Abstract
Insulin‐like growth factor 1 (IGF‐1) is a crucial mediator of body size and bone mass during growth and development. In serum, IGF‐1 is stabilized by several IGF‐1‐binding proteins (IGFBPs) and the acid labile subunit (ALS). Previous research using ALS knockout (ALSKO) mice indicated a growth retardation phenotype, and clinical reports of humans have indicated short stature and low bone mineral density (BMD) in patients with ALS deficiency. To determine the temporal and sex‐specific effects of ALS deficiency on body size and skeletal development during growth, we characterized control and ALSKO mice from 4 to 16 weeks of age. We found that female ALSKO mice had an earlier‐onset reduction in body size (4 weeks) but that both female and male ALSKO mice were consistently smaller than control mice. Interestingly, skeletal analyses at multiple ages showed increased slenderness of ALSKO femurs that was more severe in females than in males. Both male and female ALSKO mice appeared to compensate for their more slender bones through increased bone formation on their endosteal surfaces during growth, but ALSKO females had increased endosteal bone formation compared with ALSKO males. This study revealed age‐ and sex‐specific dependencies of body size and bone size on the ALS. These findings may explain the heterogeneity in growth and BMD measurements reported in human ALS‐deficient patients. © 2010 American Society for Bone and Mineral Research.
Keywords: IGF‐1, bone, transgenic mice, sex, micro–computed tomography, endocrine, mechanical properties
Introduction
During growth and development, insulin‐like growth factor 1 (IGF‐1) is a key regulator of both the amount and distribution of bone tissue formed. Endocrine IGF‐1 is produced largely by the liver under the control of growth hormone (GH) secretion. In serum, IGF‐1 is stabilized by binding to IGF‐1‐binding proteins (IGFBPs) in binary complexes. Further stability is achieved through binding of the acid labile subunit (ALS) to IGFBP‐3/5, generating a ternary complex (of IGF‐1–IGFBP‐3/5–ALS). Human cases of ALS deficiency have now been reported, and it is evident that loss of ALS in both humans and mice leads to marked reductions in serum IGF‐1 and IGFBP‐3 levels in serum.1, 2 Although the skeletal phenotype of ALS‐deficient patients has not been closely monitored, nearly all patients present with mild short stature, and several cases of low bone mineral density (BMD) have been documented,2, 3, 4, 5, 6 suggesting a link between ALS and bone accrual during growth via IGF‐1‐dependent or ‐independent mechanisms. However, partial recovery of BMD at the end of puberty in one case6 and a family history of low BMD in others5 make the connection between ALS and BMD tenuous. Further, ALS patients with normal areal BMD (aBMD) have been reported.3
Preliminary skeletal studies of ALS knockout (ALSKO) male mice support observations in human patients. ALSKO mice showed significant decreases in femoral length by 8 weeks of age, as well as decreased cross‐sectional area and cortical bone volume by 6 weeks of age.7 However, this study did not determine whether reductions in bone size and shape remain in adulthood, alter bone mechanical properties, or are associated with changes in body composition. Furthermore, previous studies of skeletal parameters in ALSKO mice were done at a single time point during growth and therefore were unable to explain the kinetics of transverse and longitudinal growth. Moreover, the reported small reductions in height of ALS patients (−2 to −3 SD)2, 3, 4, 5 and femoral length in ALSKO mice (∼6%)7 could result in significant reductions in bone slenderness and thus increase fracture risk.
To determine the mechanism by which loss of ALS impairs skeletal acquisition from puberty to adulthood, we followed both male and female ALSKO mice longitudinally on a pure genetic background (C57BL/6J) and characterized their bone traits at multiple time points during development. We followed body size and body composition at these time points and performed regression analyses to reveal how different skeletal and physiologic traits covary. Our study sheds new light on skeletal acquisition in a state of IGF‐1 deficiency and leads to a better understanding of the human phenotype.
Methods
Animals
The generation of ALS knockout (ALSKO) mice has been described previously.8 ALSKO mice were backcrossed to C57BL/6J mice for 10 generations at the Jackson Laboratory (Bar Harbor, ME, USA). Both control and ALSKO mice used in this study were littermates produced from heterozygous mating. Groups of 2 to 5 animals were housed in cages, subjected to12‐hour light/dark cycles, and given free access to water and irradiated NIH31 diet (Purina Mills International, Brentwood, MO, USA). Female and male mice were euthanized at 4, 8, 12, and 16 weeks of age for phenotypic analysis. Sixteen‐week‐old animals were injected intraperitoneally with calcein and democycline (10 mg/kg each) at 9 and 2 days before euthanasia, respectively. All procedures involving mice were reviewed and approved by the Institutional Animal Care and Use Committee of the Jackson Laboratory and the Mount Sinai School of Medicine.
Serum parameters
Serum was collected through retroorbital bleeding in the fed state at the indicated ages and immediately frozen until analysis. Osteocalcin levels in serum were measured using a commercial radioimmuno assay (American Laboratory Products Company, Inc., Salem, NH, USA). IGF‐1, GH, and insulin levels in serum were measured using commercial radioimmunoassays, as described previously.7, 9, 10 Serum glucose levels were measured using a OneTouch Ultra 2 blood glucose monitor (Lifescan, Inc., Milpitas, CA, USA).
Body composition
Groups of ALSKO and control female (n = 23 to 43 per group) and male (n = 17 to 40 per group) mice were measured at 4, 8, 12, and 16 weeks for total body weight as well as lean and fat mass using the PIXImus dual‐energy X‐ray densitometer (GE‐Lunar, Madison, WI, USA). The PIXImus was calibrated daily with a mouse phantom provided by the manufacturer. Mice were placed ventral side down with each limb and tail positioned away from the body. Full‐body scans were obtained, and X‐ray absorptiometry data were gathered and processed with manufacturer‐supplied software (Lunar PIXImus 2, Version 2.1). The head area was specifically excluded from all analyses by the PIXImus software.
Bone morphology and tissue mineral density
Femoral morphology of 4‐, 8‐, and 16‐week‐old animals was assessed using an eXplore Locus SP Pre‐Clinical Specimen Micro‐Computed Tomography system (GE Healthcare, London, Ontario, Canada) as described previously.11 Briefly, femurs were reconstructed at an 8.7‐µm voxel resolution, and distal metaphyseal bone volume fraction and microarchitecture, as well as mid‐diaphyseal cortical bone geometry, were computed from the binarized images. All regions of analysis were standardized relative to either the third trochanter (cortical bone) or fusion of the distal growth plate (trabecular bone). Cortical and trabecular regions were thresholded individually using a standard thresholding algorithm that segmented bone voxels from nonbone voxels. Trabecular parameters assessed included bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular spacing (Tb.Sp). Transverse cortical bone measurements included total cross‐sectional area (Tt.Ar), cortical bone area (Ct.Ar), polar moment of inertia (J 0), and marrow area (Ma.Ar). Tissue mineral density (TMD), which represents the average mineral value of the bone voxels expressed in hydroxyapatite (HA) density equivalents, was calculated by converting the grayscale output of bone voxels in hounsfield units (HU) to mineral values (mg/cc of HA) through the use of a calibration phantom containing air, water, and hydroxyapatite (SB3, Gamex RMI, Middleton, WI, USA). To quantify the relative changes in bone growth on different bone surfaces, we measured two indices of trait covariation—relative cortical area (RCA) and robustness. RCA is defined as Ct.Ar/Tt.Ar and is a measure of how much bone tissue is present in the total area of bone. Robustness has been discussed in previous studies of human tibias12, 13 and mouse femurs.11, 14 For the purposes of this study, robustness was defined as Tt.Ar/bone length (Le) and is a ratio that indicates the extent of transverse bone size relative to longitudinal bone size; smaller Tt.Ar/Le values indicate more slender bones.
Cortical bone histomorphometry
Right femurs of 16‐week‐old animals were cleaned of excess connective tissue and embedded in polymethyl methacrylate (PMMA) as described previously.15 For dynamic histomorphometry, embedded samples were sectioned (200 µm thickness) at the mid‐diaphysis immediately distal to the third trochanter using a low‐speed diamond‐coated wafering saw (Leica Microsystems, Inc., Bannockburn, IL, USA). Sections were adhered to acrylic slides using a nonfluorescing acrylic mounting medium, and images of each section (×20 magnification) were obtained using a digital camera (Sony Exwave HAD, 3CCD Camera, Sony, Park Ridge, NJ, USA) attached to a visible light microscope (Zeiss Axioplan2, Zeiss, Thornwood, NY, USA) with a pixel resolution of 2.1 µm. Separately for both the endosteal and periosteal surfaces, bone formation was quantified by labeled surface (L.Pm/B.Pm, %), mineral apposition rate (MAR, µm/day), and bone‐formation rate (BFR/B.Pm, µm/day × 100). All measurements were performed using an OsteoMeasure system (Osteometrics, Atlanta, GA, USA) in accordance with standard protocols.16
Mechanical properties
Left femurs from 12‐week‐old male and female ALSKO and control mice were subjected to mechanical testing at room temperature, with samples kept moist using a PBS solution. Femurs were loaded to failure in four‐point bending at 0.05 mm/s using a servohydraulic materials testing system (Instron Corp, Canton, MA, USA) as described previously.14 Load and midspan deflection were acquired by an A/D system sampling at 25 Hz. Load‐deflection curves were analyzed for stiffness (the initial slope), maximum load (breaking load), postyield deflection (PYD), and work to failure (work). PYD, a measure of bone ductility, was defined as the deflection at failure minus the deflection at yield, where yield was a 10% reduction of secant stiffness (load range normalized for deflection range) relative to the initial stiffness. Work was calculated as the area under the load‐deflection curve. To estimate whole‐bone strength of 8‐ and 16‐week‐old control and ALSKO mice based on morphologic traits, we used a modification of Selker and Carter's17 bone strength index S B, herein defined as J 0/(Tt.Ar/Le).
Statistical analysis
Means and SDs for each trait were determined separately for each strain and sex. Significant differences in mean trait values among factors (eg, strain, age, sex, bone surface) were identified using multifactor ANOVA and Bonferroni post hoc tests (p < .05; Statistica 6.0, Statsoft, Tulsa, OK, USA). To determine if sex and genotype were independent of each other for a given trait, interaction effects were calculated from ANOVA (Statistica 6.0, Statsoft). To describe the bivariate relationship of morphologic traits during growth, linear regressions were performed. Where appropriate, nonzero slopes (F test, p < .05) as well as significant differences in slopes among strains and sexes (ANCOVA, p < .05) were quantified (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Serum parameters
Serum levels of IGF‐1 were reduced in both female and male ALSKO mice compared with controls (Fig. 1 A). Female ALSKO mice had a 42% to 52% decrease in serum IGF‐1 levels from 4 to 16 weeks of age, whereas male ALSKO mice had decreases of 30% to 52% of control levels. Significant reductions in serum IGF‐1 levels compared with controls were apparent at 4 weeks of age, but only for females. By 8 weeks, both females and males showed significant reductions in IGF‐1 levels that remained through adulthood (16 weeks); serum IGF‐1 levels for female and male ALSKO mice were statistically indistinguishable. Despite the marked reductions in serum IGF‐1 levels in ALSKO mice, we did not detect elevations in serum GH levels at any of the indicated ages (Fig. 1 B). We further determined whether loss of ALS affected serum levels of insulin or glucose, but no significant differences in those markers were found when comparing the groups at multiple ages (Fig. 1 C, D). Serum osteocalcin levels decreased gradually with age. We found significant reductions in serum osteocalcin at 4 and 8 weeks in ALSKO male mice (during the late growth phase), as well as at 8 weeks in ALSKO female mice, when compared with their respective controls (Fig. 1 E). However, at 16 weeks, osteocalcin levels of both female and male ALSKO mice were identical to those of controls.
Figure 1.
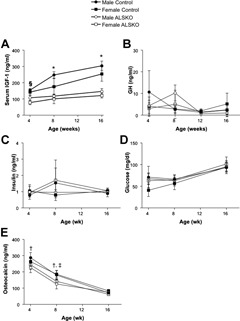
Mean serum IGF‐1 (A), GH (B), glucose (C), insulin (D) (n = 5 per group), and osteocalcin (E) (n = 8 per group) levels (±SD) for male and female control and ALSKO mice at 4, 8, and 16 weeks of age. § ALSKO females have significantly reduced IGF‐1 levels compared with both male and female controls at 4 weeks. *ALSKO males and females have significantly reduced IGF‐1 levels compared to their respective controls at 8 and 16 weeks. † ALSKO males have reduced osteocalcin compared with male controls. ‡ ALSKO females have reduced osteocalcin compared with female controls.
Body size and composition
Given that the GH/IGF‐1 axis is important in the regulation of body size and composition, we measured body weight, femoral length, fat mass, and lean mass in ALSKO and control mice during growth. In female ALSKO mice, a significant reduction in body size compared with controls was present by 4 weeks of age, whereas in males, a significant reduction appeared by 8 weeks of age (Fig. 2 A). At all ages, female mice had reduced body weight compared with males. Further, ALSKO mice of a given sex had significantly reduced weight compared with controls, except for female ALSKO mice at 12 weeks of age. Significant reductions in body weight in ALSKO mice from 8 to 16 weeks of age were paralleled by significant reductions in femoral length, although the differences for males were most apparent at 16 weeks of age (Fig. 2 B). Reduced body weight in ALSKO female and male mice did not alter body composition because relative lean and fat mass were similar to those in controls in both males and females (Fig. 2 C, D).
Figure 2.
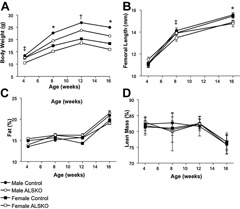
Body size and composition data for control and ALSKO mice. (A) Mean body weight (±SD) at 4, 8, 12, and 16 weeks of age. (B) Mean femoral length (±SD) at 4, 8, and 16 weeks of age. (C) Mean fat composition relative to body weight (%, ±SD) at 4, 8, 12, and 16 weeks of age. (D) Mean lean mass relative to body weight (%, ±SD) at 4, 8, 12, and 16 weeks of age. *ALSKO males and females have significantly smaller mean values than their respective controls. † ALSKO males have significantly smaller mean values than male controls. ‡ ALSKO females have significantly smaller mean values than female controls.
Bone morphology and tissue mineral density
Both female and male ALSKO mice demonstrated alterations in transverse (cross‐sectional) bone size by 8 weeks of age. In male ALSKO, mice there was a significant reduction in Tt.Ar and J 0 (Fig. 3 A, D), and in both males and females, there was a reduction in Ma.Ar (Fig. 3 C). By 16 weeks of age, female ALSKO mice had significantly reduced Tt.Ar, Ct.Ar, J 0, and Ma.Ar (−20%, −10%, −31%, and −28%, respectively; Fig. 3), whereas males showed more profound reductions in those traits (−28%, −19%, −42%, and −30%, respectively). Cortical TMD values for female and male ALSKO mice were statistically indistinguishable from those of control mice at 4, 8, and 16 weeks of age (Table 1). Statistical analysis of 16‐week data revealed a significant interaction effect between sex and genotype for nearly all cortical traits (Table 2). The largest interaction effects were seen for Tt.Ar, J 0, and robustness.
Figure 3.
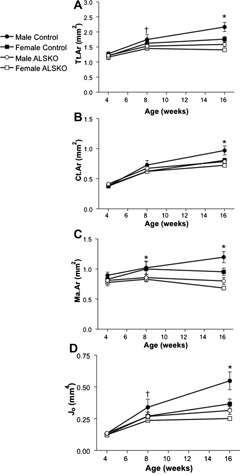
Mid‐diaphyseal cortical bone traits from control and ALSKO femurs. Means (±SD) are presented for (A) Tt.Ar, (B) Ct.Ar, (C) Ma.Ar, and (D) J 0 at 4, 8, and 16 weeks of age. *ALSKO males and females have significantly smaller mean values than their respective controls. † ALSKO males have significantly smaller mean values than male controls.
Table 1.
Mid‐diaphyseal Tissue Mineral Density (TMD) for Male and Female Control and ALSKO Mice at 4, 8, and 16 Weeks of Age
| Age (weeks) | Female ALSKO | n | Female Control | n | Male ALSKO | n | Male Control | n | |
|---|---|---|---|---|---|---|---|---|---|
| TMD | 4 | 1047 ± 33 | 12 | 1032 ± 23 | 11 | 1030 ± 22 | 8 | 1038 ± 57 | 12 |
| (mg/cc) | 8 | 1184 ± 34 | 5 | 1193 ± 40 | 6 | 1198 ± 29 | 6 | 1208 ± 45 | 6 |
| 16 | 1317 ± 73 | 9 | 1297 ± 58 | 7 | 1314 ± 59 | 7 | 1278 ± 53 | 9 |
Mean values ± SD are presented; no statistical differences were found.
Table 2.
Strain‐Sex Interaction Effects From Least Squares Means (ANOVA) for Femoral Length, Measured Cortical Bone Traits, and Derived Traits in 16‐Week‐Old Animals
| F Statistic | p‐value | |
|---|---|---|
| Femoral Length | 2.22 | 0.147 |
| Tt.Ar* | 9.58 | 0.004 |
| Ct.Ar* | 8.84 | 0.006 |
| Ma.Ar* | 7.30 | 0.012 |
| Ct.Th* | 5.10 | 0.032 |
| Jo* | 14.05 | 0.001 |
| RCA | 0.78 | 0.384 |
| Robustness* | 8.88 | 0.006 |
Significant (p < .05) strain‐sex interaction effect for the indicated trait.
Trabecular bone analyzed from the distal metaphyses revealed more subtle changes than were found for mid‐diaphyseal cortical bone. Trabecular bone fraction peaked at 8 weeks of age in both genders of control and ALSKO mice. Trabecular TMD values were similar between control and ALSKO mice at all ages (Table 3).
Table 3.
Distal Metaphyseal Trabecular Bone Traits for Male and Female Control and ALSKO Mice at 4, 8, and 16 Weeks of Age
| Age (weeks) | Male ALSKO | n | Male Control | n | Female ALSKO | n | Female Control | n | |
|---|---|---|---|---|---|---|---|---|---|
| BV/TV (%) | 4 | 11.1 ± 1 .8 | 8 | 10.2 ± 2.5 | 12 | 12.3 ± 2.0* | 12 | 9.3 ± 1.3 | 11 |
| 8 | 16.6 ± 2.6 | 6 | 17.2 ± 4.1 | 6 | 14.3 ± 1.2 | 5 | 11.6 ± 1.7 | 6 | |
| 16 | 14.0 ± 0.5 | 7 | 15.2 ± 1 .7 | 9 | 11.0 ± 1.7 | 9 | 10.9 ± 2.4 | 7 | |
| Tb.N | 4 | 4.4 ± 0.6 | 8 | 4.4 ± 1.7 | 12 | 5.2 ± 1.5 | 12 | 3.7 ± 0.8 | 11 |
| 8 | 5.6 ± 0.8 | 6 | 5.3 ± 0.6 | 6 | 5.5 ± 0.5* | 5 | 4.2 ± 0.6 | 6 | |
| 16 | 5.2 ± 0.5 | 7 | 4.7 ± 0.5 | 9 | 5.1 ± 1.1 | 9 | 4.9 ± 1.1 | 7 | |
| Tb.Th (mm) | 4 | 0.025 ± 0.001 | 8 | 0.024 ± 0.003 | 12 | 0.024 ± 0.003 | 12 | 0.025 ± 0.002 | 11 |
| 8 | 0.030 ± 0.003 | 6 | 0.032 ± 0.004 | 6 | 0.026 ± 0.002 | 5 | 0.028 ± 0.001 | 6 | |
| 16 | 0.027 ± 0.002* | 7 | 0.032 ± 0.003 | 9 | 0.022 ± 0.002 | 9 | 0.022 ± 0.001 | 7 | |
| Tb.Sp (mm−1) | 4 | 0.21 ± 0.03 | 8 | 0.22 ± 0.07 | 12 | 0.18 ± 0.05* | 12 | 0.25 ± 0.05 | 11 |
| 8 | 0.1 5 ± 0.03 | 6 | 0.16 ± 0.03 | 6 | 0.16 ± 0.01* | 5 | 0.22 ± 0.03 | 6 | |
| 16 | 0.17 ± 0.02 | 7 | 0.1 8 ± 0.02 | 9 | 0.18 ± 0.04 | 9 | 0.19 ± 0.05 | 7 | |
| TMD (mg/cc) | 4 | 413 ± 34 | 8 | 439 ± 80 | 12 | 392 ± 67 | 12 | 445 ± 47 | 11 |
| 8 | 599 ± 50 | 6 | 671 ± 94 | 6 | 504 ± 38 | 5 | 564 ± 47 | 6 | |
| 16 | 683 ± 69 | 7 | 778 ± 80 | 9 | 560 ± 93 | 9 | 542 ± 57 | 7 |
Mean values ± SD are presented.
Statistically different from control animals of the same sex and age (ANOVA, p < .05).
Femoral trait covariation during growth
To assess relative differences in transverse and longitudinal growth and in the amount of bone tissue accumulated for a given bone size among our strains and sexes, we quantified femoral robustness (Tt.Ar/Le) and relative cortical area (RCA) during growth. From 4 to 8 weeks, robustness was largely unchanged in all strains and sexes. However, by 16 weeks, females were significantly less robust (more slender) than their male counterparts (Fig. 4 A). Similarly, female and male ALSKO mice were significantly more slender than controls. Females showed increased RCA by 8 weeks of age, and by 16 weeks, both male and female ALSKO mice had an increased RCA compared with controls (Fig. 4 B).
Figure 4.
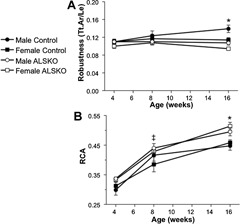
Mean (±SD) femoral (A) robustness (Tt.Ar/Le) and (B) relative cortical area (RCA) of 4‐, 8‐, and 16‐week‐old control and ALSKO mice. *ALSKO males and females have significantly smaller mean values than their respective controls. ‡ALSKO females have significantly smaller mean values than female controls.
Since functional variation in robustness and RCA can arise from variations in body weight, we examined linear relationships from 4 to 16 weeks of age. For both male and female control mice, robustness increased with body weight during growth, and the slopes of these regressions were statistically indistinguishable (Fig. 5). In contrast, in ALSKO male mice, robustness was independent of body weight (Fig. 5 A), whereas in ALSKO female mice, robustness decreased significantly with increasing body weight during growth (Fig. 5 B). Male and female control mice showed an identical increase in RCA with body weight during growth (Fig. 6). Surprisingly, ALSKO mice of both genders showed significant increases in RCA with body weight, and these increases were significantly greater than those of their respective control mice (ANCOVA, p < .001). In addition, the increase in RCA with body weight was greater in female ALSKO mice than in male ALSKO mice (ANCOVA, p < .001).
Figure 5.
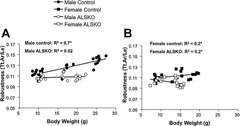
Linear regressions for robustness and body weight for (A) male and (B) female control and ALSKO mice. *Regression slope is significantly different from zero (F test, p < .05).
Figure 6.
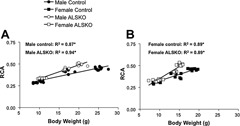
Linear regressions for relative cortical area (RCA) and body weight for (A) male and (B) female control and ALSKO mice. *Regression slope is significantly different from zero (F test, p < .05).
Femoral cortical histomorphometry
Histomorphometric measurements from 16‐week‐old femurs revealed no significant differences in mineral apposition rate (MAR) among our four groups of mice on either the endosteal or periosteal surface, but significant differences were found for labeled perimeter (L.Pm/B.Pm) and bone‐formation rate (BFR) (Table 4). Male ALSKO mice mice had significantly increased endosteal L.Pm/B.Pm compared with the endosteal and perisoteal values of control mice. Female ALSKO mice had significantly greater mean endosteal L.Pm/B.Pm and BFR values compared with all other groups except for the mean endosteal value of female control animals, which is likely a result of large sample variance in the female control group. Female control mice also tended to have significantly greater L.Pm/B.Pm and BFR values compared with other groups, the exceptions being in comparison with mean values for the endosteal surfaces of male and female ALSKO mice.
Table 4.
Mean (±SD) Histomorphometry Data From Mid‐diaphyseal Cortical Bone of 16‐Week‐Old Male and Female Control and ALSKO Mice (n = 6 to 7/Group)
| L.Pm (%) | MAR (µm/day) | BFR/B.Pm (µm/day × 100) | |
|---|---|---|---|
| Periosteal Surface | |||
| Male Control | 21.6 ± 6.5a,b,c | 1.38 ± 0.32 | 32.3 ± 16.2b,c |
| Male ALSKO | 28.0 ± 6.8b,c | 1.29 ± 0.29 | 35.6 ± 7.9b,c |
| Female Control | 28.4 ± 8.5b,c | 1.43 ± 0.28 | 41.1 ± 14.5b,c |
| Female ALSKO | 25.1 ± 2.7b,c | 1.44 ± 0.16 | 35.8 ± 4.1b,c |
| Endosteal Surface | |||
| Male Control | 10.8 ± 7.5a,b,c | 1.40 ± 0.10 | 25.1 ± 3.9b,c |
| Male ALSKO | 41.0 ± 12.7b | 1.58 ± 0.81 | 62.2 ± 27.0b |
| Female Control | 56.1 ± 18.1 | 1.39 ± 0.35 | 80.6 ± 37.6 |
| Female ALSKO | 68.3 ± 5.8 | 1.55 ± 0.15 | 105.6 ± 14.1 |
Statistically different from male ALSKO endosteal surface.
Statistically different from female ALSKO endosteal surface.
Statistically different from female control endosteal surface.
Mechanical properties
Mechanical properties from four‐point bending of female and male control and ALSKO femurs revealed significant sex‐specific differences but no genotype differences at 12 weeks of age (Table 5). Male mice of a given strain (control or ALSKO) had significantly greater maximum load values compared with their female counterparts. Similar increases were seen when comparing work values between males and females. Although differences between control and ALSKO mice of a given sex were not significant for any mechanical properties, mean values of ALSKO mechanical properties were consistently lower than those of controls. To estimate mechanical properties of 8‐and 16‐week‐old femurs based on morphologic traits, we calculated bone strength index values S B. For 8‐week‐old animals, S B values were not significantly different between control and ALSKO mice of either sex, but by 16 weeks, S B values were significantly reduced for ALSKO mice of both genders compared with their respective controls (Table 6), indicating that a decrease in whole‐bone mechanical properties as a result of ALS deficiency does not manifest until adulthood.
Table 5.
Mean (±SD) Mechanical Properties for Male and Female Control and ALSKO Mice at 12 Weeks of Age (n = 10/group except for #, where n = 9)
| Stiffness (N/mm) | Maximum Load (N) | PYD (mm) | Work to Failure (N · mm) | |
|---|---|---|---|---|
| Male Control# | 116.6 ± 23.2 | 24.3 ± 3.3a,b | 0.76 ± 0.42 | 22.3 ± 8.4a,b |
| Male ALSKO | 107.7 ± 14.9 | 21.8 ± 2c | 0.42 ± 0.23 | 14.6 ± 4.6 |
| Female Control | 116.4 ± 21.3 | 19.4 ± 2.9a | 0.52 ± 0.54 | 12.7 ± 8.8a |
| Female ALSKO | 98.5 ± 11.6 | 17.9 ± 2b,c | 0.32 ± 0.10 | 10.3 ± 2.7b |
Note: For a given mechanical property, shared letters indicate that mean values are significantly different (ANOVA, p < .05).
Table 6.
Mean (±SD) for Bone Strength Index S B at 8 and 16 Weeks of Age (ANOVA)
| 8 weeks | 16 weeks | |||
|---|---|---|---|---|
| Male Control | 2.7 ± 0.31a | n = 6 | 3.9 ± 0.29a,c | n = 9 |
| Male ALSKO | 2.4 ± 0.16 | n = 6 | 2.9 ± 0.17a | n = 7 |
| Female Control | 2.3 ± 0.10a | n = 6 | 3.2 ± 0.20b,c | n = 7 |
| Female ALSKO | 2.2 ± 0.07 | n = 5 | 2.7 ± 0.12b | n = 9 |
Note: Shared letters indicate a significant difference for a given age (p < .05).
Discussion
Female ALSKO mice have early‐onset body size reductions
This study demonstrated that ALS deficiency results in significant reductions in body size and skeletal properties in both sexes. These reductions appear as early as 4 weeks of age and exist into adulthood, where bones are disproportionately more slender (for a given body weight) and have reduced mechanical properties. In both male and female mice, ablation of the Als gene resulted in decreased body weight. However, the corresponding decreases in lean and fat mass were not statistically significant when normalized to body weight (Fig. 2), indicating that ALSKO female and male mice are proportionally smaller than controls. This proportional decrease in lean and fat mass also was observed in male mice of the liver‐specific Igf1 gene deletion (LID) mice on an FVB/N background,14 emphasizing the role of serum IGF‐1 in determining body size. Nevertheless, although both male and female ALSKO mice have proportionately reduced body size and identically reduced serum IGF‐1 levels (approximately 30% to 52%), we have demonstrated notable sex‐specific differences. For example, decreased body weight appears earlier in females (4 weeks) than in males (8 weeks). In addition, decreased femoral length appears by 8 weeks in females and not until 16 weeks in males. Male LID mice also did not show reductions in body size until the late growth phase (12 weeks).14 Thus ALSKO females appear to have an early‐onset body size phenotype compared with male ALSKO and LID mice. In humans, although most reported cases of ALS deficiency have been in males, a report including both sexes supports early‐onset body size reductions in females, as indicated by the fact that a reduction in prepubertal body weight and length was most obvious in the female patient compared with several male patients.18
ALS deficiency diminishes pubertal and postpubertal skeletal development
Given the significant reductions in body size of ALSKO mice, we expected marked reductions in skeletal size. Both female and male ALSKO mice showed significant reductions in femoral Tt.Ar, Ct.Ar and J 0 by adulthood (16 weeks; Fig. 3). Although in both males and females, ALSKO and control mice had significantly different values for Tt.Ar and J 0 by 16 weeks of age, histomorphometric data did not show any differences in L.Pm/B.Pm, MAR, or BFR. This is likely a result of very low levels of periosteal apposition in these mice given that mice with markedly different femoral sizes have been reported to show no difference in MAR and BFR at adulthood (16 weeks).19 By contrast, changes on the endosteal surface were more apparent. Female mice for both control and ALSKO strains were found to have increased L.Pm and BFR compared with their male counterparts. To our knowledge, this is the first report of sex‐specific differences in femoral histomorphometric data on different bone surfaces. These data are in line with published data comparing femurs of mice with significantly different Ma.Ar values and revealing that decreased Ma.Ar values were associated with higher BFR values on the endosteal surface.20 Further, our data are in line with prior studies indicating a tendency toward greater bone accrual on the endosteal surfaces (smaller Ma.Ar) of female long bones in humans.12, 21, 22, 23 Genotype‐specific differences also were found on the endosteum because Ma.Ar values were significantly reduced in both female and male ALSKO mice as early as 8 weeks. This indicated that during puberty, ALSKO mice of both sexes add more bone on their endosteum compared with controls (Fig. 3 C). This preferential addition of bone endosteally in ALSKO mice was further supported by our histomorphometric data, which indicated significantly greater mean L.Pm/B.Pm and BFR values for the endosteal surfaces compared with the periosteal surfaces of ALSKO mice at 16 weeks (Table 4). The decrease in Ma.Ar of male and female ALSKO femurs at 8 weeks may be a compensatory infilling response similar to that seen in male LID femurs beginning at 16 weeks of age.14 Interestingly, at 8 weeks, male ALSKO mice had significantly reduced femoral Tt.Ar and J 0 (Fig. 3 A), indicating that periosteal expansion, which is normally large for human24 and murine25 males during puberty, has been inhibited as a result of ALS deficiency. A similar reduction in femoral Tt.Ar and J 0 was seen in 8‐week‐old male LID mice.14 Also similar to the LID mice, ALSKO mice of both sexes showed no difference in their extent of tissue mineralization, and variations in trabecular bone properties were minimal and nonexistent by adulthood. Thus ALS defines the cortical skeletal phenotype, and its actions are likely mediated at least in part through reductions in serum IGF‐1 levels.
ALS deficiency results in slender bones and increased marrow infilling
To determine whether reductions in skeletal traits resulted in more slender bones in ALSKO mice, we calculated robustness (Tt.Ar/Le) for each strain. Although both Tt.Ar and Le are reduced in female and male ALSKO mice, the relative reductions (Tt.Ar/Le) were not proportional. Thus both female and male ALSKO mice had more slender bones by adulthood (Fig. 4 A). Although more slender bones are not necessarily at an increased risk of fracture, increased risk may result if compensatory mechanisms are not present to buffer the demands of loading and use.11, 13, 26 One common mechanism of compensating for more slender bones, increased mineralization, was not present in ALSKO mice. However, given the aforementioned reductions in Ma.Ar, we theorized that marrow infilling through endosteal bone apposition may serve to compensate by increasing RCA. Indeed, increased RCA values were present in female and male ALSKO mice by 16 weeks of age (Fig. 4 B). Notably, female ALSKO mice, which were more slender than male ALSKO mice, demonstrated increased RCA as early as 8 weeks. Further, the endosteal L.Pm/B.Pm and BFR values of female ALSKO mice were significantly greater than those of male ALSKO mice at 16 weeks of age (Table 4). Thus it is conceivable that by beginning to increase their RCA at an earlier age and maintaining it throughout the late growth phase (4 to 16 weeks), female ALSKO mice are able to achieve, on average, similar RCA values as male ALSKO mice.
ALSKO mice are more slender relative to body weight
The presence of increased RCA suggested that ALSKO femurs were disproportionately less robust for their altered body size. To confirm this prediction, we examined the linear relationship between robustness and body weight. We clearly show a positive relationship between robustness and body weight in control mice such that with increases in body weight, there is an increase in robustness. In sharp contrast, for both female and male ALSKO mice, regressions of femoral robustness (Tt.Ar/Le) against body weight during growth revealed nonpositive slopes (Fig. 5). Male ALSKO mice exhibited no such relationship, whereas ALSKO females actually had decreased robustness (increased slenderness) given their increases in body size. Thus, while ALS deficiency rendered both males and females susceptible to the development of femurs that are underdesigned for their size, females were even more susceptible than males. A regression of RCA versus body weight during growth revealed that both male and female ALSKO mice increased their RCA relative to body weight to a greater extent than their respective controls (Fig. 6). Therefore, both sexes may be attempting to compensate for more slender bones by adding more bone endosteally during growth. This is supported by statistically greater endosteal L.Pm/B.Pm and BFR values in male ALSKO mice compared with controls (Table 4); increases in L.Pm/B.Pm and BFR in female ALSKO mice compared with controls, although represented in the mean values, did not reach statistical significance owing to a large sample variance in the female control group. Given that both sexes demonstrated a marrow infilling response to decreased robustness, female ALSKO mice, which demonstrated significantly reduced robustness during growth compared with male ALSKO mice, were expected to a have greater increase in RCA compared with male ALSKO mice, and this was indeed the case (ANCOVA, p < .001). Compensation for a more slender bone by increasing RCA has been documented previously in male LID mice,14 as well as in human female and male tibias.26 Nevertheless, based on the significant reductions in Tt.Ar, J 0, and robustness, we predicted that ALSKO femurs still would be mechanically inferior to those of control mice. Interestingly, four‐point bending of 12‐week‐old femurs did not show any differences in mechanical properties. These data, combined with the fact that genotype‐specific differences in cortical traits do not fully manifest until 16 weeks of age, suggested that reductions in femoral whole‐bone mechanical properties would not appear until adulthood in ALSKO mice. In support of this, calculated bone strength indices S B for 8‐week‐old animals were similar to 12‐week four‐point bending data in showing no differences for ALSKO and control mice. However, at 16 weeks, significant differences in J 0, Tt.Ar, and Le did lead to significant reductions in S B in both female and male ALSKO mice, thus indicating that significant reductions in mechanical properties arising from a loss of ALS appear only when peak bone properties have been achieved.
Conclusion
The results of this study offer the first longitudinal analysis of how loss of ALS affects body size, composition, and skeletal development during growth. In both male and female mice, loss of ALS (ALSKO) results in a significant reduction in body size and a “scaling down” of fat and lean mass to match body size. However, skeletal traits in ALSKO mice do not scale with body size, and loss of ALS results in an uncoupling of longitudinal and transverse bone growth relative to body weight from puberty to adulthood. ALSKO mice of both sexes therefore are disproportionately more slender relative to body weight, and in females, the increased slenderness is greater than in males. The fact that differences in bone traits (Tt.Ar, Ct.Ar, J 0, Ma.Ar) and bone slenderness were found to be sex‐ and age‐dependent in ALSKO mice may help to explain the variability in ALS human patient phenotypes reported to date. This variation may be a result of improper regulation of sex hormones (ie, delayed puberty) arising from ALS deficiency and, if present, would warrant further developmental studies. Interestingly, both female and male ALSKO mice appear to compensate for their reduced slenderness through increased endosteal apposition (marrow infilling). The result is that the increased slenderness of ALSKO femurs is offset by an increase in cortical bone tissue. This adaptive response results in increased bone mass and may partially offset reductions in whole‐bone mechanical properties normally associated with smaller, more slender bones. However, the magnitude of changes in Tt.Ar, J 0, and robustness, as well as the differences in bone strength indices, indicate that ALSKO bones are ultimately mechanically inferior at the onset of peak bone acquisition. The mechanism by which ALS mediates periosteal bone accrual and longitudinal bone growth is currently unknown. Given the similarities between ALSKO and LID mice, a portion of the effect is likely due to reductions in serum IGF‐1. Still, loss of ALS may have unique mechanisms by which it modulates bone growth. For example, unlike marrow stromal cells from LID mice, ALSKO mice had fewer alkaline phosphatase–positive colonies in culture and were unable to support osteoclastogenesis in cocultures, suggesting that loss of ALS may have a direct effect on cell fate.27 Further, female ALSKO mice may manifest delayed puberty secondary to the reductions in serum IGF‐1 and body size.28, 29 More detailed studies in both mice and humans are needed to clarify these relationships, and longitudinal studies of ALS‐deficient patients of both sexes will be needed to verify the extent to which ALS deficiencies alter bone mass and geometry during growth and aging.
Disclosures
All the authors state that they have no conflicts of interest.
Acknowledgements
We would like to thank Dr YR Boisclair (Department of Animal Science, Cornell University, Ithaca, New York, USA) for creation of the original ALSKO line and Dr KJ Jepsen (Department of Orthopaedics, Mount Sinai School of Medicine, New York, NY, USA) for use of the Instron mechanical testing system. This work was supported by funding agencies in the United States, including NIAMS (NIH Grants AR054919 and AR055141 to SY).
References
- 1. Ueki I, Ooi GT, Tremblay ML, Hurst KR, Bach LA, Boisclair YR. Inactivation of the acid labile subunit gene in mice results in mild retardation of postnatal growth despite profound disruptions in the circulating insulin‐like growth factor system. Proc Natl Acad Sci USA. 2000; 97: 6868–6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fofanova‐Gambetti OV, Hwa V, Kirsch S, et al. Three novel IGFALS gene mutations resulting in total ALS and severe circulating IGF‐I/IGFBP‐3 deficiency in children of different ethnic origins. Horm Res. 2009; 71: 100–110. [DOI] [PubMed] [Google Scholar]
- 3. Domene HM, Scaglia PA, Lteif A, et al. Phenotypic effects of null and haploinsufficiency of acid‐labile subunit in a family with two novel IGFALS gene mutations. J Clin Endocrinol Metab. 2007; 92: 4444–4450. [DOI] [PubMed] [Google Scholar]
- 4. Heath KE, Argente J, Barrios V, et al. Primary acid‐labile subunit deficiency due to recessive IGFALS mutations results in postnatal growth deficit associated with low circulating insulin growth factor (IGF. 1 IGF binding protein‐3 levels, and hyperinsulinemia. J Clin Endocrinol Metab. 2008; 93: 1616–1624. [DOI] [PubMed] [Google Scholar]
- 5. van Duyvenvoorde HA, Kempers MJ, Twickler TB, et al. Homozygous and heterozygous expression of a novel mutation of the acid‐labile subunit. Eur J Endocrinol. 2008; 159: 113–120. [DOI] [PubMed] [Google Scholar]
- 6. Domene HM, Bengolea SV, Jasper HG, Boisclair YR. Acid‐labile subunit deficiency: phenotypic similarities and differences between human and mouse. J Endocrinol Invest. 2005; 28 (5 Suppl): 43–46. [PubMed] [Google Scholar]
- 7. Yakar S, Rosen CJ, Beamer WG, et al. Circulating levels of IGF‐1 directly regulate bone growth and density. J Clin Invest. 2002; 110: 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yakar S, Liu JL, Stannard B, et al. Normal growth and development in the absence of hepatic insulin‐like growth factor I. Proc Natl Acad Sci U S A. 1999; 96: 7324–7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yakar S, Bouxsein ML, Canalis E, et al. The ternary IGF complex influences postnatal bone acquisition and the skeletal response to intermittent parathyroid hormone. J Endocrinol. 2006; 189: 289–299. [DOI] [PubMed] [Google Scholar]
- 10. Yakar S, Setser J, Zhao H, et al. Inhibition of growth hormone action improves insulin sensitivity in liver IGF‐1‐deficient mice. J Clin Invest. 2004; 113: 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jepsen KJ, Hu B, Tommasini SM, et al. Genetic randomization reveals functional relationships among morphologic and tissue‐quality traits that contribute to bone strength and fragility. Mamm Genome. 2007; 18: 492–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tommasini SM, Nasser P, Jepsen KJ. Sexual dimorphism affects tibia size and shape but not tissue‐level mechanical properties. Bone. 2007; 40: 498–505. [DOI] [PubMed] [Google Scholar]
- 13. Tommasini SM, Nasser P, Schaffler MB, Jepsen KJ. Relationship between bone morphology and bone quality in male tibias: implications for stress fracture risk. J Bone Miner Res. 2005; 20: 1372–1380. [DOI] [PubMed] [Google Scholar]
- 14. Yakar S, Canalis E, Sun H, et al. Serum IGF‐1 determines skeletal strength by regulating sub‐periosteal expansion and trait interactions. J Bone Miner Res. 2009; 24: 1481–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jepsen KJ, Pennington DE, Lee YL, Warman M, Nadeau J. Bone brittleness varies with genetic background in A/J and C57BL/6J inbred mice. J Bone Miner Res. 2001; 16: 1854–1862. [DOI] [PubMed] [Google Scholar]
- 16. Parfitt AM, Drezner MK, Glorieux FH, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987; 2: 595–610. [DOI] [PubMed] [Google Scholar]
- 17. Selker F, Carter DR. Scaling of long bone fracture strength with animal mass. J Biomech. 1989; 22: 1175–1183. [DOI] [PubMed] [Google Scholar]
- 18. Wikland KA, Luo ZC, Niklasson A, Karlberg J. Swedish population‐based longitudinal reference values from birth to 18 years of age for height, weight and head circumference. Acta Paediatr. 2002; 91: 739–754. [DOI] [PubMed] [Google Scholar]
- 19. Akhter MP, Iwaniec UT, Covey MA, Cullen DM, Kimmel DB, Recker RR. Genetic variations. in bone density, histomorphometry, and strength in mice. Calcif Tissue Int. 2000; 67: 337–344. [DOI] [PubMed] [Google Scholar]
- 20. Sheng MH, Baylink DJ, Beamer WG, et al. Histomorphometric studies show that bone formation and bone mineral apposition rates are greater in C3H/HeJ (high‐density) than C57BL/6J (low‐density) mice during growth. Bone. 1999; 25: 421–429. [DOI] [PubMed] [Google Scholar]
- 21. Hogler W, Blimkie CJ, Cowell CT, et al. A comparison of bone geometry and cortical density at the mid‐femur between prepuberty and young adulthood using magnetic resonance imaging. Bone. 2003; 33: 771–778. [DOI] [PubMed] [Google Scholar]
- 22. Pandey N, Bhola S, Goldstone A, et al. Interindividual variation in functionally adapted trait sets is established during postnatal growth and predictable based on bone robustness. J Bone Miner Res. 2009; 24: 1969–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garn SM, Nagy JM, Sandusky ST. Differential sexual dimorphism in bone diameters of subjects of European and African ancestry. Am J Phys Anthropol. 1972; 37: 127–129. [DOI] [PubMed] [Google Scholar]
- 24. Hogler W, Blimkie CJ, Cowell CT, et al. Sex‐specific developmental changes in muscle size and bone geometry at the femoral shaft. Bone. 2008; 42: 982–989. [DOI] [PubMed] [Google Scholar]
- 25. Richman C, Kutilek S, Miyakoshi N, et al. Postnatal and pubertal skeletal changes contribute predominantly to the differences in peak bone density between C3H/HeJ and C57BL/6J mice. J Bone Miner Res. 2001; 16: 386–397. [DOI] [PubMed] [Google Scholar]
- 26. Tommasini SM, Nasser P, Hu B, Jepsen KJ. Biological co‐adaptation of morphological and composition traits contributes to mechanical functionality and skeletal fragility. J Bone Miner Res. 2008; 23: 236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fritton JC, Kawashima Y, Mejia W, et al. The insulin‐like growth factor‐1 (IGF‐1) binding protein acid‐labile subunit (ALS) alters mesenchymal stromal cell fate. J Biol Chem. 2010; 285: 4709–4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tam CS, de Zegher F, Garnett SP, Baur LA, Cowell CT. Opposing influences of prenatal and postnatal growth on the timing of menarche. J Clin Endocrinol Metab. 2006; 91: 4369–4373. [DOI] [PubMed] [Google Scholar]
- 29. Blogowska A, Krzyzanowska‐Swiniarska B, Zielinska D, Rzepka‐Gorska I. Body composition and concentrations of leptin, neuropeptide Y, beta‐endorphin, growth hormone, insulin‐like growth factor‐I and insulin at menarche in girls with constitutional delay of puberty. Gynecol Endocrinol. 2006; 22: 274–278. [DOI] [PubMed] [Google Scholar]


