Abstract
There is growing evidence that insulin‐like growth factor 1 (IGF‐1) and parathyroid hormone (PTH) have synergistic actions on bone and that part of the anabolic effects of PTH is mediated by local production of IGF‐1. In this study we analyzed the skeletal response to PTH in mouse models with manipulated endocrine or autocrine/paracrine IGF‐1. We used mice carrying a hepatic IGF‐1 transgene (HIT), which results in a threefold increase in serum IGF‐1 levels and normal tissue IGF‐1 expression, and Igf1 null mice with blunted IGF‐1 expression in tissues but threefold increases in serum IGF‐1 levels (KO‐HIT). Evaluation of skeletal growth showed that elevations in serum IGF‐1 in mice with Igf1 gene ablation in all tissues except the liver (KO‐HIT) resulted in a restoration of skeletal morphology and mechanical properties by adulthood. Intermittent PTH treatment of adult HIT mice resulted in increases in serum osteocalcin levels, femoral total cross‐sectional area, cortical bone area and cortical bone thickness, as well as bone mechanical properties. We found that the skeletal response of HIT mice to PTH was significantly higher than that of control mice, suggesting synergy between IGF‐1 and PTH on bone. In sharp contrast, although PTH‐treated KO‐HIT mice demonstrated an anabolic response in cortical and trabecular bone compartments compared with vehicle‐treated KO‐HIT mice, their response was identical to that of PTH‐treated control mice. We conclude that (1) in the presence of elevated serum IGF‐1 levels, PTH can exert an anabolic response in bone even in the total absence of tissue IGF‐1, and (2) elevations in serum IGF‐1 levels synergize PTH action on bone only if the tissue IGF‐1 axis is intact. Thus enhancement of PTH anabolic actions depends on tissue IGF‐1. © 2010 American Society for Bone and Mineral Research.
Keywords: IGF‐1, bone, transgenic mice, IGF1KO, micro–computed tomography, endocrine IGF‐1, intermittent PTH
Introduction
Insulin‐like growth factor 1 (IGF‐1) is an important regulator of skeletal growth and development. IGF‐1 acts in an endocrine/autocrine/paracrine fashion. Studies with transgenic and knockout mice have proven that IGF‐1 modulates linear and transverse bone growth, as well as bone mineralization. Endocrine (serum) IGF‐1 is secreted mainly by the liver and largely regulated by pituitary growth hormone (GH), whereas autocrine/paracrine (tissue) IGF‐1 is regulated by a number of tissue factors, as well as by GH. In our previous studies we performed skeletal characterization of liver IGF‐1‐deficient (LID) mice, which exhibit 75% reductions in serum IGF‐1 levels but have otherwise normal skeletal expression of IGF‐1.1 We found that reduced serum levels of IGF‐1 in male LID mice were associated with the development of slender bones during growth. The slender phenotype resulted mostly from inhibition of transverse bone growth (subperiosteal expansion); there were only minimal affects on linear growth. In a subsequent study we assessed how elevated serum IGF‐1 affected skeletal development of females in the presence or absence of tissue IGF‐1 expression.2 We studied mice carrying a hepatic IGF‐1 transgene (HIT), which exhibit threefold increases in serum IGF‐1 levels and normal tissue IGF‐1 expression, as well as Igf1 null mice with blunted IGF‐1 expression in tissues but threefold increases in serum IGF‐1 levels (KO‐HIT). We found that increased serum IGF‐1 in the presence of normal tissue IGF‐1 levels (HIT mice) led to enhancement of morphologic and mechanical properties of bone during development such that at 16 weeks of age bones were longer, cortices were thicker, and tissue‐level mineralization increased. Strikingly, we demonstrated that in the total absence of tissue Igf1 gene expression, elevated serum levels of IGF‐1 (KO‐HIT mice) restored body weight, augmented growth rate, and led to normal skeletal development.2 Thus an increase in serum IGF‐1 was able to counteract the deficits in body size and skeletal development associated with an absence of tissue IGF‐1.
Parathyroid hormone (PTH) exerts both anabolic and catabolic3 effects on the skeleton in humans and animals. Upon binding to its receptors on osteoblasts, PTH initiates a signaling cascade leading to activation of cAMP protein kinase A (PKA) and phosphoinositide protein kinase C (PKC).3, 4 These, in turn, eventually lead to transcriptional activation of genes involved in osteoblast differentiation and activity, such as Runx2, osteocalcin, and ALP, or genes involved in osteoclast activity, such as RANKL. 3, 4 There is growing evidence that IGF‐1 and PTH have synergistic actions on bone and that part of the anabolic effects of PTH is in fact mediated by local production of IGF‐1.5, 6, 7, 8, 9
Intermittent administration of PTH has been shown to activate anabolic pathways in bone, and previous studies have shown that these pathways are mediated in part through the IGF‐1 signaling system.6, 8, 9 As such, the anabolic effects of PTH on the skeleton of the Igf1 null mice were suppressed compared with controls.5, 7 Similarly, PTH effects on the skeleton of mice with osteoblast‐specific IGF‐1 receptor gene ablation (Igf1robKO) were blunted in cortical bone and partially inhibited in trabecular bone.10 Despite the valuable insights gained from these studies, the mechanisms by which IGF‐1 mediates PTH anabolic effects on the skeleton are still obscure. Moreover, in humans, there is significant heterogeneity in the skeletal response to PTH. Previously, in a randomized, controlled study, we noted that baseline IGF‐1 levels did not predict the changes in trabecular bone mass in response to PTH, nor did the change in circulating IGF‐1.11 Thus the role of circulating and skeletal IGF‐1 in the skeletal response to PTH remains unclear. In this study we set out to determine whether PTH exerts its anabolic effects on bone in the total absence of autocrine/paracrine IGF‐1 and to evaluate whether PTH, in the presence of elevated serum IGF‐1, can exert synergistic anabolic effects on the adult skeleton.
Materials and Methods
Animals
Male HIT and KO‐HIT mice (on FVB/N background) were generated as described previously.12 Male mice were housed four per cage in a clean mouse facility, fed standard mouse chow (Purina Laboratory Chow 5001, Purina Mills, Brentwood, MO, USA) and water ad libitum and kept on a 12‐hour light/dark cycle. Animal care and maintenance were provided through the Mount Sinai School of Medicine's AAALAC Accredited Animal Facility. All procedures were approved by the Animal Care and Use Committee of the Mount Sinai School of Medicine.
Serum hormones
Mice were bled through the mandibular vein, and serum samples were collected between 7 and 9 a.m. on a fed state at the indicated ages. Serum IGF‐1 and osteocalcin levels were determined using commercial radioimmunoassays, as described previously.13, 14, 15
Micro–computed tomography (µCT)
Cortical bone morphology at the midfemoral diaphysis and trabecular bone volume fraction and microarchitecture in the excised distal femoral metaphysis were assessed as described previously.1 Femurs were reconstructed at an 8.7‐µm voxel resolution. For trabecular bone regions, we assessed the bone volume fraction (BV/TV), trabecular thickness (Tb.Th, mm), trabecular number (Tb.N, 1/mm), and trabecular spacing (Tb.Sp, mm). For cortical bone at the femoral midshaft, we measured the average total cross‐sectional area inside the periosteal envelope (Tt.Ar, mm2), the cortical bone and medullary area within this same envelope (Ct.Ar and Ma.Ar, mm2, respectively), the relative cortical area (RCA: Ct.Ar/Tt.Ar), the average cortical thickness (Ct.Th, mm), and the polar moment of inertia (J 0, mm4). Robustness was defined as Tt.Ar/Le; a higher ratio denotes a more robust, less slender bone. All regions of analysis were standardized according to anatomic landmarks. Tissue mineral density (TMD) was defined as the average mineral value of the bone voxels and was expressed in hydroxyapatite density equivalents (HA mg/cm3).
Mechanical testing
Mouse femurs from 16‐week‐old control, HIT, and KO‐HIT mice were tested to failure by four‐point bending using a servohydraulic materials testing system (Instron Corp., Canton, MA, USA). From these tests measurements of whole‐bone stiffness, maximum load, postyield deflection, and work to failure were calculated. Femurs were placed with the anterior surface down on two lower supports. The two lower and two upper supports were set apart by 6.35 and 2.2 mm, respectively. Loading was centered over the midshaft at a displacement of 0.05 mm/s until failure. All mechanical properties were calculated from the load‐displacement curves, as described previously.16
PTH treatment
Twelve‐week‐old male mice of all genotypes were treated for 4 weeks with PTH (50 ng/g of body weight; Bachem Bioscience, Inc., Torrance, CA, USA). PTH was injected intraperitoneally (5 mg/mL in saline solution with 2% heat‐inactivated mouse serum; Innovative Research Inc., Plymouth, MN, USA, C57BL6 Mouse Serum) 7 days a week for 4 weeks.
Histomorphometry
Animals were injected with calcein (20 mg/kg) 12 and 2 days before killing. Right femurs from PTH‐treated male mice of all genotypes were fixed in 10% neutral buffered formalin and embedded in polymethyl methacrylate. Thick sections (approximately 120 µm) were cut using a low‐speed band saw and diamond‐coated wafering blade (Buehler, Inc., Lake Bluff, IL, USA). Sections were adhered to glass slides and polished to 50 µm for fluorescent imaging. Histomorphometric parameters were measured on the periosteal surface at the femoral midshaft. Measurements of labeled perimeter (L.Pm), bone‐formation rate (BFR), and mineral apposition rate (MAR) were made using an OsteoMeasure System (Osteometrics, Atlanta, GA, USA).17
Statistical analysis
All bone traits, body weight (BW), serum hormone, and µCT measurements are presented as means ± SEM. One‐way analysis of variance (ANOVA) was used to test for differences among groups at each age (Statview Software Version 5.0, SAS Institute, Inc., Cary, NC, USA). If ANOVA revealed significant effects, the means were compared by Fisher's test, considering p < .05 as significant.
Results
Elevated serum IGF‐1 levels promote skeletal acquisition independent of local IGF‐1 production
Male HIT and KO‐HIT mice were generated as described previously.18 Both HIT and KO‐HIT mice show twofold increases in serum IGF‐1 during growth and development (Fig. 1 A) owing to the rat Igf1 transgene expressed specifically in liver. Similar to our findings in females,2 male HIT mice, with elevated serum IGF‐1 but otherwise normal autocrine/paracrine IGF‐1 expression, exhibited greater body weights (Fig. 1 B) than controls: +4.6% at 4 weeks of age (p < .05), +22.3% by 8 weeks (p < .0001), and +25.4% by 16 weeks (p < .0001). On the other hand, KO‐HIT male mice, with elevated serum IGF‐1 but no autocrine/paracrine Igf1 gene expression, were smaller (–18%) at 4 weeks of age but exhibited a “catch‐up” growth phenotype such that by 8 weeks their body weight reached its plateau and by 16 weeks did not differ significantly from that of controls. Body length (Fig. 1 C) and femoral length (Fig. 1 D) in HIT and KO‐HIT mice followed body weight such that HIT mice were longer than control mice at 8 and 16 weeks of age, whereas KO‐HIT mice were smaller than controls and reached adult control length by 8 weeks of age.
Figure 1.
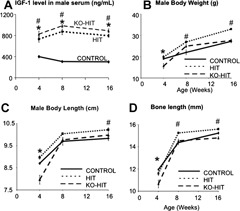
Growth characteristics of the HIT and KO‐HIT male mice. (A) Serum IGF‐1 levels were measured at 4, 8, and 16 weeks of age and show a twofold increase in both HIT and KO‐HIT mice compared with controls. (B) Body weight of HIT mice increased significantly throughout growth compared with control mice, whereas KO‐HIT mice show reductions in body weight at 4 weeks but normalize (indistinguishable from controls) thereafter. (C) Body length (nose to anus) decreased significantly in 4‐week‐old KO‐HIT mice but normalized at 8 weeks. HIT mice showed significant increases in body length at 16 weeks of age. (D) Femur length (assessed by µCT) decreased significantly in 4‐week‐old KO‐HIT mice but normalized at 8 weeks. HIT mice showed significant increases in bone length at 8 and 16 weeks of age. Data presented as mean ± SEM of n = 10 to 15 mice in each group at each time point. *p < .05 comparing KO‐HIT mice with control. # p < .05 comparing HIT mice with control.
To understand how modulations in serum and tissue IGF‐1 affect skeletal morphology in male mice, we performed a longitudinal analysis of the femoral middiaphyseal cortical bone from 4 to 16 weeks of age. Similar to what we found in females,2 elevations in serum IGF‐1 in HIT male mice led to increases in cross‐sectional total area (Tt.Ar) (+16% at 8 weeks and +19% at 16 weeks of age), cortical area (Ct.Ar) (+29% at 8 weeksand +16% at 16 weeks of age), and cortical thickness (Ct.Th) (+19% at 8 weeks and +7.5% at 16 weeks of age) at 8 and 16 weeks of age (Fig. 2). Tissue mineral density (TMD) increased similarly with age in all groups until 16 weeks, when KO‐HIT mice showed significant increases (+3.3%) compared with controls. Despite twofold increases in serum IGF‐1 levels, KO‐HIT male mice exhibited reduced Tt.Ar (–19%) and Ct.Ar (–18%) at 4 weeks, indicating that tissue IGF‐1 is essential for establishment of skeletal morphology during early postnatal growth. Nonetheless, KO‐HIT male mice showed a rapid increase in linear (+1.8 fold) and transverse (+5.9 fold) growth rates between 4 and 8 weeks of age such that by 16 weeks their cortical bone morphology was similar to that of controls (Fig. 3).
Figure 2.
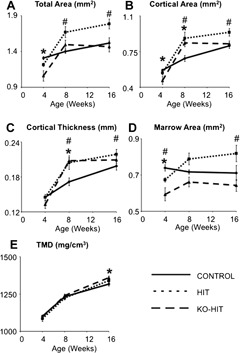
Cortical bone morphology was analyzed at the femoral midshaft by µCT. (A) Total cross‐sectional area (Tt.Ar), (B) cortical bone area (Ct.Ar), (C) cortical bone thickness (Ct.Th), (D) marrow area (Ma.Ar), and (E) tissue mineral density (TMD) were measured at 4, 8, and 16 weeks of age. Data presented as mean ± SEM of n = 10 to 15 mice in each group at each time point. *p < .05 comparing KO‐HIT mice with control. # p < .05 comparing HIT mice with control.
Figure 3.
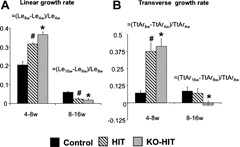
Linear and transverse bone growth rates. Linear growth rate indicated by increases in femur length (Le) (A) and transverse growth rate indicated by increases in Tt.Ar (B) were analyzed over two growth periods (4 to 8 weeks and 8 to 16 weeks of age) and expressed as ratios [ie, linear growth rate between 4 and 8 weeks = (Le8w –Le4w)/Le4w]. Data presented as mean ± SEM of n = 10 to 15 mice in each group over each period of time. *p < .05 comparing KO‐HIT mice with control. # p < .05 comparing HIT mice with control.
Trabecular architecture was assessed at the femoral distal metaphysis of 4, 8, and 16‐week‐old mice and revealed minor changes between groups. We found that trabecular bone volume/total volume (BV/TV) peaked at 8 weeks of age for all groups and decreased by approximately 13% to 27% with age (Fig. 4). At 16 weeks of age, both HIT and KO‐HIT mice exhibited decreases in trabecular number (Tb.N) and subsequent increases in trabecular spacing (Tb.Sp). However, while trabecular thickness (Tb.Th) in HIT mice did not differ from controls, KO‐HIT male mice showed significant decreases in Tb.Th at 16 weeks of age.
Figure 4.
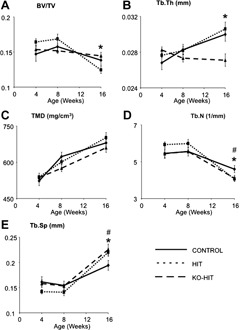
Trabecular bone morphology was analyzed at the distal femur by µCT. Bone volume fraction (BV/TV) (A), trabecular thickness (Tb.Th) (B), tissue mineral density (TMD) (C), trabecular number (Tb.N) (D), and trabecular spacing (Tb.Sp.) (E) were measured at 4, 8, and 16 weeks of age. Data presented as mean ± SEM of n = 10 to 15 mice in each group at each time point. *p < .05 comparing KO‐HIT mice with control. # p < .05 comparing HIT mice with control.
Whole‐bone mechanical properties examined by four‐point bending tests of cortical bone at the femoral midshaft showed a significant increase in maximum load (+19%) and stiffness (+18%) in HIT mice at 16 weeks of age (Fig. 5), as expected from the enhanced cortical architecture of those mice. KO‐HIT mice, however, showed increases in maximum load only at 8 weeks (+28%) but otherwise were similar to controls (data not shown).
Figure 5.
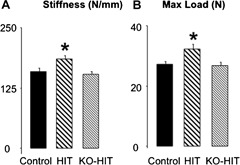
Mechanical properties of femurs were assessed by four‐point bending test on 16‐week‐old mice. Stiffness (A) indicates resistance to bending, and maximum load (B) indicates the maximum force the sample can bear before failure. Data presented as mean ± SEM of n = 10 to 15 mice in each group at each time point. *p < .05 comparing KO‐HIT mice with control. # p < .05 comparing HIT mice with control.
Elevated levels of serum IGF‐1 synergize PTH anabolic actions on bone
To understand how modulation of serum and tissue IGF‐1 affects the anabolic actions of intermittent PTH on the skeleton, we treated control, HIT, and KO‐HIT mice from 12 to 16 weeks of age with PTH. As shown in Table 1, body weight and femoral length were not affected by PTH treatment in all groups. Similarly, serum IGF‐1 levels were not affected by 4 weeks of intermittent PTH treatment. On the other hand, serum osteocalcin levels increased significantly in all groups treated with PTH (Table 1).
Table 1.
Changes in Serum Hormones and Bone Morphology Following PTH Treatment
| Control | HIT | KO‐HIT | ||||
|---|---|---|---|---|---|---|
| Treatment | Vehicle | PTH | Vehicle | PTH | Vehicle | PTH |
| Serum IGF‐1 (ng/mL) | 299 ± 11 | 289 ± 12 | 797 ± 31b | 904 ± 35c | 841 ± 43b | 842 ± 35c |
| Serum osteocalcin (ng/mL) | 76 ± 6 | 134 ± 11a | 95 ± 5b | 179 ± 16a,c | 74 ± 4 | 130 ± 6a |
| Body weight (g) | 26.8 ± 0.7 | 26.0 ± 0.7 | 33.0 ± 0.7b | 32.0 ± 1.0c | 27.6 ± 0.8 | 28.2 ± 0.6 |
| Femur length (cm) | 15.2 ± 0.1 | 15.2 ± 0.1 | 15.6 ± 0.1 | 15.7 ± 0.2 | 14.8 ± 0.1 | 15.1 ± 0.1 |
| Cortical bone morphology | ||||||
| Tt.Ar. (mm2) | 1.52 ± 0.04 | 1.64 ± 0.03 | 1.76 ± 0.06b | 2.00 ± 0.08a,c | 1.46 ± 0.05 | 1.64 ± 0.05a |
| Ct.Ar. (mm2) | 0.81 ± 0.03 | 0.96 ± 0.03a | 0.94 ± 0.04b | 1.19 ± 0.06a,c | 0.83 ± 0.03 | 0.97 ± 0.04a |
| Ma.Ar. (mm2) | 0.71 ± 0.03 | 0.68 ± 0.01 | 0.82 ± 0.03b | 0.81 ± 0.04c | 0.64 ± 0.02 | 0.67 ± 0.02 |
| Ct.Th. (mm) | 0.199 ± 0.006 | 0.229 ± 0.005a | 0.214 ± 0.006 | 0.257 ± 0.010a,c | 0.209 ± 0.004 | 0.234 ± 0.008a |
| Robustness (Tt.Ar./Le) (mm) | 0.102 ± 0.002 | 0.108 ± 0.002 | 0.113 ± 0.003b | 0.127 ± 0.005a,c | 0.099 ± 0.003 | 0.109 ± 0.003a |
| Cortical TMD (mg/cm3) | 1316 ± 10 | 1318 ± 14 | 1339 ± 9 | 1329 ± 9 | 1359 ± 15b | 1370 ± 8c |
| Trabecular bone morphology | ||||||
| BV/TV | 0.137 ± 0.007 | 0.155 ± 0.009 | 0.122 ± 0.005 | 0.148 ± 0.007a | 0.114 ± 0.006b | 0.145 ± 0.008a |
| Tb.Th. (µm) | 29.8 ± 0.7 | 30.1 ± 1.0 | 30.3 ± 0.8b | 30.3 ± 1.3 | 27.2 ± 0.8b | 29.3 ± 1.1 |
| Tb.N. (1/mm) | 4.60 ± 0.21 | 5.14 ± 0.21 | 4.02 ± 0.13b | 4.89 ± 0.13a | 4.21 ± 0.18 | 4.92 ± 0.12a |
| Tb.Sp. (mm) | 0.195 ± 0.010 | 0.167 ± 0.011 | 0.223 ± 0.008b | 0.176 ± 0.006a | 0.217 ± 0.011b | 0.175 ± 0.005a |
| Trabecular TMD (mg/cm3) | 676 ± 15 | 687 ± 22 | 694 ± 21 | 663 ± 33 | 662 ± 23 | 709 ± 21 |
| Mechanical testing | ||||||
| Stiffness (N/mm) | 158 ± 8 | 162 ± 7 | 186 ± 7b | 232 ± 15a,c | 152 ± 6 | 191 ± 11a,c |
| Maximum load (N) | 27.1 ± 0.9 | 30.8 ± 0.9 | 32.2 ± 1.5b | 38.6 ± 2.0a,c | 26.6 ± 1.1 | 32.5 ± 1.7a |
Different from vehicle of the same genotype.
Different from vehicle‐treated control.
Different from PTH‐treated control.
Analysis of cortical bone following intermittent PTH treatment revealed increases in total cross‐sectional area (Tt.Ar), Ct.Ar, and Ct.Th in both HIT and KO‐HIT mice and an increase in relative cortical area (RCA) in HIT mice (+10.5%) compared with vehicle‐treated mice. The analysis of bone robustness (Tt.Ar/length) and polar moment of inertia also revealed that PTH treatment increased bone robusticity in both HIT and KO‐HIT mice (+10%) compared with vehicle‐treated mice. These increases in cortical bone traits in response to PTH treatment were in accordance with increased mechanical properties. HIT and KO‐HIT male mice showed increases in maximum load (∼20%) and stiffness (∼24%; Table 1), suggesting that PTH anabolic actions on cortical bone in the absence of tissue IGF‐1 can be compensated by elevations in serum IGF‐1 levels. It is important to note that bone traits of PTH‐treated HIT mice were significantly higher than those in PTH‐treated control mice (Table 1; significance labeled with c), whereas no differences were detected between PTH‐treated KO‐HIT and control mice.
Analysis of trabecular bone response to intermittent PTH revealed significant increases in BV/TV in both HIT and KO‐HIT mice (+22%, and +25%, respectively), accompanied by an increase in Tb.N (+20%, and +18%, respectively) and a decrease in Tb.Sp (–21%, and –20%, respectively; Table 1). Unlike the synergy between PTH and IGF‐1 that was observed in the cortical envelope of HIT mice, trabecular bone exhibited no enhancement of morphologic traits when comparing PTH‐treated HIT and control mice. These data indicate that PTH response in the trabecular bone compartment is minimally mediated by serum/tissue IGF‐1.
Histomorphometric analysis at the femoral midshaft was performed after 4 weeks of intermittent PTH treatment at 16 weeks of age (Table 2). We found that percent labeled perimeter (%L.Pm) and bone‐formation rate (BFR) at the periosteal surface increased in all groups treated with PTH. This was in accordance with increased serum osteocalcin levels following intermittent PTH treatment. These results, although not statistically significant, are in accordance with increased morphologic traits assessed by µCT analyses.
Table 2.
Mean Histomorphometric Parameters Following PTH Treatment
| Control | HIT | KO‐HIT | ||||
|---|---|---|---|---|---|---|
| Vehicle | PTH | Vehicle | PTH | Vehicle | PTH | |
| L.Pm (%) | 70.9 ± 0.8 | 80.5 ± 4.8 | 61.7 ± 11.5 | 73.2 ± 6.5 | 53.4 ± 6.1a | 62.3 ± 6.2 |
| MAR (µm/day) | 3.0 ± 0.8 | 2.0 ± 0.2 | 1.3 ± 0.2 | 2.9 ± 0.8 | 1.4 ± 0.1 | 2.0 ± 0.4 |
| BFR/P.Pm (µm/day × 100) | 226.4 ± 83.6 | 165.9 ± 20.0 | 81.6 ± 24.4 | 229.3 ± 75.8 | 78.0 ± 13.4 | 131.4 ± 29.7 |
Different from PTH‐treated control.
Discussion
In this study we used mouse models to manipulate the endocrine and autocrine/paracrine modes of IGF‐1 action. We found that elevations in serum IGF‐1 levels during growth in male mice lead to enhanced bone accrual and to the development of a robust, mechanically superior bone (HIT mice). However, elevations in serum IGF‐1 in mice with Igf1gene ablation in all tissues except the liver (KO‐HIT) demonstrated no morphologic or mechanical gains beyond the normal skeletal morphology and mechanical properties exhibited by adult control mice. These findings are in agreement with our previous study of the development of the female HIT and KO‐HIT skeleton,2 which demonstrated augmentation of all skeletal properties of HIT females and normalization of KO‐HIT females skeletal properties to those of control females by adulthood. Together data from male (this study) and female2 mice suggest that acceleration of skeletal acquisition through increased endocrine IGF‐1 requires autocrine/paracrine IGF‐1. The fact that the linear and transverse femoral growth rates of mice with elevated serum IGF‐1 levels (HIT and KO‐HIT) were statistically indistinguishable between 4 and 8 weeks of age (approximately two‐ and fourfold increases) indicates that the lack of skeletal acceleration in the absence of autocrine/paracrine IGF‐1 is due to abrogation of early growth (before 4 weeks) processes, which result in an attenuated “start point” for enhanced skeletal acquisition by the hepatic Igf1 transgene.
A second focus of this study was the interaction between serum/tissue IGF‐1 and PTH on bone accrual. Here we show that increases in serum IGF‐1 levels (HIT mice) synergized PTH anabolic effects on bone such that PTH‐treated HIT mice exhibited further increases in several skeletal phenotypes compared with PTH‐treated control mice. Four weeks of treatment with intermittent PTH of adult mice did not alter body weight, femur length, or serum IGF‐1 levels but increased significantly serum osteocalcin (a marker of bone formation). This was accompanied by augmentation of cortical and trabecular bone morphologic indices. HIT males showed increases in Tt.Ar., Ct.Ar., and Ct.Th., leading to increased whole‐bone stiffness and maximum load, as evaluated by four‐point bending. Unlike the Igf1 null mice, which had no skeletal response to PTH,5, 7 KO‐HIT mice showed an anabolic response in both cortical and trabecular bone compartments. However, this anabolic response did not differ from that of PTH‐treated control mice, even though serum IGF‐1 levels were similar to those of HIT mice. It should be noted that although histomorphometric indices of increased cortical bone accrual (L.Pm and BFR) were increased in PTH‐treated HIT and KO‐HIT mice, these differences were not statistically significant. This was not unexpected because significant cortical bone enhancement from PTH treatment, with no corresponding changes in histomorphometric indices, has been reported previously10, 19 and appears to arise from increased sample variation as a result of PTH action on cortical surfaces. Together these observations and published data5, 7 suggest that (1) PTH can exert an anabolic effect in bone in the absence of tissue IGF‐1 but only in the presence of elevated serum IGF‐1 levels and (2) elevations in serum IGF‐1 levels synergize PTH action on bone only if the tissue IGF‐1 axis is intact. Thus enhancement of PTH anabolic actions depends on tissue IGF‐1. It should be noted that PTH has significant activity in the kidney both to enhance tubular reabsorption of calcium and to stimulate production of 1,25‐dihydroxyvitamin D3. The latter directly promotes calcium absorption in the intestine. High circulating levels of IGF‐1 also can increase glomerular filtration rates, and independently, IGF‐1 stimulates 1α‐hydroxylase activity in the kidney.20, 21, 22, 23, 24, 25 Therefore, it is conceivable that the PTH anabolic effects in HIT and KO‐HIT mice are also mediated through improved calcium balance.
Clinical studies show significant heterogeneity in the skeletal response to intermittent PTH treatment, implying that local or systemic factors contribute to the variation in anabolic effects of this agent (reviewed in refs. 3, 4, and 26, 27, 28, 29, 30). One such factor may be IGF‐1. Indeed, PTH treatment of Igf1 null mice failed to induce anabolic responses in bone.5, 7 Similarly, PTH treatment of Igf1robKO mice with selective depletion of the IGF‐1 receptor (IGF‐1R) in mature osteoblasts (but normal levels of serum IGF‐1) failed to increase bone formation or resorption markers.10 Furthermore, mice lacking the insulin‐like receptor substrate 1 (IRS‐1), a secondary messenger that transmits IGF‐1R signaling, did not respond to 4 weeks of intermittent PTH treatment.31 In contrast, PTH treatment of the LID mice produced skeletal anabolic responses that were similar to or greater than controls13 despite a 75% reduction in serum IGF‐1 levels. Together these studies suggest that autocrine/paracrine actions of IGF‐1 (via the IGF‐1R) in bone play important roles in mediating PTH anabolic actions. This study extends our understanding of these IGF‐1/PTH interactions by demonstrating that PTH can in fact exert robust anabolic responses on bones in the absence of autocrine/paracrine IGF‐1 (KO‐HIT mice) as long as serum IGF‐1 levels are high. The reasons for this endocrine effect are unclear at the present time. One possibility is that very high circulating concentrations of IGF‐1 may lead to tissue redistribution, thereby compensating for the absence of local IGF‐1 expression. It is also conceivable that the very high levels of hepatic and circulating IGF‐1 in KO‐HIT mice induce expression of IGF‐binding proteins (IGFBPs) in bone that might promote IGF‐1 activity. For example, IGFBP‐2 and IGFBP‐5 are upregulated in response to IGF‐1, and IGFBP‐2 has been shown to have anabolic properties in bone when coupled with IGF‐1.32, 33, 34 Notwithstanding, the interaction between circulating IGF‐1 and the skeletal response to PTH administration in HIT mice is impressive and suggests that both tissue IGF‐1 and the circulating pool of IGF‐1 contribute to a robust skeletal phenotype. Although our previous human study11 was unable to demonstrate a relationship between the trabecular response to PTH by quantitative computed tomography (QCT) and circulating IGF‐1, the animal models presented in this study differ dramatically in their relative serum IGF‐1 levels. Indeed, the only time circulating IGF‐1 concentrations reach levels attained by the HIT model are during a short span of puberty. As such, it would be interesting to determine if the skeletal response to PTH during transient periods of very high circulating IGF‐1 and rapid growth compares with the skeletal responsiveness to PTH administration in aging animals.
Finally, it is conceivable that in our mouse models there may be significant compensatory changes, particularly in response to very high circulating levels of IGF‐1. Serum levels of IGF‐1 in these two strains are at least twice those observed normally in wild‐type mice and markedly greater than during maximal pubertal growth. Further studies are needed to define how these changes affect the skeletal response to PTH.
In summary, we have shown that the skeletal response to PTH depends on tissue IGF‐1 and that circulating levels of IGF‐1 can compensate for the absence of skeletal IGF‐1 only when concentrations are quite high. The mechanism for this compensation requires further study.
Disclosures
All the authors state that they have no conflicts of interest.
Acknowledgements
We would like to thank Mordechai Bet‐On for his assistance in processing tissues for histomorphometry. Financial support for this study was received from NIH Grants AR054919 (SY), AR055141 (SY), and AR 53853 (CJR).
References
- 1. Yakar S, Canalis E, Sun H, et al. Serum IGF‐1 determines skeletal strength by regulating subperiosteal expansion and trait interactions. J Bone Miner Res. 2009; 24: 1481–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sebastien Elis H‐WC, Wu Y, Rosen CJ, et al. Elevated serum levels of IGF‐1 are sufficient to establish normal body size and skeletal properties, even in the absence of tissue IGF‐1. J Bone Miner Res. 2010; 25: 1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Poole KE, Reeve J. Parathyroid hormone: a bone anabolic and catabolic agent. Curr Opin Pharmacol. 2005; 5: 612–617. [DOI] [PubMed] [Google Scholar]
- 4. Martin TJ, Quinn JM, Gillespie MT, Ng KW, Karsdal MA, Sims NA. Mechanisms involved in skeletal anabolic therapies. Ann NY Acad Sci. 2006; 1068: 458–470. [DOI] [PubMed] [Google Scholar]
- 5. Bikle DD, Sakata T, Leary C, et al. Insulin‐like growth factor 1 is required for the anabolic actions of parathyroid hormone on mouse bone. J Bone Miner Res. 2002; 17: 1570–1578. [DOI] [PubMed] [Google Scholar]
- 6. Canalis E, Centrella M, Burch W, McCarthy TL. Insulin‐like growth factor I mediates selective anabolic effects of parathyroid hormone in bone cultures. J Clin Invest. 1989; 83: 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miyakoshi N, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S. Evidence that anabolic effects of PTH on bone require IGF‐I in growing mice. Endocrinology. 2001; 142: 4349–4356. [DOI] [PubMed] [Google Scholar]
- 8. Pfeilschifter J, Laukhuf F, Muller‐Beckmann B, Blum WF, Pfister T, Ziegler R. Parathyroid hormone increases the concentration of insulin‐like growth factor‐I and transforming growth factor beta 1 in rat bone. J Clin Invest. 1995; 96: 767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Watson P, Lazowski D, Han V, Fraher L, Steer B, Hodsman A. Parathyroid hormone restores bone mass and enhances osteoblast insulin‐like growth factor I gene expression in ovariectomized rats. Bone. 1995; 16: 357–365. [DOI] [PubMed] [Google Scholar]
- 10. Wang Y, Nishida S, Boudignon BM, Burghardt A, Elalieh HZ, Hamilton MM, Majumdar S, Halloran BP, Clemens TL, Bikle DD. IGF‐I receptor is required for the anabolic actions of parathyroid hormone on bone. J Bone Miner Res. 2007; 22: 1329–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sellmeyer DE, Black DM, Palermo L, Greenspan S, Ensrud K, Bilezikian J, Rosen CJ. Hetereogeneity in skeletal response to full‐length parathyroid hormone in the treatment of osteoporosis. Osteoporos Int. 2007; 18: 973–979. [DOI] [PubMed] [Google Scholar]
- 12. Wu Y, Sun H, Yakar S, Leroith D. Elevated levels of IGF‐1 in serum rescue the severe growth retardation of IGF‐1 null mice. Endocrinology. 2009; 150: 4395–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yakar S, Bouxsein ML, Canalis E, Sun H, Glatt V, Gundberg C, Cohen P, Hwang D, Boisclair Y, Leroith D, Rosen CJ. The ternary IGF complex influences postnatal bone acquisition and the skeletal response to intermittent parathyroid hormone. J Endocrinol. 2006; 189: 289–299. [DOI] [PubMed] [Google Scholar]
- 14. Yakar S, Rosen CJ, Beamer WG, Ackert‐Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D. Circulating levels of IGF‐1 directly regulate bone growth and density. J Clin Invest. 2002; 110: 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yakar S, Setser J, Zhao H, Stannard B, Haluzik M, Glatt V, Bouxsein ML, Kopchick JJ, LeRoith D. Inhibition of growth hormone action improves insulin sensitivity in liver IGF‐1‐deficient mice. J Clin Invest. 2004; 113: 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jepsen KJ, Hu B, Tommasini SM, Courtland HW, Price C, Terranova CJ, Nadeau JH. Genetic randomization reveals functional relationships among morphologic and tissue‐quality traits that contribute to bone strength and fragility. Mamm Genome. 2007; 18: 492–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987; 2: 595–610. [DOI] [PubMed] [Google Scholar]
- 18. Wu Y, Sun H, Yakar S, LeRoith D. Elevated levels of insulin‐like growth factor (IGF)‐I in serum rescue the severe growth retardation of IGF‐I null mice. Endocrinology. 2009; 150: 4395–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iida‐Klein A, Lu SS, Cosman F, Lindsay R, Dempster DW. Effects of cyclic vs. daily treatment with human parathyroid hormone (1‐34) on murine bone structure and cellular activity. Bone. 2007; 40: 391–398. [DOI] [PubMed] [Google Scholar]
- 20. Bianda T, Glatz Y, Bouillon R, Froesch ER, Schmid C. Effects of short‐term insulin‐like growth factor‐I (IGF‐I) or growth hormone (GH) treatment on bone metabolism and on production of 1,25‐dihydroxycholecalciferol in GH‐deficient adults. J Clin Endocrinol Metab. 1998; 83: 81–87. [DOI] [PubMed] [Google Scholar]
- 21. Menaa C, Vrtovsnik F, Friedlander G, Corvol M, Garabedian M. Insulin‐like growth factor I, a unique calcium‐dependent stimulator of 1,25‐dihydroxyvitamin D3 production. Studies in cultured mouse kidney cells. J Biol Chem. 1995; 270: 25461–25467. [DOI] [PubMed] [Google Scholar]
- 22. Nesbitt T, Drezner MK. Insulin‐like growth factor‐I regulation of renal 25‐hydroxyvitamin D‐1‐hydroxylase activity. Endocrinology. 1993; 132: 133–138. [DOI] [PubMed] [Google Scholar]
- 23. Wong MS, Sriussadaporn S, Tembe VA, Favus MJ. Insulin‐like growth factor I increases renal 1,25(OH)2D3 biosynthesis during low‐P diet in adult rats. Am J Physiol. 1997; 272: F698–703. [DOI] [PubMed] [Google Scholar]
- 24. Wong MS, Tembe VA, Favus MJ. Insulin‐like growth factor‐I stimulates renal 1, 25‐ dihydroxycholecalciferol synthesis in old rats fed a low calcium diet. J Nutr. 2000; 130: 1147–1152. [DOI] [PubMed] [Google Scholar]
- 25. Zoidis E, Gosteli‐Peter M, Ghirlanda‐Keller C, Meinel L, Zapf J, Schmid C. IGF‐I and GH stimulate Phex mRNA expression in lungs and bones and 1,25‐dihydroxyvitamin D(3) production in hypophysectomized rats. Eur J Endocrinol. 2002; 146: 97–105. [DOI] [PubMed] [Google Scholar]
- 26. Sato K. [Therapeutic agents for disorders of bone and calcium metabolism. Development of nasal formulation of hPTH (1‐34)]. Clin Calcium. 2007; 17: 64–71. [PubMed] [Google Scholar]
- 27. Compston JE. Skeletal actions of intermittent parathyroid hormone: effects on bone remodelling and structure. Bone. 2007; 40: 1447–1452. [DOI] [PubMed] [Google Scholar]
- 28. Thomas T. Intermittent parathyroid hormone therapy to increase bone formation. Joint Bone Spine. 2006; 73: 262–269. [DOI] [PubMed] [Google Scholar]
- 29. Rosen CJ. The cellular and clinical parameters of anabolic therapy for osteoporosis. Crit Rev Eukaryot Gene Expr. 2003; 13: 25–38. [DOI] [PubMed] [Google Scholar]
- 30. Swarthout JT, D'Alonzo RC, Selvamurugan N, Partridge NC, Parathyroid hormone‐dependent signaling pathways regulating genes in bone cells. Gene. 2002; 282: 1–17. [DOI] [PubMed] [Google Scholar]
- 31. Yamaguchi M, Ogata N, Shinoda Y, Akune T, Kamekura S, Terauchi Y, Kadowaki T, Hoshi K, Chung UI, Nakamura K, Kawaguchi H. Insulin receptor substrate‐1 is required for bone anabolic function of parathyroid hormone in mice. Endocrinology. 2005; 146: 2620–2628. [DOI] [PubMed] [Google Scholar]
- 32. DeMambro VE, Clemmons DR, Horton LG, Bouxsein ML, Wood TL, Beamer WG, Canalis E, Rosen CJ. Gender‐specific changes in bone turnover and skeletal architecture in igfbp‐2‐null mice. Endocrinology. 2008; 149: 2051–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Amin S, Riggs BL, Melton LJ 3rd, Achenbach SJ, Atkinson EJ, Khosla S. High serum IGFBP‐2 is predictive of increased bone turnover in aging men and women. J Bone Miner Res. 2007; 22: 799–807. [DOI] [PubMed] [Google Scholar]
- 34. Conover CA, Johnstone EW, Turner RT, Evans GL, John Ballard FJ, Doran PM, Khosla S. Subcutaneous administration of insulin‐like growth factor (IGF)‐II/IGF binding protein‐2 complex stimulates bone formation and prevents loss of bone mineral density in a rat model of disuse osteoporosis. Growth Horm IGF Res. 2002; 12: 178–183. [DOI] [PubMed] [Google Scholar]


