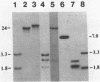
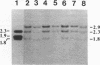
Abstract
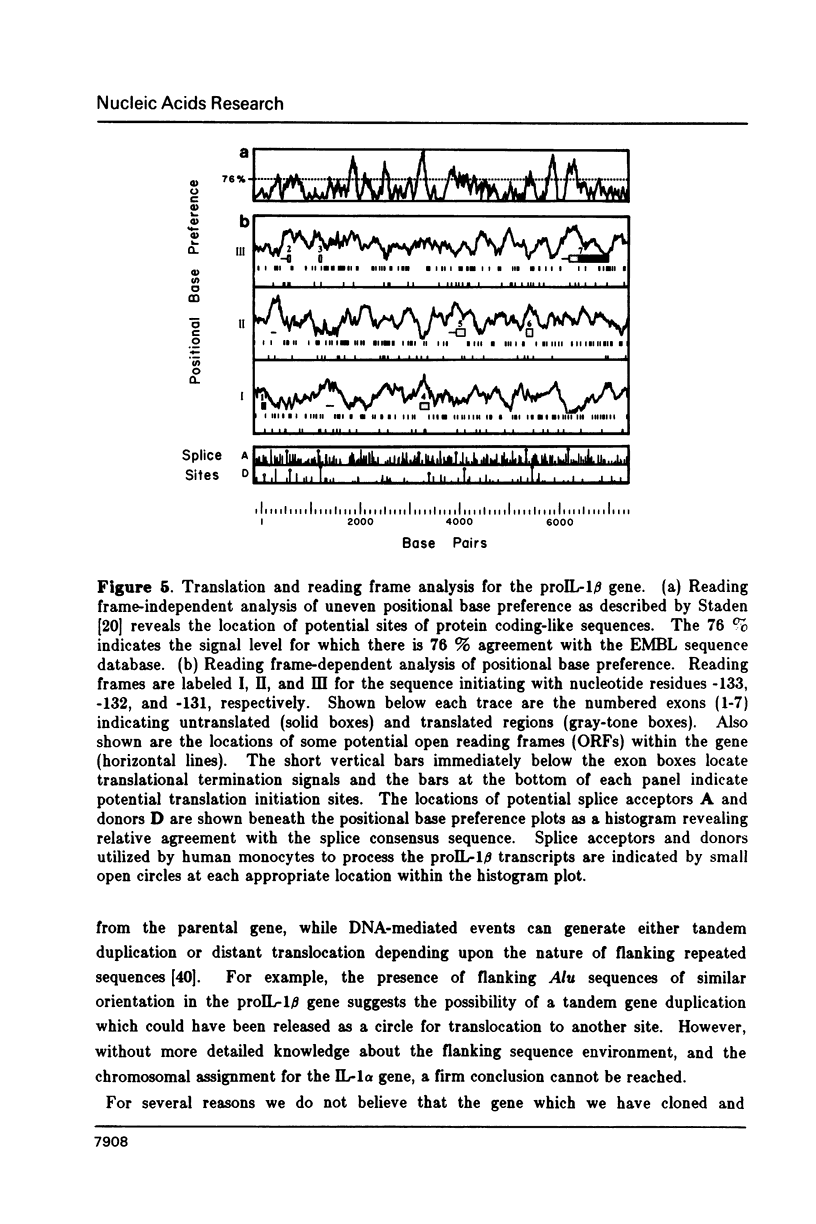
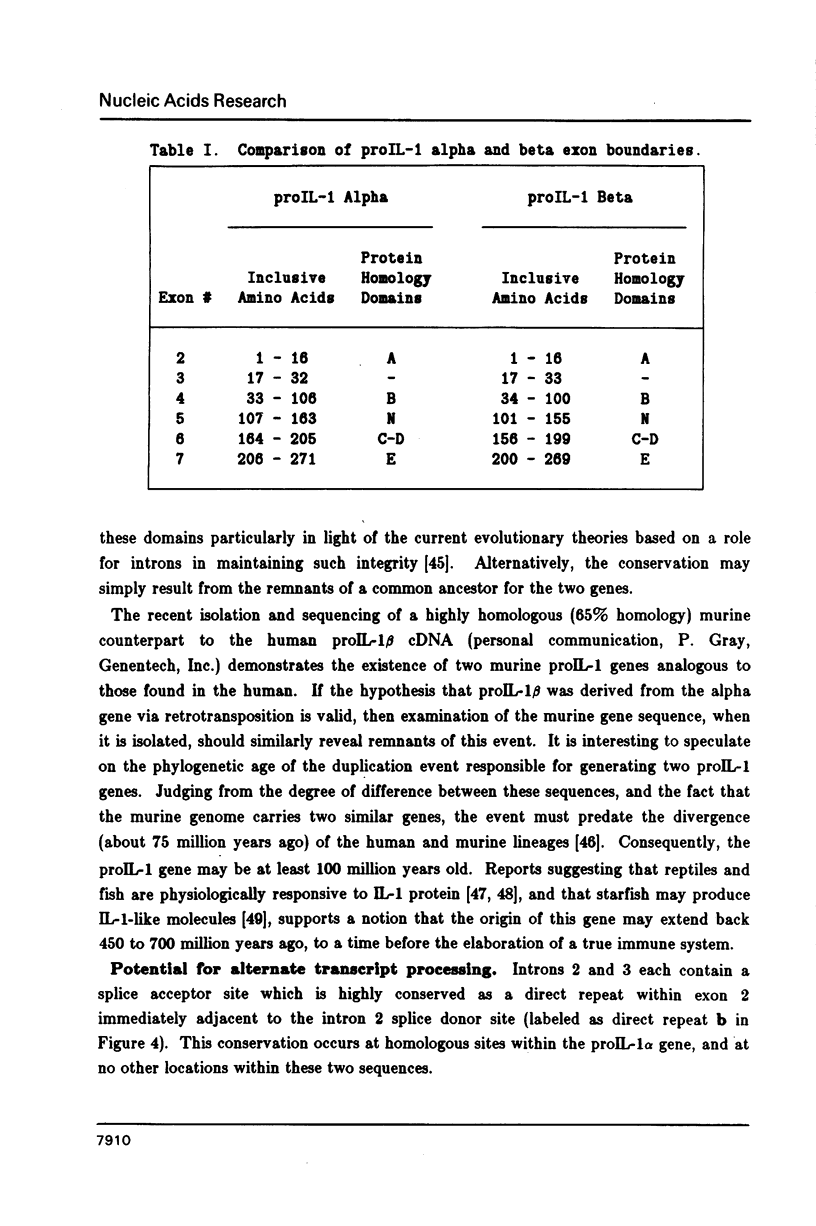
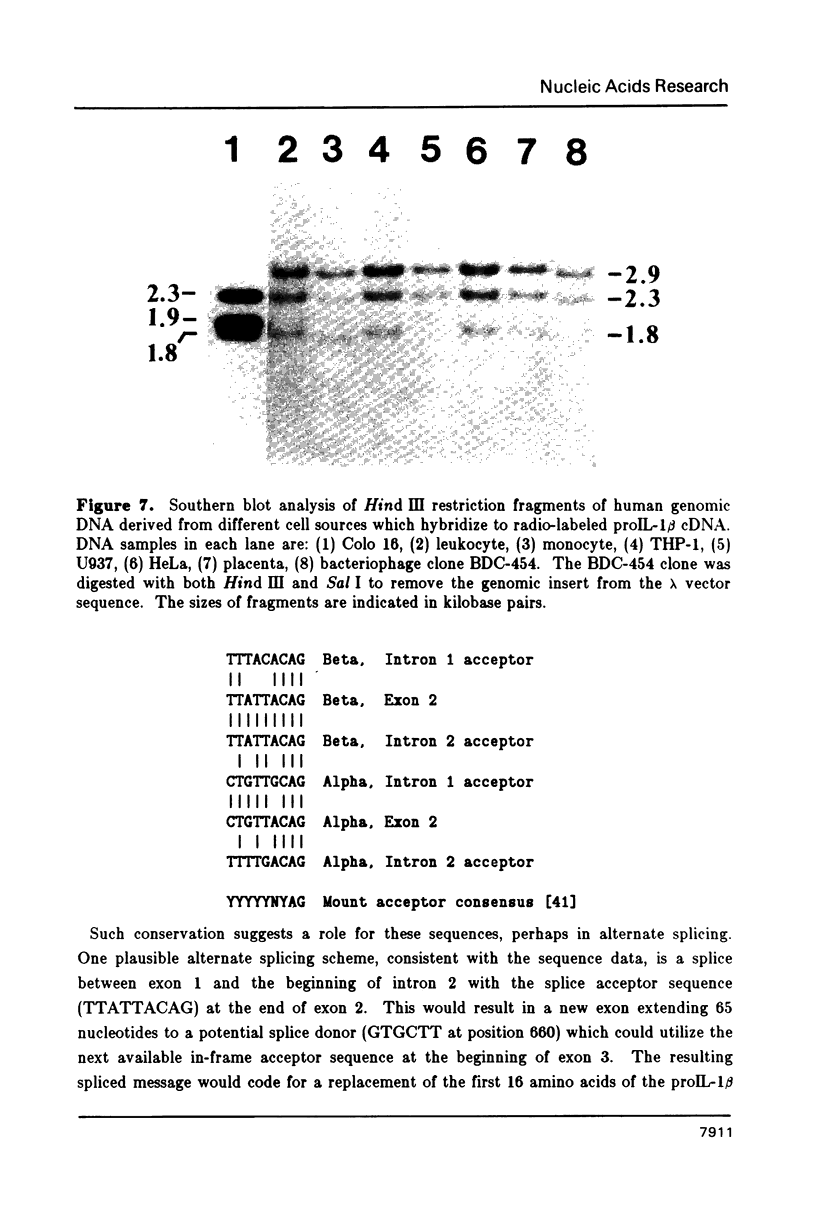
We have isolated the human prointerleukin 1 (proIL-1) beta gene from leukocyte and fetal liver libraries. The nucleotide sequence and its gene organization reveals that the proIL-1 beta gene is composed of seven exons with a primary transcription product length of 7,008 nucleotides. The exon sequence agrees well with that of the human proIL-1 beta cDNA. Features of interest within the transcriptional unit include positioned TATA, CAT, and poly-adenylation signals for gene regulation, as well as the signatures of gene duplication via retrotransposition in the form of flanking direct repeats and a genomic poly A tail. The genomic organization of the proIL-1 beta gene with respect to the number and position of exon boundaries is strikingly similar to that of the recently reported human proIL-1 alpha gene. Therefore, we hypothesize that the proIL-1 beta may have arisen by a reverse transcriptase mediated duplication of the related alpha gene.
Full text
PDF



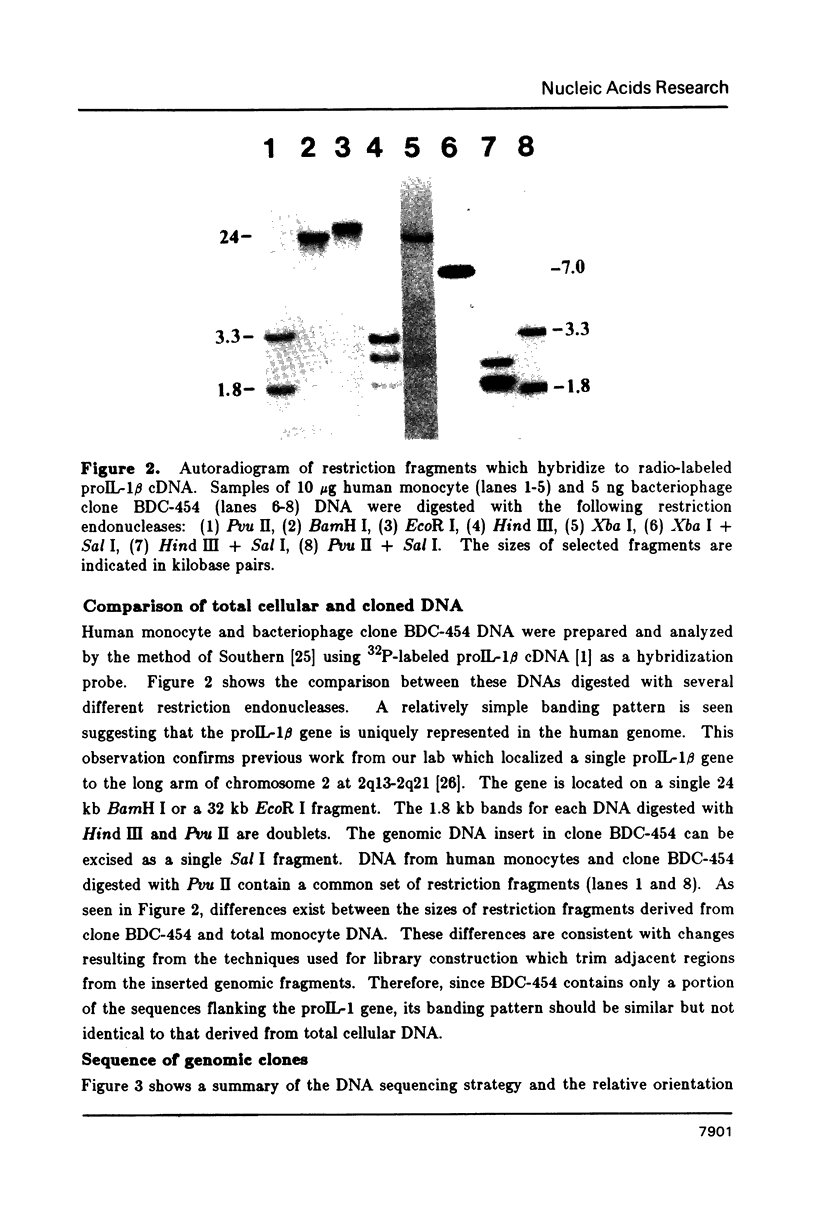
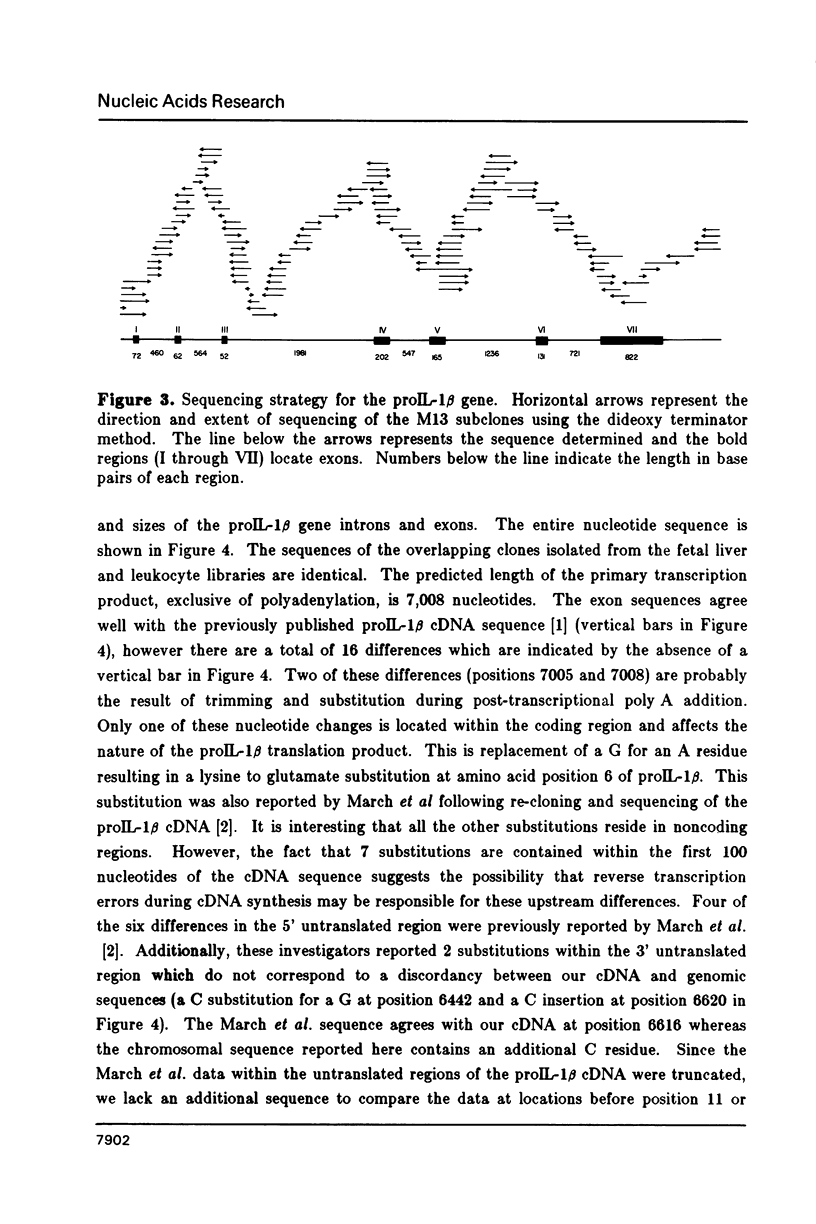









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auron P. E., Rosenwasser L. J., Matsushima K., Copeland T., Dinarello C. A., Oppenheim J. J., Webb A. C. Human and murine interleukin 1 possess sequence and structural similarities. J Mol Cell Immunol. 1985;2(3):169–177. [PubMed] [Google Scholar]
- Auron P. E., Webb A. C., Rosenwasser L. J., Mucci S. F., Rich A., Wolff S. M., Dinarello C. A. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battey J., Max E. E., McBride W. O., Swan D., Leder P. A processed human immunoglobulin epsilon gene has moved to chromosome 9. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5956–5960. doi: 10.1073/pnas.79.19.5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bernheim H. A., Kluger M. J. Endogenous pyrogen-like substance produced by reptiles. J Physiol. 1977 Jun;267(3):659–666. doi: 10.1113/jphysiol.1977.sp011831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly J. J., Vogel S. N., Prendergast R. A. Down-regulation of Ia expression on macrophages by sea star factor. Cell Immunol. 1985 Feb;90(2):408–415. doi: 10.1016/0008-8749(85)90205-9. [DOI] [PubMed] [Google Scholar]
- Dower S. K., Call S. M., Gillis S., Urdal D. L. Similarity between the interleukin 1 receptors on a murine T-lymphoma cell line and on a murine fibroblast cell line. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1060–1064. doi: 10.1073/pnas.83.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Fontana A., Grob P. J. Lymphokines and the brain. Springer Semin Immunopathol. 1984;7(4):375–386. doi: 10.1007/BF00201967. [DOI] [PubMed] [Google Scholar]
- Furutani Y., Notake M., Fukui T., Ohue M., Nomura H., Yamada M., Nakamura S. Complete nucleotide sequence of the gene for human interleukin 1 alpha. Nucleic Acids Res. 1986 Apr 25;14(8):3167–3179. doi: 10.1093/nar/14.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutani Y., Notake M., Yamayoshi M., Yamagishi J., Nomura H., Ohue M., Furuta R., Fukui T., Yamada M., Nakamura S. Cloning and characterization of the cDNAs for human and rabbit interleukin-1 precursor. Nucleic Acids Res. 1985 Aug 26;13(16):5869–5882. doi: 10.1093/nar/13.16.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Folsom V., Tonegawa S. Cell type-specific enhancer element associated with a mouse MHC gene, E beta. Nature. 1984 Aug 16;310(5978):594–597. doi: 10.1038/310594a0. [DOI] [PubMed] [Google Scholar]
- Hamby B. A., Huggins E. M., Jr, Lachman L. B., Dinarello C. A., Sigel M. M. Fish lymphocytes respond to human IL-1. Lymphokine Res. 1986 Spring;5(2):157–162. [PubMed] [Google Scholar]
- Hollis G. F., Hieter P. A., McBride O. W., Swan D., Leder P. Processed genes: a dispersed human immunoglobulin gene bearing evidence of RNA-type processing. Nature. 1982 Mar 25;296(5855):321–325. doi: 10.1038/296321a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Harris S. Processes of gene duplication. Nature. 1982 Mar 4;296(5852):9–10. doi: 10.1038/296009a0. [DOI] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Karin M., Richards R. I. Human metallothionein genes--primary structure of the metallothionein-II gene and a related processed gene. Nature. 1982 Oct 28;299(5886):797–802. doi: 10.1038/299797a0. [DOI] [PubMed] [Google Scholar]
- Keller E. B., Noon W. A. Intron splicing: a conserved internal signal in introns of animal pre-mRNAs. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7417–7420. doi: 10.1073/pnas.81.23.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Lewis S. A., Wilde C. D., Cowan N. J. Evolutionary history of a multigene family: an expressed human beta-tubulin gene and three processed pseudogenes. Cell. 1983 Jun;33(2):477–487. doi: 10.1016/0092-8674(83)90429-4. [DOI] [PubMed] [Google Scholar]
- Lomedico P. T., Gubler U., Hellmann C. P., Dukovich M., Giri J. G., Pan Y. C., Collier K., Semionow R., Chua A. O., Mizel S. B. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. 1984 Nov 29-Dec 5Nature. 312(5993):458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- Luger T. A., Stadler B. M., Katz S. I., Oppenheim J. J. Epidermal cell (keratinocyte)-derived thymocyte-activating factor (ETAF). J Immunol. 1981 Oct;127(4):1493–1498. [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- McLauchlan J., Gaffney D., Whitton J. L., Clements J. B. The consensus sequence YGTGTTYY located downstream from the AATAAA signal is required for efficient formation of mRNA 3' termini. Nucleic Acids Res. 1985 Feb 25;13(4):1347–1368. doi: 10.1093/nar/13.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P., Cavender D., Ziff M. Production of interleukin 1 by human endothelial cells. J Immunol. 1986 Apr 1;136(7):2486–2491. [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M., Yoshimura N., Ichimori Y., Kishimoto S., Nakai S., Nishino N., Kishimoto T. Immunologic and molecular characterizations of T cell-derived T cell activating factor. J Immunol. 1986 Feb 15;136(4):1288–1294. [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Reed R., Maniatis T. Intron sequences involved in lariat formation during pre-mRNA splicing. Cell. 1985 May;41(1):95–105. doi: 10.1016/0092-8674(85)90064-9. [DOI] [PubMed] [Google Scholar]
- Rosenwasser L. J., Webb A. C., Clark B. D., Irie S., Chang L., Dinarello C. A., Gehrke L., Wolff S. M., Rich A., Auron P. E. Expression of biologically active human interleukin 1 subpeptides by transfected simian COS cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5243–5246. doi: 10.1073/pnas.83.14.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauder D. N., Mounessa N. L., Katz S. I., Dinarello C. A., Gallin J. I. Chemotactic cytokines: the role of leukocytic pyrogen and epidermal cell thymocyte-activating factor in neutrophil chemotaxis. J Immunol. 1984 Feb;132(2):828–832. [PubMed] [Google Scholar]
- Sharp P. A. Conversion of RNA to DNA in mammals: Alu-like elements and pseudogenes. Nature. 1983 Feb 10;301(5900):471–472. doi: 10.1038/301471a0. [DOI] [PubMed] [Google Scholar]
- Soares M. B., Schon E., Henderson A., Karathanasis S. K., Cate R., Zeitlin S., Chirgwin J., Efstratiadis A. RNA-mediated gene duplication: the rat preproinsulin I gene is a functional retroposon. Mol Cell Biol. 1985 Aug;5(8):2090–2103. doi: 10.1128/mcb.5.8.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staden R. Computer methods to locate signals in nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):505–519. doi: 10.1093/nar/12.1part2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Graphic methods to determine the function of nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):521–538. doi: 10.1093/nar/12.1part2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Measurements of the effects that coding for a protein has on a DNA sequence and their use for finding genes. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):551–567. doi: 10.1093/nar/12.1part2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S., Nakai S., Nishida Y., Hisajima H., Honjo T. Long terminal repeat-like elements flank a human immunoglobulin epsilon pseudogene that lacks introns. EMBO J. 1982;1(12):1539–1544. doi: 10.1002/j.1460-2075.1982.tb01352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb A. C., Collins K. L., Auron P. E., Eddy R. L., Nakai H., Byers M. G., Haley L. L., Henry W. M., Shows T. B. Interleukin-1 gene (IL1) assigned to long arm of human chromosome 2. Lymphokine Res. 1986 Spring;5(2):77–85. [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]