Abstract
Background
Circulating 25-hydroxyvitamin D [25(OH)D] concentration is inversely associated with peripheral arterial disease and hypertension. Vascular remodeling may play a role in this association, however, data relating vitamin D level to specific remodeling biomarkers among ESRD patients is sparse. We tested whether 25(OH)D concentration is associated with markers of vascular remodeling and inflammation in African American ESRD patients.
Methods
We conducted a cross-sectional study among ESRD patients receiving maintenance hemodialysis within Emory University-affiliated outpatient hemodialysis units. Demographic, clinical and dialysis treatment data were collected via direct patient interview and review of patients records at the time of enrollment, and each patient gave blood samples. Associations between 25(OH)D and biomarker concentrations were estimated in univariate analyses using Pearson's correlation coefficients and in multivariate analyses using linear regression models. 25(OH) D concentration was entered in multivariate linear regression models as a continuous variable and binary variable (<15 ng/ml and ≥15 ng/ml). Adjusted estimate concentrations of biomarkers were compared between 25(OH) D groups using analysis of variance (ANOVA). Finally, results were stratified by vascular access type.
Results
Among 91 patients, mean (standard deviation) 25(OH)D concentration was 18.8 (9.6) ng/ml, and was low (<15 ng/ml) in 43% of patients. In univariate analyses, low 25(OH) D was associated with lower serum calcium, higher serum phosphorus, and higher LDL concentrations. 25(OH) D concentration was inversely correlated with MMP-9 concentration (r = -0.29, p = 0.004). In multivariate analyses, MMP-9 concentration remained negatively associated with 25(OH) D concentration (P = 0.03) and anti-inflammatory IL-10 concentration positively correlated with 25(OH) D concentration (P = 0.04).
Conclusions
Plasma MMP-9 and circulating 25(OH) D concentrations are significantly and inversely associated among ESRD patients. This finding may suggest a potential mechanism by which low circulating 25(OH) D functions as a cardiovascular risk factor.
Background
The chronic pro-inflammatory state of end-stage renal disease (ESRD) is associated with increased risk for cardiovascular disease and death [1-3]. Observational studies suggest an inverse association between circulating 25-hydroxyvitamin D [25(OH) D] concentration and serum inflammatory biomarkers. Low 25(OH)D concentrations are associated with elevated tumor necrosis factor-alpha (TNF-α) concentrations [4], and randomized controlled studies among critically ill patients and those with congestive heart failure report that vitamin D supplementation leads to significant reductions in interleukin 6 ( IL-6) and C-reactive protein (CRP) concentrations [5], and resulted in lower tumor necrosis factor-alpha (TNF-α) and increased anti-inflammatory IL-10 concentrations [6]. However, few studies have addressed the association between 25(OH)D concentration and increased inflammation on biomarkers of vascular remodeling. Matrix metalloproteinases (MMP's) are important in extracellular matrix remodeling, are major effector molecules of inflammatory cells, and play a key role in many vascular diseases, including hypertension and aneurysm formation [7,8]. Recent in vitro studies indicate that vitamin D down-regulates MMP-9 production by TNF-α [9] and suppresses production of MMP-7 and MMP-9 [10-12]. Moreover, MMP-2 and MMP-9 activation are associated with collagen deposition and cardiac fibrosis [13].
Previous studies document the high prevalence of 25(OH) D deficiency within the U.S. ESRD and general population [14,15], and the association of 25(OH)D deficiency with vascular disorders [16,17]. Therefore, examination of the relationship between circulating 25(OH)D and biomarkers of vascular remodeling is warranted, as identification of a link between 25(OH) D concentration and vessel remodeling may suggest a mechanism for vitamin D deficiency as a risk factor for cardiovascular disease, and with further study, may offer promising therapy to improve vascular function among chronic kidney disease and ESRD patients.
We sought to determine whether circulating 25(OH) D concentration is associated with serum markers of vascular remodeling and inflammation in a cross-sectional study of prevalent hemodialysis African American patients.
Methods
Study Population
Adult ESRD patients receiving in-center maintenance hemodialysis at one of six Emory University-affiliated Davita dialysis centers between September 2006-November 2008, were eligible to enroll in the study. The majority of patients within the Emory dialysis centers are African American; of 94 patients enrolled, 91 were AA, thus we limited our analyses to AA patients. Patients were excluded from the study for the following reasons: 1) presence of known malignancy or active vasculitis; 2) evidence of current infection or inflammation; or 3) current or recent use of steroids, calcineurin inhibitors or antimetabolite medications (methotrexate, azathioprine, 5-fluorouracil, mercaptopurine, sulfadiazine). These exclusion criteria were chosen because they provide inflammatory or anti-inflammatory therapy that could alter the association between vitamin D and inflammatory markers. Demographic, clinical and dialysis treatment data were collected via direct patient interview and review of patients records at the time of enrollment, and each patient gave baseline blood samples. The Institutional Review Board (IRB) of Emory University Medical Center approved the study protocol and informed consent was obtained from each patient prior to study enrollment. Human subjects procedures followed were in accordance with the ethical standards of the institutional IRB and with the Helsinki Declaration of 1975.
Definition of variables
All assessments were conducted at a single baseline visit. We analyzed several covariates including age, gender, self-reported race, length of time on dialysis, body mass index (BMI), smoking status (never, former current), vascular access type (arteriovenous fistula, arteriovenous graft, central venous catheter), season of blood collection, and activated vitamin D treatment. Comorbidities included diabetes (defined by use of diabetes medications), hypertension, stroke or transient ischemic event, congestive heart failure, peripheral arterial disease (PAD), and HIV or AIDS. Results of calcium, phosphorus, intact PTH, albumin, hemoglobin, KT/V (used to quantify hemodialysis adequacy), total cholesterol, LDL and HDL cholesterol, and triglycerides obtained within two weeks of the baseline blood sample measurement were abstracted from the outpatient dialysis records and included in the analysis.
Study Measurements
Serum Cytokine Measurement
All patients received hemodialysis treatment three times per week. Blood for the analysis of serum biomarkers was drawn specifically for this study protocol, and was obtained before a routine, midweek hemodialysis session within 5 days of study enrollment. After collection, serum and plasma were aliquoted and stored at -80 C for subsequent analysis.
All assays were performed on plasma as directed by the manufacturer and samples were centrifuged for 10 minutes at 10000rpm before testing. All the assays had quality controls, plasma controls and a non-plasma sample that was added to each plate. The dilution of the samples (always done with the Calibration diluent in which the Standard Cocktail was reconstituted ) depended on the individual assays and of the expected concentration range for the samples tested.
The Fluorokine® MultiAnalyte Profiling (MAP) Kits from R&D Systems (Minneapolis, USA) were used to determine the concentration of analytes. The Fluorokine® MAP MultiAnalyte Profiling Human MMP Base Kit (R&D Systems) was used to determine the matrix metalloproteinase 9 (MMP-9) and MMP-2 concentrations (the inter-assay variability is 3.0% and 6.2% respectively). Il-6 and Il-10 concentrations were measured with the Fluorokine® MAP MultiAnalyte Profiling Human Base Kit A (R&D Systems) (the inter-assay variability is 4.6% and 5.2% respectively). The CardioPhase hsCRP reagents from Siemens Diagnostics (Dade Behring product) were used to determine the concentration of hsCRP in plasma samples on a BNII (nephelometry systems) System. The Internal Quality Controls (Controls SL/1 and SL/2 - lower-higher range of CRP) and Standard SL curve were run with the assay.
25(OH)D Measurement
Serum 25(OH) D concentrations were measured using chemiluminescence immunoassay (Diasorin Inc, Stillwater, MD, USA; coefficient of variation over multiple runs 6.3-12.9%) by ARUP Laboratories (Salt Lake City, UT). 25(OH) D concentration was categorized using a cutoff of 15 ng/ml, the point below which there is an increased risk for cardiovascular events [18,19].
Statistical Analysis
Categorical variables were reported by using the number or percent of observations. Continuous variables were expressed as means with standard deviations. For comparative evaluations, Chi-square tests (Fisher's exact test when appropriate) and student unpaired independent t tests were performed for categorical and continuous variables, respectively. Distribution figures and Kolmogorov-Smirnov tests were used to assess inflammatory marker normality. Non-normally distributed markers were successfully normalized by natural logarithmic transformation. We used figures plots to verify that relationships, if any, between biomarkers and 25(OH)D concentrations were linear. Associations between 25(OH)D and biomarker concentrations were first estimated in univariate analyses using Pearson's correlation coefficients and corresponding P values.
To adjust for possible confounding of these associations we then used multivariate linear regression models. Included in the models were previously reported confounders as well as variables significant at the 5% level from the univariate analyses. 25(OH) D concentration was first entered in multivariate linear regression models as a continuous variable. Adjusted associations between continuous vitamin D concentration and biomarkers were reported as beta regression coefficients where the null value (β = 0) represented no association. We then categorized 25(OH) D using a binary variable (<15 ng/ml and ≥15 ng/ml). Adjusted estimate concentrations of biomarkers were compared between 25(OH) D groups using analysis of variance (ANOVA) with SAS generalized linear model procedures. Finally, results were stratified by vascular access type. Two-tailed P values P < 0.05 were considered statistically significant. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
Patient Characteristics
The characteristics of the entire study cohort and of those classified by serum 25(OH) D concentrations are summarized in Table 1. Lower 25(OH) D concentration was significantly associated with lower serum calcium, higher serum phosphorus, and higher LDL concentration. There were no significant differences in other characteristics between vitamin D status groups. Among the study cohort, all subjects were treated with activated (D3) vitamin D, with the exception of 17 subjects, who took no form of vitamin D (activated or nutritional), and 3 subjects who took only nutritional (D2) vitamin D. The characteristics of the cohort by vascular access type are summarized in Table 2. Vascular access type was significantly associated with diabetes, stroke or TIA, HIV or AIDS, 25(OH)D and hemoglobin concentrations.
Table 1.
Characteristics of African American participants by circulating 25(OH) D concentration
| Characteristic | All patients | 25(OH) D < 15 ng/ml | 25(OH) D ≥ 15 ng/ml | |
|---|---|---|---|---|
| N = 91 | N = 39 | N = 52 | p value | |
| Age (years) | 59.3 ± 12.4 | 56.4 ± 12.6 | 61.4 ± 12.0 | 0.05 |
| Sex | 0.27 | |||
| Female | 43 (47.3) | 21 (48.5) | 22 (51.2) | |
| Male | 48 (52.7) | 18 (37.5) | 30 (62.5) | |
| Dialysis vintage (yrs) | 5.7 ± 4.1 | 6.2 ± 4.5 | 5.3 ± 3.9 | 0.33 |
| KT/V* | 1.4 ± 0.3 | 1.4 ± 0.2 | 1.5 ± 0.4 | 0.38 |
| Body mass index (kg/m2) | 0.10 | |||
| Under (<18.5) | 1 (1.2) | 0 (0.0) | 1 (100.0) | |
| Normal (18.5 to <25) | 23 (26.7) | 6 (26.09) | 17 (73.9) | |
| Overweight (25 to <30) | 37 (43.0) | 18 (48.6) | 19 (51.4) | |
| Obese (> = 30) | 25 (29.1) | 14 (56.0) | 11 (44.0) | |
| Smoking status | 0.08 | |||
| Never | 55 (60.4) | 28 (50.9) | 27 (49.09) | |
| Former | 23 (25.7) | 7 (30.4) | 16 (69.57) | |
| Current | 13 (14.3) | 4 (30.8) | 9 (69.23) | |
| Vascular access type | 0.34 | |||
| AVF | 38 (41.8) | 13 (34.2) | 25 (65.8) | |
| Graft | 44 (48.4) | 21 (47.7) | 23 (52.3) | |
| Catheter | 9 (9.0) | 5 (55.6) | 4 (44.4) | |
| Activated vitamin D treatment | 39 (42.9) | 27 (29.7) | 44 (48.3) | 0.08 |
| Comorbidities | ||||
| Diabetes | 45 (49.4) | 23 (51.1) | 22 (48.9) | 0.11 |
| Hypertension | 89 (97.8) | 38 (42.7) | 51 (57.3) | 0.84 |
| Stroke or TIA | 16 (17.6) | 10 (62.5) | 6 (37.5) | 0.08 |
| Congestive heart failure | 21 (23.1) | 9 (42.9) | 12 (57.1) | 0.99 |
| Peripheral arterial disease | 13 (14.3) | 7 (53.9) | 6 (46.1) | 0.79 |
| HIV or AIDS | 3 (3.3) | 0 (0.0) | 3 (100.0) | 0.26 |
| Laboratory tests | ||||
| 25(OH)D level (ng/ml) | 18.8 ± 9.6 | 11.1 ± 1.9 | 24.5 ± 9.0 | |
| Calcium (mg/dl) | 8.8 ± 0.8 | 8.5 ± 0.9 | 9.0 ± 0.8 | <0.001 |
| Phosphorus (mg/dl) | 5.3 ± 2.0 | 5.8 ± 1.8 | 5.0 ± 2.1 | 0.05 |
| intact PTH (pg/dl) | 478.5 ± 537.09 | 569.9 ± 693.5 | 409.9 ± 373.6 | 0.16 |
| Albumin (g/dl) | 3.9 ± 0.4 | 3.9 ± 0.1 | 3.9 ± 0.1 | 0.74 |
| Alkaline Phosphatase (U/L) | 143.0 ± 113.0 | 164.9 ± 53.0 | 126.5 ± 66.7 | 0.15 |
| Hemoglobin (g/l) | 12.1 ± 1.4 | 12.0 ± 1.5 | 12.2 ± 1.3 | 0.63 |
| Total Cholesterol (mg/dl) | 154.4 ± 32.8 | 160.6 ± 35.6 | 149.7 ± 29.9 | 0.13 |
| Triglycerides (mg/dl) | 133.2 ± 75.3 | 135.6 ± 66.0 | 131.3 ± 82.6 | 0.79 |
| HDL cholesterol (mg/dl) | 48.1 ± 19.4 | 44.4 ± 15.8 | 51.0 ± 21.6 | 0.12 |
| LDL cholesterol (mg/dl) | 79.1 ± 31.8 | 87.1 ± 36.5 | 72.8 ± 26.3 | 0.04 |
Results are expressed as number (%) or mean ± standard deviation
*KT/V = dialysis adequacy
Table 2.
Characteristics of African American participants by vascular access type
| Characteristic | Arteriovenous fistula
(AVF) |
Arteriovenous graft
(AVG) |
Catheter | |
|---|---|---|---|---|
| N = 38 | N = 44 | N = 9 | p value | |
| Age (years) | 57.2 ± 13.0 | 60.8 ± 12.4 | 60.7 ± 9.6 | 0.41 |
| Sex | 0.07 | |||
| Female | 13 (34.2) | 26 (29.9) | 4 (44.4) | |
| Male | 25 (65.8) | 18 (40.9) | 5 (55.6) | |
| Dialysis vintage (yrs) | 4.9 ± 3.4 | 6.4 ± 4.5 | 6.1 ± 5.2 | 0.25 |
| KT/V* | 1.5 ± 0.4 | 1.4 ± 0.3 | 1.4 ± 0.2 | 0.49 |
| Body mass index (kg/m2) | 0.67 | |||
| Under (<18.5) | 0 (0.0) | 0 (0.0) | 1 (11.1) | |
| Normal (18.5 to <25) | 10 (28.6) | 13 (30.9) | 0 (0.0) | |
| Overweight (25 to <30) | 13 (37.1) | 18 (42.9) | 6 (66.7) | |
| Obese (> = 30) | 12 (34.3) | 11 (26.2) | 2 (22.2) | |
| Smoking status | 0.45 | |||
| Never | 21 (55.3) | 31 (70.5) | 3 (33.3) | |
| Former | 8 (21.0) | 10 (22.7) | 5 (55.6) | |
| Current | 9 (23.7) | 3 (6.8) | 1 (11.1) | |
| Activated vitamin D treatment | ||||
| 29 (76.3) | 34 (77.3) | 8 (88.9) | 0.70 | |
| Comorbidities | ||||
| Diabetes | 13 (34.2) | 26 (59.1) | 6 (66.7) | 0.02 |
| Hypertension | 37 (97.4) | 44 (100.0) | 8 (88.9) | 0.48 |
| Stroke or TIA | 2 (5.3) | 11 (25.0) | 3 (33.3) | 0.03 |
| Congestive heart failure | 10 (26.3) | 10 (22.7) | 1 (11.1) | 0.62 |
| Peripheral arterial disease | 4 (10.5) | 8 (18.2) | 2 (22.2) | 0.60 |
| HIV or AIDS | 0 (0.0) | 1 (2.3) | 2 (22.2) | <0.01 |
| Laboratory tests | ||||
| 25(OH)D level (ng/ml) | 21.6 ± 11.3 | 16.7 ± 7.3 | 17.1 ± 9.7 | 0.05 |
| Calcium (mg/dl) | 8.9 ± 0.8 | 8.7 ± 0.9 | 8.8 ± 1.1 | 0.65 |
| Phosphorus (mg/dl) | 5.5 ± 2.2 | 5.1 ± 1.6 | 5.5 ± 2.7 | 0.63 |
| intact PTH (pg/dl) | 401.6 ± 268.2 | 515.1 ± 693.0 | 624.4 ± 668.8 | 0.44 |
| Albumin (g/dl) | 4.1 ± 0.4 | 6.1 ± 0.2 | 3.7 ± 0.2 | 0.63 |
| Alkaline Phosphatase (U/L) | 115.4 ± 57.0 | 171.2 ± 146.6 | 120.9 ± 67.1 | 0.07 |
| Hemoglobin (g/l) | 12.13 ± 1.1 | 12.1 ± 1.6 | 11.0 ± 1.6 | 0.04 |
| Total Cholesterol (mg/dl) | 152.1 ± 31.2 | 153.7 ± 31.3 | 168.9 ± 46.4 | 0.42 |
| Triglycerides (mg/dl) | 132.4 ± 68.5 | 136.3 ± 82.1 | 121.4 ± 77.5 | 0.88 |
| HDL cholesterol (mg/dl) | 47.4 ± 20.5 | 48.6 ± 17.1 | 48.9 ± 27.2 | 0.96 |
| LDL cholesterol (mg/dl) | 79.3 ± 28.7 | 75.4 ± 32.3 | 95.6 ± 41.4 | 0.27 |
Results are expressed as number (%) or mean ± standard deviation
*KT/V = dialysis adequacy
Serum 25(OH) D Concentrations
Mean 25(OH) D concentration was 18.8 ± 9.6 ng/ml. Thirty-nine (42.9%) subjects had a 25(OH) D concentration <15 ng/ml (Table 1). The mean serum 25(OH) D concentration of subjects in the higher (≥15 ng/ml) and lower (<15 ng/ml) vitamin D category were 24.5 ± 9.0 ng/ml and 11.1 ± 1.9 ng/ml, respectively.
Serum Inflammatory Markers
Continuous 25(OH) D
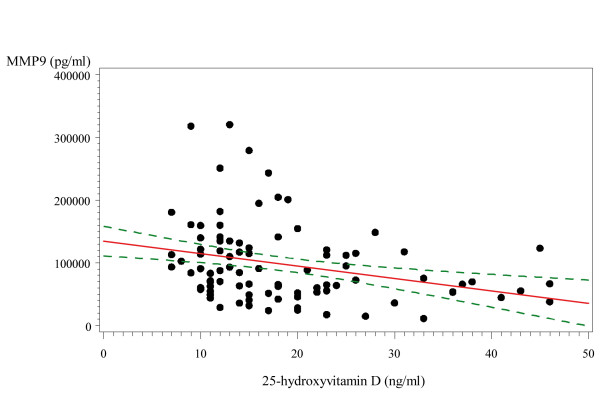
25(OH) D concentration was inversely and significantly correlated with MMP-9 concentration (r = -0.29, p = 0.004) (Figure 1), while the remaining biomarkers were not associated with 25(OH) D by correlation (Pearson) analysis.
Figure 1.
Correlation between serum 25(OH)D and MMP-9 concentration. Pearson correlation coefficient -0.29, P value = 0.004 Note: Continuous line represents regression line and dashed lines represent 95% confidence intervals
Categorical 25(OH) D
The distribution of serum inflammatory markers by 25(OH) D concentration are shown in Table 3. In the univariate analyses and compared to patients with 25(OH)D ≥15 ng/ml, MMP-9 and CRP concentrations were significantly greater among subjects with 25(OH)D < 15 ng/ml. The remaining inflammatory markers were not associated with 25(OH)D categories.
Table 3.
Distribution of inflammatory factors by serum 25(OH)D level
| All patients | 25(OH) D <15 ng/ml | 25(OH) D ≥ 15 ng/ml | P value | |
|---|---|---|---|---|
| TNF- α (pg/ml) | 8.7 ± 3.5 | 8.1 ± 2.9 | 9.0 ± 3.9 | 0.2 |
| MMP-9 (pg/ml) | 97517 ± 66188 | 115566 ± 66626 | 83981 ± 59392 | 0.01 |
| MMP-2 (pg/ml) | 548224 ± 156828 | 537353 ± 182886 | 488938 ± 165330 | 0.2 |
| CRP (mg/L) | 8.8 ± 16.0 | 10.5 ± 19.0 | 7.7 ± 13.0 | 0.03 |
| IL-10 (pg/ml) | 0.5 ± 0.6 | 0.4 ± 0.4 | 0.5 ± 0.6 | 0.6 |
Results are expressed as mean ± standard deviation
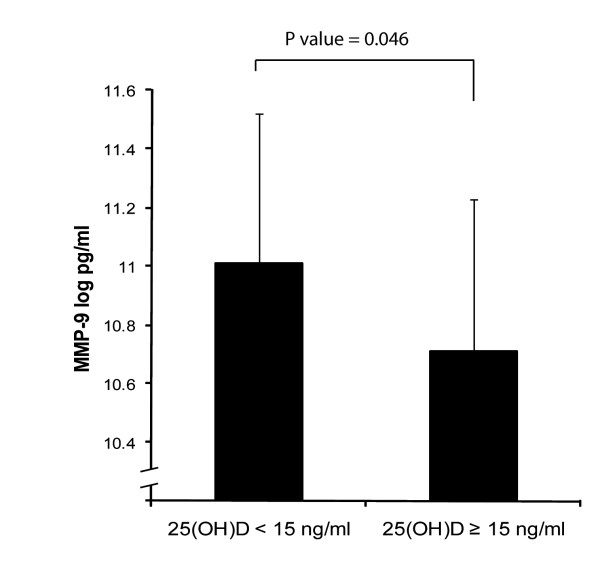
We compared the difference in MMP-9 concentration between the lowest and highest 25(OH) D groups (Figure 2). Compared to the highest group and after adjustment for smoking, gender, BMI, age, diabetes, season and activated vitamin D treatment, the log MMP-9 concentration was greater in the lowest 25(OH)D group (P = 0.046). For every unit increase in 25(OH) D concentration, there was a log decrease in pro-inflammatory MMP-9 concentration of 0.018 pg/ml (p = 0.03), while there was a log increase in anti-inflammatory IL-10 concentration of 0.02 pg/ml (p = 0.04) (Table 4).
Figure 2.
Difference in Log Concentration of MMP-9 by 25(OH)D group
Table 4.
Adjusted* multivariate associations between 25(OH) D concentration and inflammatory markers
| Model 1 | Model 1 + vitamin D treatment
adjustment |
Model 1 + vitamin D treatment + season
adjustment |
||||
|---|---|---|---|---|---|---|
|
Beta coefficient ± standard
error |
P value |
Beta coefficient ± standard
error |
P value |
Beta coefficient ± standard
error |
P value | |
| Log TNF-α (pg/ml) | -0.003 ± 0.006 | 0.5 | -0.002 ± 0.006 | 0.6 | -0.003 ± 0.006 | 0.5 |
| Log MMP-9 (pg/ml) | -0.017 ± 0.008 | 0.04 | -0.019 ± 0.008 | 0.02 | -0.018 ± 0.008 | 0.03 |
| Log MMP-2 (pg/ml) | -0.003 ± 0.004 | 0.5 | -0.004 ± 0.004 | 0.3 | -0.004 ± 0.004 | 0.3 |
| Log CRP (mg/ml) | -0.013 ± 0.015 | 0.3 | -0.014 ± 0.014 | 0.4 | -0.013 ± 0.014 | 0.3 |
| Log IL-10 (pg/ml) | 0.022 ± 0.010 | 0.04 | 0.021 ± 0.010 | 0.04 | 0.021 ± 0.010 | 0.04 |
Model 1 is adjusted for smoking, gender, BMI, age, and diabetes
Upon stratification of vascular access type (arteriovenous fistula (AVF) vs. arteriovenous graft (AVG)), it appears that the association between 25(OH) D concentration and MMP-9 was mostly driven by AVG's, as 25(OH)D and MMP-9 and IL-10 no longer remained associated among AVF users. In contr ast, for every unit increase in 25(OH) D concentration, the log concentration of MMP-9 decreased by 0.052 pg/ml (P = 0.002) among patients using an AVG, while the association of IL-10 to 25(OH) D no longer remained significant among AVG users (Table 5).
Table 5.
Adjusted* associations between vitamin D level and inflammatory markers, stratified by vascular groups
| Arteriovenous fistula | P value | Arteriovenous graft | P value | |
|---|---|---|---|---|
| Log MMP-9 (pg/ml) | 0.004 ± 0.010 | 0.7 | -0.052 ± 0.016 | 0.002 |
| Log IL-10 (pg/ml) | 0.016 ± 0.010 | 0.1 | 0.041 ± 0.023 | 0.08 |
*Adjusted for smoking, gender, BMI, age, diabetes, season and activated vitamin D treatment
Results are expressed as beta coefficient + standard error
Discussion
Our results indicate that plasma MMP-9 expression is inversely and independently associated with circulating 25(OH) D concentration among ESRD patients. We also found anti-inflammatory IL-10 concentration to be associated with 25(OH) D concentration. This is the first report to our knowledge to assess the relationship of 25(OH)D concentration with MMP-9 and IL-10 in an ESRD population, and may help to shed light on mechanisms by which vitamin D deficiency is associated with an increased risk of atherosclerosis and cardiovascular disease.
Our results are consistent with previous data among non-ESRD populations that report a strong association between 25(OH) D deficiency and elevated markers of vascular remodeling: Vitamin D supplementation of vitamin D deficient, healthy British Bangladeshi adults resulted in significant reductions in plasma MMP-9 and serum CRP concentrations [20]. Vitamin D supplementation of congestive heart failure patients significantly increased serum concentrations of anti-inflammatory cytokine IL-10 and suppressed TNF-α synthesis over a nine month period [6]. Further, previous reports show that anti-inflammatory IL-10 is increased by vitamin D supplementation in vitro and in mice [11]. Vitamin D acts on dendritic cells, T-cells, and B-cells to enhance IL-10 expression [21-23].
While others have reported reductions in CRP concentrations following vitamin D supplementation [5,6,24], and negative correlations between serum 25(OH)D concentration and TNF-α concentrations [4], this was not observed in our study, possibly due to our sample size or that a greater differential in 25(OH)D concentration may be needed in order to observe an association with these biomarkers. Whilst we identified associations between both MMP-9 and IL-10 with 25(OH) D concentration, we also observed several important negative findings in this cross-sectional study following multivariate adjustment. First, while PTH concentrations were greater among patients with 25(OH)D < 15 ng/dL, they were not significantly different among the vitamin D cohorts, as we would have expected to observe, possibly due to our relatively small sample size. Second, there was no significant association observed between 25(OH) D and CRP, the most common biomarker of inflammation. This may be due to our relatively small sample size, however, other cross-sectional reports among non-ESRD populations have also failed to find an association between 25(OH)D concentration and CRP concentration following multivariate analysis, supporting our conclusion [4,14,18]. We did not observe elevations in MMP-9 and CRP concentrations as large as those reported previously in comparable patients [25], likely because MMP-9 gene expression is upregulated by several factors that were not included in our analysis. Finally, while MMP-2 concentrations tended to be higher with 25(OH)D concentrations <15 ng/dL, this did not reach significance, likely due to our sample size.
MMP's promote the remodeling of connective tissue and basement membranes via degradation of collagen, and act as important regulatory molecules in inflammation and vascular diseases. MMP-9 is released by neutrophils and is a key effector molecule of inflammatory cells, aiding migration of inflammatory leukocytes through tissue barriers, lysing protein substrates, modulating smooth muscle cell migration, and promoting angiogenesis [26,27]. MMP's also regulate inflammation by directly and indirectly acting on pro-inflammatory cytokines, such as TNF-α and TGF-β, to control chemokine activity [27]. 25(OH)D may act to reduce MMP-9 in several ways. An increase in IL-10 concentration, as we observed, may also act to suppress MMP-9 secretion [28]. Pulmonary tuberculosis in vitro studies suggest that vitamin D blunts MMP-9 expression by inhibition of c-Jun-N-terminal kinase(JNK) and NFkappaB signaling cascades [9]. MMP-9 concentrations are increased in the setting of several chronic inflammatory diseases, and MMP elevations are associated with abdominal aortic aneurysm rupture [29] and acute myocardial infarction [30]. These findings suggest that the risk for acquiring or further progression of these disorders may be positively impacted by limiting MMP concentrations.
Another important finding of our study is that vascular access type modifies the relationship between plasma MMP-9 and circulating 25(OH) D concentrations. When we stratified AVF versus AVG, the association between MMP-9 and 25(OH) D concentrations no longer remained significant among AVF patients, whereas the relationship remained significant among patients using an AVG, suggesting that MMP-9 concentrations in AVF patients were too low for a relationship with 25(OH)D to be detected. This may occur because the prosthetic material within an AVG may induce increased systemic inflammation. This finding is supported by data examining the role of vascular access type on systemic inflammation among prevalent HD patients, which noted that CRP concentrations were significantly lower among autogenous AVF users compared with patients using a prosthetic AVG [31].
Limitations of this study should be considered. First, our relatively small sample size may affect our results, however the 25(OH) D concentrations observed among our subjects are comparable to those reported among larger ESRD patient cohorts, and the observed inverse association between 25(OH) D and MMP-9 has been reported in a larger, non-renal patient cohort [18]. Second, the cross-sectional design of our study does not allow for causal inference. We cannot exclude the possibility that an association exists between 25(OH) D and the additional inflammatory biomarkers examined in our study, but due to our relatively small sample size, we may be underpowered to detect it. For example, the power to detect a statistical difference, if any, between 25(OH)D groups was less than 50% for TNF-α and MMP-2. Third, although we have attempted to reduce the effect of confounding by limiting our study population to include only patients free of infection or inflammation, residual confounding inherent to the cross-sectional design cannot be excluded.
Conclusions
In conclusion, the results of our study reveal a new finding of a significant inverse relationship between plasma MMP-9 and circulating 25(OH) D concentrations among AA ESRD patients, and suggest that there may be value in measuring 25(OH) D concentrations in ESRD patients. In addition, we observed a positive association between 25(OH)D concentration and anti-inflammatory IL-10 concentration. Our findings extend data obtained from previous studies in linking low vitamin 25(OH)D concentrations to elevated concentrations of the cardiovascular risk marker, circulating MMP-9, in ESRD patients. Given the high prevalence of nutritional vitamin D deficiency and cardiovascular disease among ESRD patients, our findings suggest that future studies are warranted which further characterize the relationship between nutritional vitamin D therapy and vascular remodeling biomarker synthesis.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
HW: design of protocol, evaluation of data, draft of the manuscript. IG: statistical analyses, collaborating in drafting of the manuscript. FC: data collection, preparation of database. CDE: laboratory analyses, immunoassays. CH: laboratory analyses, immunoassays. EV: preparation of database, statistical analyses. All authors read and approved the final manuscript
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Haimanot Wasse, Email: hwasse@emory.edu.
Francesca Cardarelli, Email: francesca.cardarelli@libero.it.
Christine De Staercke, Email: cld7@CDC.GOV.
Craig Hooper, Email: chooper@cdc.gov.
Emir Veledar, Email: eveleda@emory.edu.
Idris Guessous, Email: idris.guessous@chuv.ch.
Acknowledgements
This study was supported by an NIH K23 Grant DK65634 (H.W.), a Davita Clinical Research Grant (H.W.), and a PHS Grant (UL1 RR02008, KL2 RR025009 or TL1 RR025010) from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources. Additional support (I.G.) was provided by a grant from the Swiss Foundation for Science (33CM30-124087).
References
- Segarra A, Chacon P, Martinez-Eyarre C, Argelaguer X, Vila J, Ruiz P, Fort J, Bartolome J, Camps J, Moliner E. et al. Circulating levels of plasminogen activator inhibitor type-1, tissue plasminogen activator, and thrombomodulin in hemodialysis patients: biochemical correlations and role as independent predictors of coronary artery stenosis. J Am Soc Nephrol. 2001;12:1255–1263. doi: 10.1681/ASN.V1261255. [DOI] [PubMed] [Google Scholar]
- Yeun JY, Levine RA, Mantadilok V, Kaysen GA. C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2000;35:469–476. doi: 10.1016/S0272-6386(00)70200-9. [DOI] [PubMed] [Google Scholar]
- Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Fermo I, Foca A, Paroni R, Malatino LS. Inflammation is associated with carotid atherosclerosis in dialysis patients. Creed Investigators. Cardiovascular Risk Extended Evaluation in Dialysis Patients. J Hypertens. 2000;18:1207–1213. doi: 10.1097/00004872-200018090-00006. [DOI] [PubMed] [Google Scholar]
- Peterson CA, Heffernan ME. Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J Inflamm (Lond) 2008;5:10. doi: 10.1186/1476-9255-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berghe G, Van Roosbroeck D, Vanhove P, Wouters PJ, De Pourcq L, Bouillon R. Bone turnover in prolonged critical illness: effect of vitamin D. J Clin Endocrinol Metab. 2003;88:4623–4632. doi: 10.1210/jc.2003-030358. [DOI] [PubMed] [Google Scholar]
- Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- Aziz F, Kuivaniemi H. Role of matrix metalloproteinase inhibitors in preventing abdominal aortic aneurysm. Ann Vasc Surg. 2007;21:392–401. doi: 10.1016/j.avsg.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese RS, Rao F, Khandrika S, Thomas B, Ziegler MG, Schmid-Schonbein GW, O'Connor DT. Matrix metalloproteinases: discrete elevations in essential hypertension and hypertensive end-stage renal disease. Clin Exp Hypertens. 2009;31:521–533. doi: 10.3109/10641960802668730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar-Shany K, Ravid A, Koren R. Upregulation of MMP-9 production by TNFalpha in keratinocytes and its attenuation by vitamin D. J Cell Physiol. 2010;222:729–737. doi: 10.1002/jcp.22004. [DOI] [PubMed] [Google Scholar]
- Anand SP, Selvaraj P. Effect of 1, 25 dihydroxyvitamin D(3) on matrix metalloproteinases MMP-7, MMP-9 and the inhibitor TIMP-1 in pulmonary tuberculosis. Clin Immunol. 2009;133:126–131. doi: 10.1016/j.clim.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Coussens A, Timms PM, Boucher BJ, Venton TR, Ashcroft AT, Skolimowska KH, Newton SM, Wilkinson KA, Davidson RN, Griffiths CJ. et al. 1alpha,25-dihydroxyvitamin D3 inhibits matrix metalloproteinases induced by Mycobacterium tuberculosis infection. Immunology. 2009;127:539–548. doi: 10.1111/j.1365-2567.2008.03024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetlow LC, Woolley DE. Expression of vitamin D receptors and matrix metalloproteinases in osteoarthritic cartilage and human articular chondrocytes in vitro. Osteoarthritis Cartilage. 2001;9:423–431. doi: 10.1053/joca.2000.0408. [DOI] [PubMed] [Google Scholar]
- Rahman A, Hershey S, Ahmed S, Nibbelink K, Simpson RU. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J Steroid Biochem Mol Biol. 2007;103:416–419. doi: 10.1016/j.jsbmb.2006.12.081. [DOI] [PubMed] [Google Scholar]
- Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA Jr, Tonelli M, Thadhani R. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-Hydroxyvitamin D Levels Inversely Associate with Risk for Developing Coronary Artery Calcification. J Am Soc Nephrol. 2009. [DOI] [PMC free article] [PubMed]
- Wang Y, Krishnamoorthy M, Banerjee R, Zhang J, Rudich S, Holland C, Arend L, Roy-Chaudhury P. Venous stenosis in a pig arteriovenous fistula model--anatomy, mechanisms and cellular phenotypes. Nephrol Dial Transplant. 2008;23:525–533. doi: 10.1093/ndt/gfm547. [DOI] [PubMed] [Google Scholar]
- Timms PM, Mannan N, Hitman GA, Noonan K, Mills PG, Syndercombe-Court D, Aganna E, Price CP, Boucher BJ. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM. 2002;95:787–796. doi: 10.1093/qjmed/95.12.787. [DOI] [PubMed] [Google Scholar]
- Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O'Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adorini L. Intervention in autoimmunity: the potential of vitamin D receptor agonists. Cell Immunol. 2005;233:115–124. doi: 10.1016/j.cellimm.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Heine G, Niesner U, Chang HD, Steinmeyer A, Zugel U, Zuberbier T, Radbruch A, Worm M. 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur J Immunol. 2008;38:2210–2218. doi: 10.1002/eji.200838216. [DOI] [PubMed] [Google Scholar]
- Alborzi P, Patel NA, Peterson C, Bills JE, Bekele DM, Bunaye Z, Light RP, Agarwal R. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double-blind pilot trial. Hypertension. 2008;52:249–255. doi: 10.1161/HYPERTENSIONAHA.108.113159. [DOI] [PubMed] [Google Scholar]
- Preston GA, Barrett CV, Alcorta DA, Hogan SL, Dinwiddie L, Jennette JC, Falk RJ. Serum matrix metalloproteinases MMP-2 and MMP-3 levels in dialysis patients vary independently of CRP and IL-6 levels. Nephron. 2002;92:817–823. doi: 10.1159/000065464. [DOI] [PubMed] [Google Scholar]
- Jorde R, Haug E, Figenschau Y, Hansen JB. Serum levels of vitamin D and haemostatic factors in healthy subjects: the Tromso study. Acta Haematol. 2007;117:91–97. doi: 10.1159/000097383. [DOI] [PubMed] [Google Scholar]
- Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Lacraz S, Nicod LP, Chicheportiche R, Welgus HG, Dayer JM. IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. J Clin Invest. 1995;96:2304–2310. doi: 10.1172/JCI118286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WR, Anderton M, Choke EC, Dawson J, Loftus IM, Thompson MM. Elevated plasma MMP1 and MMP9 are associated with abdominal aortic aneurysm rupture. Eur J Vasc Endovasc Surg. 2008;35:580–584. doi: 10.1016/j.ejvs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Hlatky MA, Ashley E, Quertermous T, Boothroyd DB, Ridker P, Southwick A, Myers RM, Iribarren C, Fortmann SP, Go AS. Matrix metalloproteinase circulating levels, genetic polymorphisms, and susceptibility to acute myocardial infarction among patients with coronary artery disease. Am Heart J. 2007;154:1043–1051. doi: 10.1016/j.ahj.2007.06.042. [DOI] [PubMed] [Google Scholar]
- Movilli E, Brunori G, Camerini C, Vizzardi V, Gaggia P, Cassamali S, Scolari F, Parrinello G, Cancarini GC. The kind of vascular access influences the baseline inflammatory status and epoetin response in chronic hemodialysis patients. Blood Purif. 2006;24:387–393. doi: 10.1159/000093681. [DOI] [PubMed] [Google Scholar]