in human serum and urine]. Hoppe Seylers Z Physiol Chem. 1976 Feb;357(2):153–162. doi: 10.1515/bchm2.1976.357.1.153. [DOI] [PubMed] [Google Scholar]
- Hochstrasser K., Feuth H., Steiner O. Zur Charakterisierung der sürestabilen Proteaseninhibitoren aus Humanplasma. Hoppe Seylers Z Physiol Chem. 1973 Aug;354(8):927–932. [PubMed] [Google Scholar]
- Hochstrasser K., Reisinger P., Albrecht G. J., Wachter E., Schönberger O. L. Isolation of acid-resistant urinary trypsin inhibitors by high performance liquid chromatography and their characterization by N-terminal amino-acid sequence determination. Hoppe Seylers Z Physiol Chem. 1984 Sep;365(9):1123–1130. doi: 10.1515/bchm2.1984.365.2.1123. [DOI] [PubMed] [Google Scholar]
- Hochstrasser K., Schönberger O. L., Rossmanith I., Wachter E. Kunitz-type proteinase inhibitors derived by limited proteolysis of the inter-alpha-trypsin inhibitor, V. Attachments of carbohydrates in the human urinary trypsin inhibitor isolated by affinity chromatography. Hoppe Seylers Z Physiol Chem. 1981 Oct;362(10):1357–1362. doi: 10.1515/bchm2.1981.362.2.1357. [DOI] [PubMed] [Google Scholar]
- Hochstrasser K., Wachter E. Kunitz-type proteinase inhibitors derived by limited proteolysis of the inter-alpha-trypsin inhibitor, I. Determination of the amino acid sequence of the antitryptic domain by solid-phase Edman degradation. Hoppe Seylers Z Physiol Chem. 1979 Sep;360(9):1285–1296. doi: 10.1515/bchm2.1979.360.2.1285. [DOI] [PubMed] [Google Scholar]
- Jaye M., de la Salle H., Schamber F., Balland A., Kohli V., Findeli A., Tolstoshev P., Lecocq J. P. Isolation of a human anti-haemophilic factor IX cDNA clone using a unique 52-base synthetic oligonucleotide probe deduced from the amino acid sequence of bovine factor IX. Nucleic Acids Res. 1983 Apr 25;11(8):2325–2335. doi: 10.1093/nar/11.8.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson B. M., Löffler C., Ohlsson K. Human granulocyte elastase is inhibited by the urinary trypsin inhibitor. Hoppe Seylers Z Physiol Chem. 1982 Oct;363(10):1167–1175. doi: 10.1515/bchm2.1982.363.2.1167. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopez C., Grubb A., Soriano F., Mendez E. The complete amino acids sequence of human complex-forming glycoprotein heterogeneous in charge (protein HC). Biochem Biophys Res Commun. 1981 Dec 15;103(3):919–925. doi: 10.1016/0006-291x(81)90898-6. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morii M., Travis J. The reactive site of human inter-alpha-trypsin inhibitor is in the amino-terminal half of the protein. Biol Chem Hoppe Seyler. 1985 Jan;366(1):19–21. doi: 10.1515/bchm3.1985.366.1.19. [DOI] [PubMed] [Google Scholar]
- Méndez E., Fernández-Luna J. L., Grubb A., Leyva-Cobián F. Human protein HC and its IgA complex are inhibitors of neutrophil chemotaxis. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1472–1475. doi: 10.1073/pnas.83.5.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisinger P., Hochstrasser K., Albrecht G. J., Lempart K., Salier J. P. Human inter-alpha-trypsin inhibitor: localization of the Kunitz-type domains in the N-terminal part of the molecule and their release by a trypsin-like proteinase. Biol Chem Hoppe Seyler. 1985 May;366(5):479–483. doi: 10.1515/bchm3.1985.366.1.479. [DOI] [PubMed] [Google Scholar]
- Salier J. P., Martin J. P., Lambin P., McPhee H., Hochstrasser K. Purification of the human serum inter-alpha-trypsin inhibitor by zinc chelate and hydrophobic interaction chromatographies. Anal Biochem. 1980 Dec;109(2):273–283. doi: 10.1016/0003-2697(80)90649-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbuch M. The inter-alpha-trypsin inhibitor. Methods Enzymol. 1976;45:760–772. doi: 10.1016/s0076-6879(76)45069-3. [DOI] [PubMed] [Google Scholar]
- Takagi T., Takagi K., Kawai T. Complete amino acid sequence of human alpha 1-microglobulin. Biochem Biophys Res Commun. 1981 Feb 27;98(4):997–1001. doi: 10.1016/0006-291x(81)91209-2. [DOI] [PubMed] [Google Scholar]
- Tejler L., Grubb A. O. A complex-forming glycoprotein heterogeneous in charge and present in human plasma, urine, and cerebrospinal fluid. Biochim Biophys Acta. 1976 Jul 19;439(1):82–94. doi: 10.1016/0005-2795(76)90164-1. [DOI] [PubMed] [Google Scholar]
- Traboni C., Cortese R. Sequence of a full length cDNA coding for human protein HC (alpha 1 microglobulin). Nucleic Acids Res. 1986 Aug 11;14(15):6340–6340. doi: 10.1093/nar/14.15.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Berman C. H., Dull T. J., Gray A., Lee J. M. Isolation of the human insulin-like growth factor I gene using a single synthetic DNA probe. EMBO J. 1984 Feb;3(2):361–364. doi: 10.1002/j.1460-2075.1984.tb01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent C., Bouic P., Revillard J. P., Bataille R. Complexes of alpha 1-microglobulin and monomeric IgA in multiple myeloma and normal human sera. Mol Immunol. 1985 Jun;22(6):663–673. doi: 10.1016/0161-5890(85)90096-3. [DOI] [PubMed] [Google Scholar]
- Wachter E., Hochstrasser K. Kunitz-type proteinase inhibitors derived by limited proteolysis of the inter-alpha-trypsin inhibitor, IV. The amino acid sequence of the human urinary trypsin inhibitor isolated by affinity chromatography. Hoppe Seylers Z Physiol Chem. 1981 Oct;362(10):1351–1355. doi: 10.1515/bchm2.1981.362.2.1351. [DOI] [PubMed] [Google Scholar]