Abstract
We previously have described a model of MS in which constitutive expression of murine IL-2 by HSV-1 (HSV-IL-2) causes CNS demyelination in different strains of mice (Zandian et al 2009, IOVS, 50:3275). In the current study, we investigated whether this HSV-IL-2-induced demyelination can be blocked using recombinant viruses expressing different cytokines or by injection of plasmid DNA. We have found that co-infection of HSV-IL-2-infected mice with recombinant viruses expressing IL-12p35, IL-12p40, or IL-12p35 + IL-12p40 did not block the CNS demyelination, and that co-infection with a recombinant virus expressing IFN-γ exacerbated it. In contrast, co-infection with a recombinant virus expressing IL-4 reduced demyelination, while co-infection of HSV-IL-2 infected mice with a recombinant HSV-1 expressing the IL-12 heterodimer (HSV-IL-12p70) blocked the CNS demyelination in a dose-dependent manner. Similarly, injection of IL-12p70 DNA blocked HSV-IL-2-induced CNS demyelination in a dose-dependent manner and injection of IL-35 DNA significantly reduced CNS demyelination. Injection of mice with IL-12p35 DNA, IL-12p40 DNA, IL-12p35 + IL-12p40 DNA, or IL-23 DNA did not have any effect on HSV-IL-2-induced demyelination, while injection of IL-27 DNA increased the severity of the CNS demyelination in the HSV-IL-2 infected mice. This study demonstrates for the first time that IL-12p70 can block HSV-IL-2-induced CNS demyelination and that IL-35 can also reduce this demyelination, whereas IFN-γ and IL-27 exacerbated the demyelination in the CNS of the HSV-IL-2-infected mice. Our results suggest a potential role for IL-12p70 and IL-35 signaling in the inhibition of HSV-IL-2-induced immunopathology by preventing development of autoaggressive T cells.
Keywords: Recombinant virus, IL-12, IL-35, ocular, multiple sclerosis
INTRODUCTION
Demyelinating diseases constitute a spectrum of immunopathologic syndromes in which, myelin, the fatty covering of nerve cell fibers in the brain, optic nerve, and spinal cord is destroyed 1. While the causes and pathogenesis of demyelination are unknown, one hypothesis is that autoimmunity to antigens of the CNS is triggered by environmental factors, such as viral infections, in genetically susceptible individuals and that the activated immune response leads to myelin destruction. One of the major diseases associated with degradation of the myelin sheath is multiple sclerosis (MS) 2, 3. An early manifestation of MS is visual disturbances and optic neuropathy due to demyelination of the optic nerve. This is a common cause of visual and neurological dysfunction in young adults diagnosed with MS 4–8. Thus, the occurrence of optic neuropathy can be used as a prognostic indicator early in the course of MS 4–8.
Epidemiologic studies have implicated interleukin (IL)-2 in the pathology of MS and patients with MS have elevated levels of IL-2 in their cerebrospinal fluid and sera 9–12. IL-2-deficient mice are more resistant to experimental autoimmune encephalitis (EAE) than their heterozygote and wild-type counterparts 13. To explore the possibility that IL-2 plays a role in the pathology of MS in conjunction with viral infection, we constructed a recombinant herpes simplex virus type 1 (HSV-1) that expresses murine IL-2 constitutively 14 as well as a panel of control recombinant viruses that express murine IL-4, interferon (IFN)-γ, IL-12p35, IL-12p40, or IL-12p70 15–18. Ocular infection of different strains of mice (i.e., BALB/c, C57BL/6, SJL/6, and 129SVE) with the HSV-IL-2 virus results in demyelination of the optic nerves, the spinal cords, and brains of the infected mice as determined by histologic examination of tissues obtained at necropsy 19, 20. The HSV-IL-2-infected mice also develop optic neuropathy as determined by changes in the visual-evoked cortical potentials (VECPs) 20. Demyelination in mice is not detectable after infection with wild-type HSV-1 alone, HSV-IL-4 or HSV-IFN-γ viruses, which are identical to HSV-IL-2 except that they express IL-4 or IFN-γ instead of IL-2 19, 20.
We have previously found that both CD8+ and CD4+ T cells contribute to HSV-IL-2-induced CNS demyelination with CD8+ T cells being the primary inducers of demyelination (manuscript in preparation). In the adoptive transfer studies, all of the transferred T cells were positive for expression of FoxP3, irrespective of their CD25 status, and depletion of FoxP3 blocked CNS demyelination by HSV-IL-2. Furthermore, we have shown previously that HSV-IL-2 induced a TH0 response in infected mice 19, which suggested that the presence of a TH0 response may be a contributing factor to HSV-IL-2-induced demyelination. It is known that IL-12 favors the production of TH1 cells through its ability to drive the differentiation of TH0 cells into TH1 cells 21–23, whereas IL-4 favors a TH2 response 24, 25. Thus, it is possible that by manipulating the cytokine environment such that it can push the TH0 responses toward a TH1 and/or TH2, demyelination may be prevented. We therefore undertook the current studies to determine whether IL-4, IFN-γ, IL-12p35, IL-12p40, IL-12p70, IL-23, IL-27, or IL-35 modulate or exacerbate the CNS demyelination that occurs in mice that are infected ocularly with HSV-IL-2.
RESULTS
Demyelination in HSV-IL-2-infected mice is not blocked by co-infection with HSV-IL-4 virus
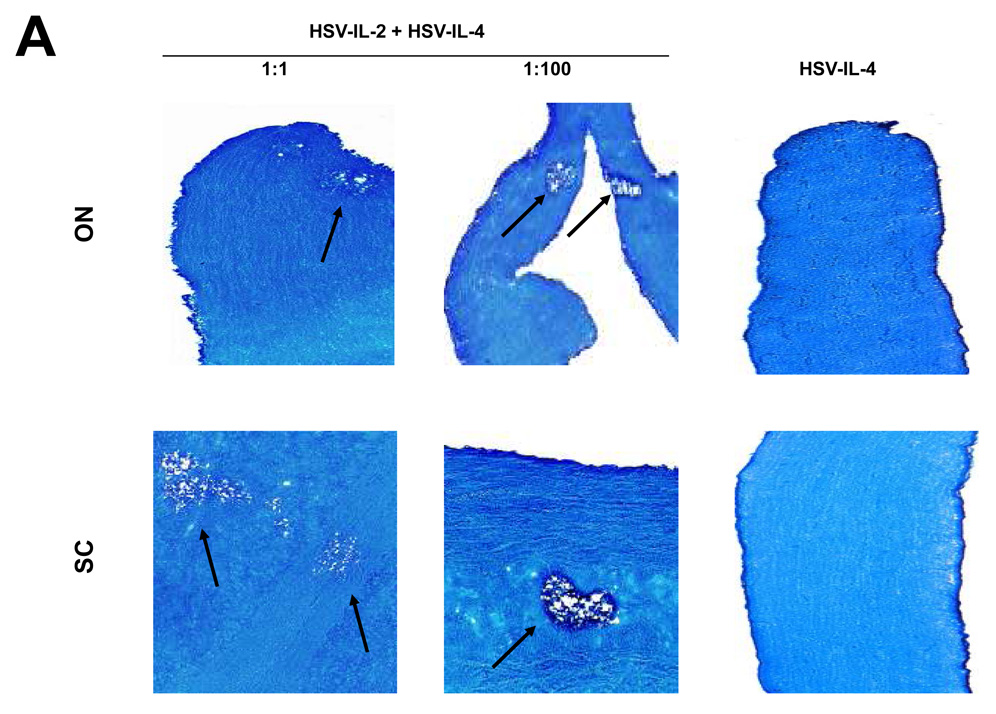
We have shown previously that ocular infection of mice with HSV-IL-2 recombinant virus induces demyelination of CNS in different strains of mouse, including BALB/c and C57BL/6 mice 20. Other investigators have reported that a recombinant HSV-1 expressing IL-4 protected rhesus monkeys 26 and mice 27, 28 from autoimmune encephalomyelitis. Thus, to determine if HSV-IL-2-induced CNS demyelination can be blocked by IL-4, BALB/c mice were infected ocularly with equal amounts (2×105 per eye) of HSV-IL-2 and HSV-IL-4, or with HSV-IL-2 alone or HSV-IL-4 alone. At day 14 post infection, mice were sacrificed and the brain, spinal cord, and optic nerves collected, post-fixed and stained with the myelin stain, LFB. Representative photomicrographs of the LFB-stained sections are shown in Figure 1A. The results indicated that demyelination was not inhibited in the spinal cord or optic nerves of the mice that were co-infected with HSV-IL-2 and HSV-IL-4 at a 1:1 ratio (Fig. 1A, Left panels). Similarly, demyelination was also detected in brains of HSV-IL-2 infected mice (not shown). No demyelination was detected in the CNS of mice infected with HSV-IL-4 alone (Fig. 1A, Right Panels).
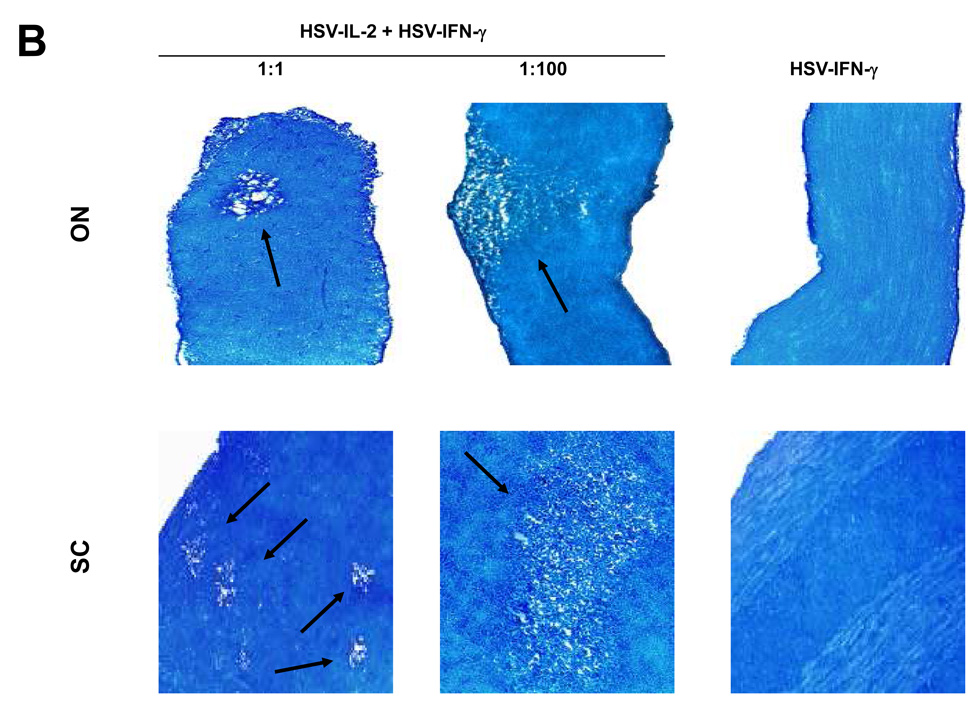
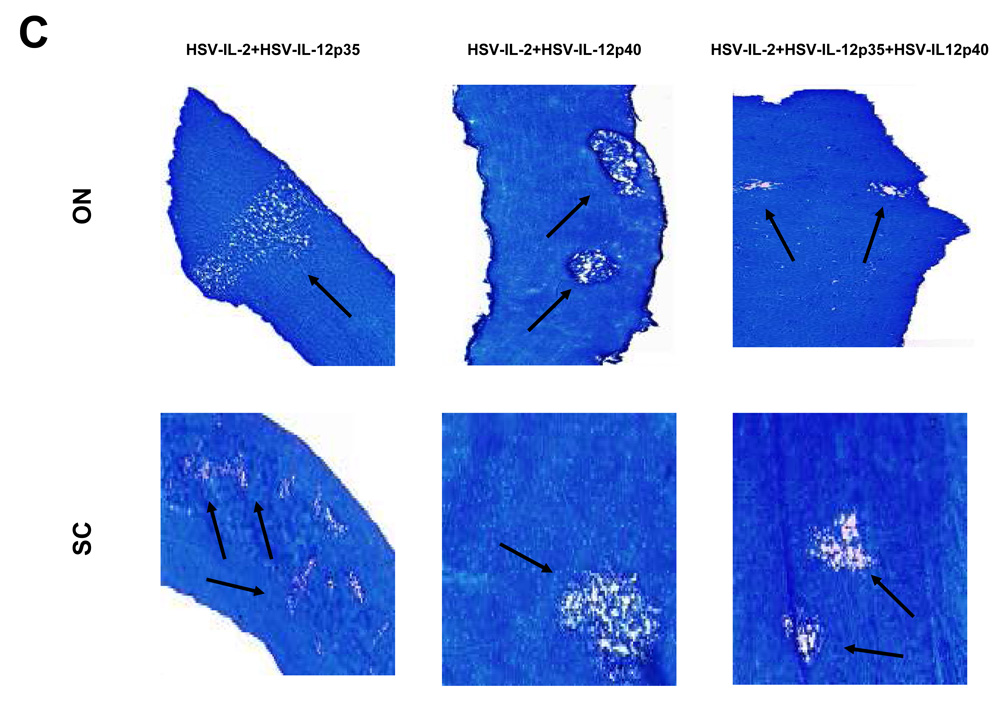
Fig. 1. Effects of IL-4, IFN-γ, IL-12p35, and IL-12p40 on HSV-IL-2-induced CNS demyelination.
Mice were co-infected ocularly with HSV-IL-2 + HSV-IL-4 or HSV-IL-2 + HSV-IFN-γ at 1:1 or 1:100 ratios as described in Materials and Methods. In addition, some mice were co-infected ocularly with HSV-IL-2 + HSV-IL-12p35, HSV-IL-2 + HSV-IL-12p40, or HSV-IL-2 + HSV-IL-12p35 + HSV-IL-12p40 as a 1:1 ratio as described in Materials and Methods. Control mice were infected with 2×107 PFU/eye of HSV-IL-4 or HSV-IFN-γ. On day 14 post infection, optic nerves and spinal cords were collected, fixed, sectioned, and stained with Luxol fast blue (LFB). Representative photomicrographs are shown. Arrows indicate areas of demyelination. 10X direct magnification. Panels: (A) Effects of IL-4 on HSV-IL-2-induced CNS demyelination; (B) Effects of IFN-γ on HSV-IL-2-induced CNS demyelination; and (C) Effects of IL-12p35 and IL-12p40 on HSV-IL-2-induced CNS demyelination.
To determine if higher levels of HSV-IL-4 were capable of affecting HSV-IL-2-induced CNS demyelination, groups of BALB/c mice were subjected to the same experimental protocol except that they were co-infected with HSV-IL-4 at a dose (2×107 PFU/eye) that was 100-fold higher than the dose of HSV-IL-2 (2×105 PFU/eye) used. Demyelination was not blocked in the brains, spinal cords, or optic nerves of the mice that were co-infected with HSV-IL-2 and HSV-IL-4 at a 1:100 ratio (Fig. 1A, Middle panels). Similar results were obtained when female C57BL/6 were co-infected with HSV-IL-2 and HSV-IL-4 recombinant viruses (data not shown). Collectively, these results indicate that IL-4 does not protect against IL-2-induced CNS demyelination in this mouse model.
Demyelination in HSV-IL-2-infected mice is not blocked following co-infection with HSV-IFN-γ virus
The roles of IFN-γ in MS remain controversial and a clinical trial of rIFN-γ for MS was discontinued prematurely due to worsening of disease 29. To evaluate the effects of HSV-IFN-γ on CNS demyelination induced by HSV-IL-2, BALB/c or C57BL/6 mice were infected ocularly with 2×105 per eye of HSV-IL-2 and 2×105 per eye of HSV-IFN-γ, or HSV-IFN-γ alone. At day 14 post infection, the mice were sacrificed and the brains, spinal cords, and optic nerves were stained with LFB. Representative photomicrographs of the stained sections of spinal cords and optic nerves are shown in Figure 1B. Demyelination was not inhibited in the spinal cords or optic nerves of the mice co-infected with the HSV-IL-2 plus HSV-IFN-γ at a 1:1 ratio (Fig. 1B, Left Panels, 1:1). Similarly, demyelination was also detected in brains of HSV-IL-2 infected mice (not shown). To determine if higher doses of HSV-IFN-γ were able to block HSV-IL-2-induced CNS demyelination additional mice were subjected to the same experimental protocol except that they were co-infected with 100-fold higher dose of HSV-IFN-γ (2×107 PFU/eye) relative to HSV-IL-2 (2×105 PFU/eye). Demyelination was not blocked in the brain, spinal cords, or optic nerves of the mice co-infected with a 1 to 100 ratio of HSV-IL-2 to HSV-IFN-γ (Fig. 1A, Middle panels). These results suggest that HSV-IFN-γ, even at a 100-fold higher dose, does not block HSV-IL-2-induced CNS demyelination in either mouse strain tested.
Demyelination in HSV-IL-2-infected mice is not blocked by co-infection with HSV-IL-12p35, HSV-IL-12p40, or HSV-IL-12p35 + HSV-IL-12p40
In preliminary studies using the HSV-1 recombinant viruses expressing murine IL-12p35 and IL-12p40 that we have constructed 30, we observed that infection of macrophages with HSV-IL-2, but not wild-type HSV-1 or recombinant HSV-1 expressing IL-4 or IFN-γ, altered the ratio of the IL-12p35 and IL-12p40 transcripts (unpublished observation). We therefore tested the possibility that co-infection of HSV-IL-2-infected mice with HSV-IL-12p35, HSV-IL-12p40, or HSV-IL-12p35 + HSV-IL12p40 may prevent demyelination. As a control some mice were co-infected with HSV-IL-2 and wild-type (wt) virus. BALB/c mice were infected with HSV-IL-2 + HSV-IL-12p35, HSV-IL-2 + HSV-IL-12p40, HSV-IL-2 + HSV-IL-12p35 + HSV-IL12p40, or HSV-IL-2 + wt at 2×105 PFU/eye of each virus. As shown in Figure 1C, at day 14 post infection, demyelination was observed in the spinal cords and optic nerves of all mice infected ocularly with HSV-IL-2 + HSV-IL-12p35, HSV-IL-2 + HSV-IL-12p40, HSV-IL-2 + HSV-IL-12p35 + HSV-IL12p40, or HSV-IL-2 + wt and similar results were obtained on analysis of the brains of the infected mice (data not shown). None of these recombinant viruses (HSV-IL-12p35, HSV-IL-12p40, or HSV-IL-12p35 + HSV-IL12p40) protected the mice from HSV-IL-2-induced demyelination, even when a higher dose (2×107 PFU/eye) of the recombinant viruses were used (data not shown). Moreover, multiple intraperitoneal injections of mice with the recombinant viruses did not inhibit CNS demyelination from HSV-IL-2 infection (data not shown). The biological activity characteristic of the IL-12 heterodimer cannot be detected in the absence of either subunit 33, 34 as the individual subunits do not exhibit IL-12 heterodimer activity by themselves 35, 36. Thus, our observation of lack of positive effect by the individual subunits is consistent with the literature. The absence of a protective effect by these recombinant viruses against HSV-IL-2-induced CNS demyelination suggests that these viruses do not promote the formation of the IL-12 heterodimer in vivo.
Demyelination in HSV-IL-2-infected mice can be blocked by co-infection with the IL-12 heterodimer in a dose-dependent manner
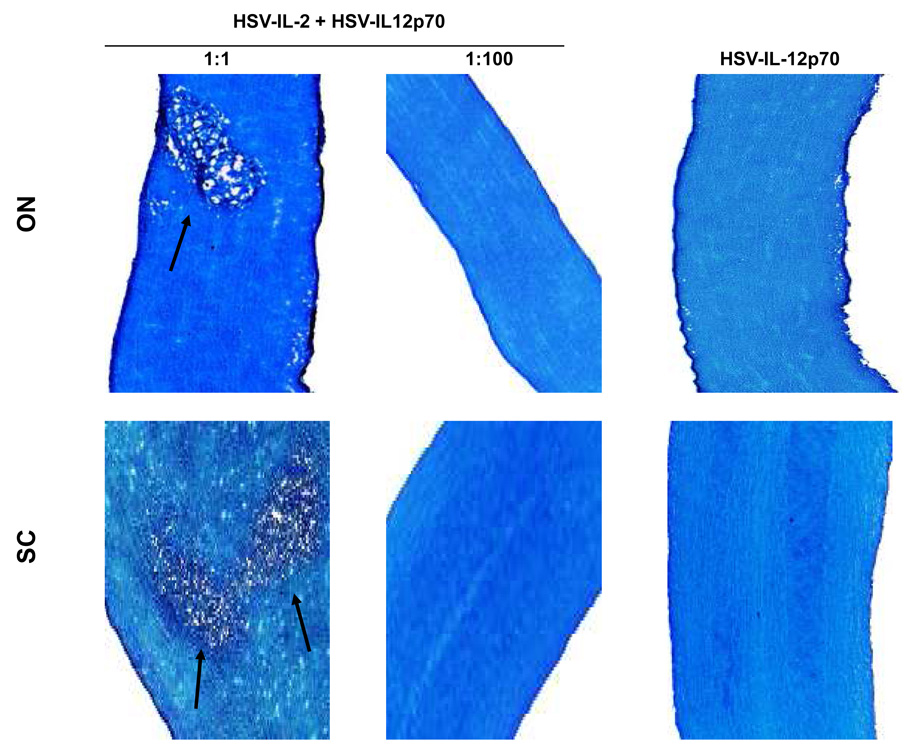
In contrast to HSV-IL-12p35 or HSV-IL-12p40, a recombinant HSV-1 that expresses both IL-12p35 and IL-12p40 in-frame as a heterodimer (HSV-IL-12p70, also called M002) has biological activity in vivo 18, 37. Therefore, we used an HSV-IL-12p70 recombinant virus to determine whether co-infection of mice with this virus would prevent HSV-IL-2-induced demyelination in infected mice. BALB/c mice were infected with HSV-IL-2 and HSV-IL-12p70 at a 1:1, 1:10, or 1:100 ratio and demyelination in the optic nerves and spinal cords of the infected mice was evaluated on day 14 post infection. As expected, infection of the mice with HSV-IL-12p70 alone did not induce demyelination (Fig. 2, IL-12p70, right panels at 2×107 PFU/eye). Demyelination was observed in the optic nerves and spinal cords of all BALB/c mice infected with HSV-IL-2 and HSV-IL-12p70 at a 1:1 ratio (Fig. 2, 1:1 column, Table 1), while only 1 mouse showed demyelination in its spinal cord but not brain or optic nerve (Table 1). In marked contrast, none of the mice infected with a 1:100 ratio of HSV-IL-2 to HSV-IL12p70 showed any signs of demyelination (Fig. 2, 1:100 column, Table 1). These results suggest that the IL-12 heterodimer can inhibit HSV-IL-2-induced CNS demyelination in ocularly infected mice in a dose-dependent manner, and high enough concentrations can block disease pathology in its entirety.
Fig. 2. Blocking of demyelination in HSV-IL-2 infected mice by IL-12p70 HSV-1 recombinant virus.
BALB/c mice were co-infected with HSV-IL-2 + HSV-IL-12p70 at 1:1, 1:10, or 1:100 ratios of HSV-IL-2 to HSV-IL-12p70. On day 14 post-infection, optic nerves and spinal cords were collected, fixed, sectioned, and stained with LFB. Representative photomicrographs are shown. Arrows indicate areas of demyelination. 10X direct magnification.
Table 1.
Role of IL-12 in protection from CNS demyelination by HSV-IL-2 recombinant virus.a
| Mice with CNS demyelination/Total number of mice in treated group | ||||||
|---|---|---|---|---|---|---|
| HSV-IL-2 + HSV-IL-12p70 | IL-12p70 DNA immunization | |||||
| CNS | 1:1 | 1:10 | 1:100 | 1X | 2X | 3X |
| Brain | 5/5 (100%) | 0/5 (0%) | 0/5 (0%) | 1/5 (20%) | 1/5 (20%) | 0/5 (0%) |
| Optic nerve | 3/5 (60%) | 1/5 (20%) | 0/5 (0%) | 1/5 (20%) | 1/5 (20%) | 0/5 (0%) |
| Spinal cord | 5/5 (100%) | 0/5 (0%) | 0/5 (0%) | 1/5 (20%) | 1/5 (20%) | 0/5 (0%) |
Mice were co-infected with 1:1,1:10, or 1:100 ratio of HSV-IL-2 to HSV-IL-12p70, while some mice were immunized 1X, 2X, or 3X and ocularly infected with 2×105 PFU/eye of HSV-IL-2. The presence of demyelination in the optic nerves, spinal cords, and brains of 5 mice per group was assessed on day 14 post infection.
Demyelination in HSV-IL-2-infected mice can also be blocked by injection of IL-12p70 DNA
To further explore the possibility that IL-12p70 can block HSV-IL-2 induced demyelination, we treated HSV-IL-2-infected mice with IL-12p70 DNA. It has previously been shown that DNA vaccine–encoded immunogen is produced by dendritic cells and macrophages residing at the site of inoculation with subsequent elicitation of immune response either by directly transfected antigen-presenting cells (APC) or via cross presentation of antigen expressed from transfected somatic cells by APCs 31. Thus, BALB/c mice were injected with IL-12p70 DNA or vector DNA 1X, 2X, or 3X and infected with HSV-IL-2 4h after the first, second, or third injection. Demyelination in the optic nerves, spinal cords, and brains of infected mice were measured on day 14 post-infection and the data are summarized in Table 1. Eighty percent (4 out of 5) mice injected once with IL-12p70 DNA were protected from HSV-IL-2-induced CNS demyelination and similar results were obtained when the mice were injected twice and infected with HSV-IL-2 (Table 1). Demyelination was not observed in the optic nerves, spinal cords, or brains of the BALB/c mice that had been injected with IL-12p70 DNA three times and infected with HSV-IL-2 (Table 1). As expected mice injected with vector DNA and infected with HSV-IL-2 exhibited demyelination in their optic nerves, spinal cords, and brains (not shown). These experiments also were carried out using C57BL/6 mice and similar results were obtained (data not shown). Collectively, these results suggest that IL-12p70 DNA can inhibit HSV-IL-2-induced CNS demyelination in ocularly infected mice in a dose-dependent manner as does HSV-IL-12p70 virus.
Roles of other IL-12 family members in blocking CNS demyelination by HSV-IL-2
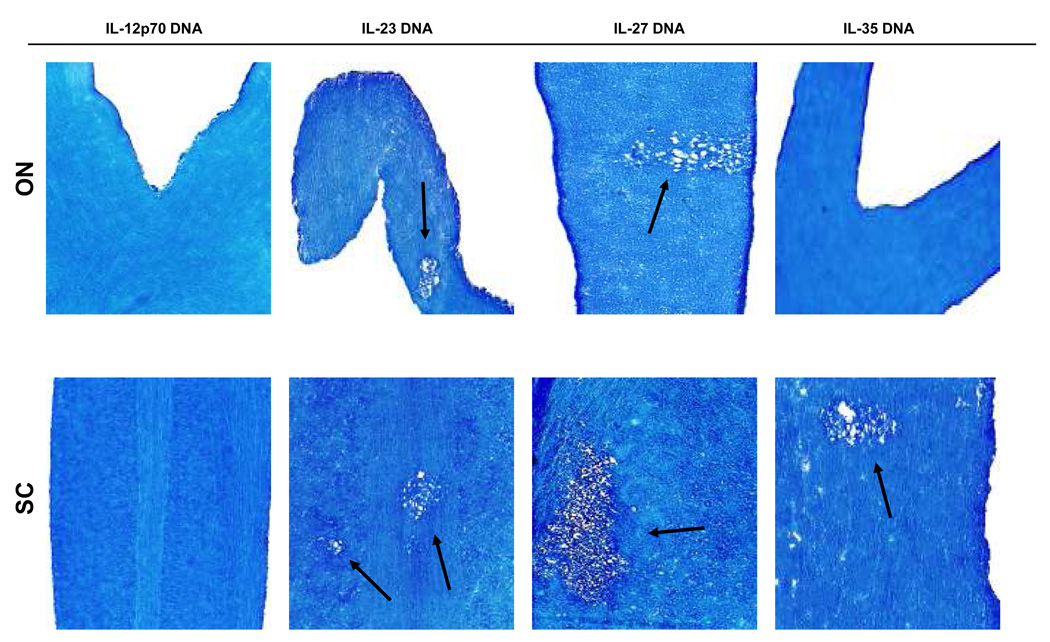
To determine if any other members of the IL-12 family can alter demyelination, BALB/c mice were injected with IL-23 DNA, IL-27 DNA, or IL-35 DNA, either once or three times before ocular infection with HSV-IL-2. Demyelination in the optical nerves, spinal cord, and brain of infected mice was evaluated on day 14 post-infection. Demyelination was detected in the optical nerves, spinal cords, and brain of all BALB/c mice injected with IL-23 DNA (Fig. 3 and Table 2, IL-23) and IL-27 DNA (Fig. 3 and Table 2, IL-27,). In contrast, demyelination was detected in the spinal cord and brain but not optic nerves of one of the mice injected once with IL-35 DNA, while after three injections demyelination was only detected in the spinal cord of the injected mice (Fig. 3 and Table 2 IL-35). Thus, our results suggest that IL-35 DNA reduced the severity of demyelination in injected mice, whereas neither IL-23 nor IL-27 blocked HSV-IL-2-induced CNS demyelination in infected mice.
Fig. 3. Effects of IL-12 family members on HSV-IL-2-induced demyelination.
BALB/c mice were injected intramuscularly 3X with IL-12p70 DNA, IL-23 DNA, IL-27 DNA, IL-35 DNA as described in Materials and Methods. Four hours after the third DNA injection, mice were infected ocularly with HSV-IL-2. On day 14 post infection, optic nerves and spinal cords were collected, fixed, sectioned, and stained with LFB. Representative photomicrographs are shown. Arrows indicate areas of demyelination. 10X direct magnification.
Table 2.
Role of IL-12 family in protection from CNS demyelination by HSV-IL-2 recombinant virus.a
| Mice with CNS demyelination/Total number of mice in the treated group | ||||||
|---|---|---|---|---|---|---|
| 1 Immunization | 3 Immunizations | |||||
| CNS | IL-23 | IL-27 | IL-35 | IL-23 | IL-27 | IL-35 |
| Brain | 5/5 (100%) | 5/5 (100%) | 1/5 (20%) | 5/5 (100%) | 5/5 (100%) | 0/5 (0%) |
| Optic nerve | 5/5 (100%) | 5/5 (100%) | 0/5 (0%) | 5/5 (100%) | 5/5 (100%) | 0/5 (0%) |
| Spinal cord | 5/5 (100%) | 5/5 (100%) | 1/5 (20%) | 5/5 (100%) | 5/5 (100%) | 1/5 (20%) |
Mice were injected 1X or 3X with IL-23, IL-27, or IL-35 DNA as described in Materials and Methods. Injected mice were ocularly infected with 2×105 PFU/eye of HSV-IL-2 4 hr after the 1X or the 3X injection. Presence of demyelination in the optic nerves, spinal cords, and brains of 5 mice per group was assessed on day 14 post infection.
Quantitation of demyelination severity in infected mice
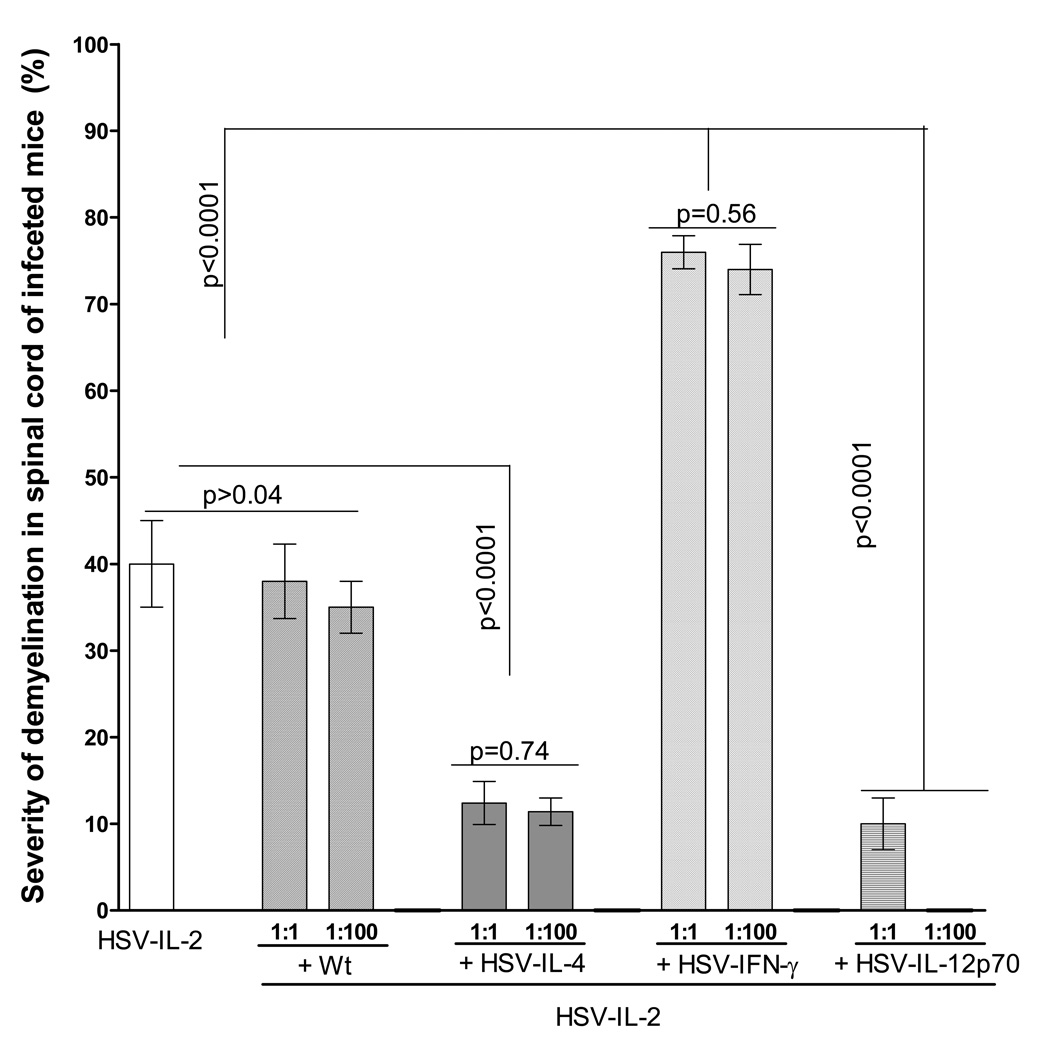
We observed that the severity of the HSV-IL-2-induced demyelination appeared to be greater in the presence of HSV-IFN-γ (Fig. 1B) than in the presence of HSV-IL-4 (Fig. 1A) or parental virus. To determine if more plaques are present in the CNS of HSV-IL-2 + HSV-IFN-γ-infected mice as compared with HSV-IL-2 alone, HSV-IL-2 + HSV-IL-4, or HSV-IL-2 + wt HSV-1, we counted the number and size of the observed plaques in the spinal cords of infected mice. The data are shown as the area of demyeli nation per total stained sections in Figure 4. The area of demyelination in HSV-IL-2 + wt HSV-1-infected mice was similar to mice that were infected with HSV-IL-2 alone (at a 1:1 ratio) (Fig. 4). Also, the level of demyelination was not increased when mice were infected with a 100-fold higher dose of wt HSV-1 (ratio of wt to HSV-IL-2 = 1:100) (Fig. 4). However, the area of demyelination in HSV-IL-2 + HSV-IL-4-infected mice was significantly lower than mice infected with HSV-IL-2 alone (at a 1:1 ratio) (Fig. 4). The level of demyelination was not altered when mice were infected with a 100-fold higher dose of HSV-IL-4 (Fig. 4). In contrast to co-infection with HSV-IL-4 recombinant virus, the area of demyelination was greater in the CNS of mice co-infected with HSV-IL-2 + HSV-IFN-γ (at a 1:1 ratio) than in the CNS of mice infected with HSV-IL-2 alone, HSV-IL-2 + HSV-IL-4, or HSV-IL-2 + wt HSV-1 (Fig. 4). However, the area of demyelination in HSV-IL-2 + HSV-IFN-γ infected mice was not increased when mice were infected with a 100-fold higher dose of HSV-IFN-γ (ratio of HSV-IFN-γ to HSV-IL-2 = 1:100). Furthermore, co-infection of HSV-IL-2 infected mice with HSV-IL-12p70 virus reduced severity of demyelination in co-infected mice (at a 1:1 ratio) than in the CNS of mice infected with HSV-IL-2 alone (Fig. 4), while no demyelination was detected in HSV-IL-2 + HSV-IL-12p70 infected mice when mice were infected with a 100-fold higher dose of HSV-IL-12p70 (Fig. 4). Thus, our results suggest that co-infection of HSV-IL-2-infected mice with HSV-IFN-γ increases CNS demyelination in a dose-independent manner, IL-4 affect the IL-2-induced demyelination by decreasing its severity, wt HSV-1 does not affect the HSV-IL-2-induced demyelination, neither increasing nor decreasing its severity, while HSV-IL-12p70 affected the HSV-IL-2-induced demyelination by decreasing its severity in a dose-dependent manner.
Fig. 4. Severity of CNS demyelination in mice co-infected with HSV-IL-2 and recombinant viruses expressing different cytokine genes.
The spinal cords of each of the 5 animals in each treatment group described in Figures 1, 2, 3, and 4 were sectioned, every fourth section was stained, and the size of the demyelination plaques in the entire sections of the spinal cords was determined as described in Materials and Methods. Data are presented as the percentage of the demyelination area in each section per total sections stained using a total of 150 sections from 5 mice per group.
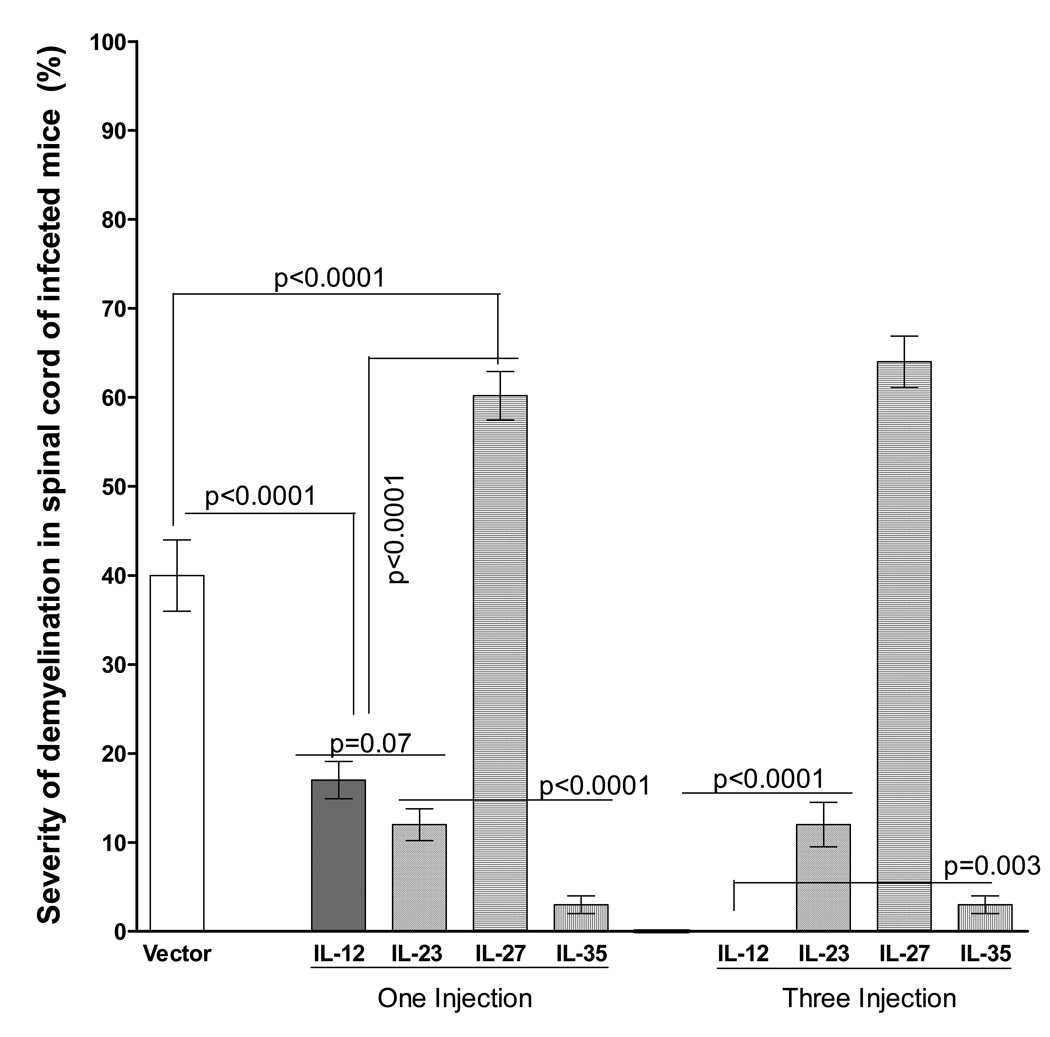
HSV-IL-2 infection of mice that were injected with IL-12, IL-23, IL-27, or IL-35 DNA also produced different patterns of demyelination (Fig. 3). We quantitated the area of demyelination (the number and size of observed plaques in the spinal cords) in mice injected either once or three times with each of these cytokine DNAs. As shown in Figure 5 (one injection), mice injected once with either IL-12, IL-23, or IL-35 DNA had significantly smaller areas of demyelination than mice injected with vector control or mice injected with IL-27. A greater area of demyelination was detected in the CNS of mice injected with IL-27 once than other groups (Fig. 5, One Injection). No demyelination was detected in the CNS of mice injected three times with IL-12 DNA, and no changes were detected in the level of demyelination in mice injected three times with either IL-23 or IL-23 (Fig. 5, Three injections). Similarly, a smaller area of demyelination was detected in the CNS of mice injected three times with IL-35 DNA (Fig. 5). Thus, our results suggest that injection of mice with IL-27 increased the severity of CNS demyelination, whereas IL-12, IL-23 and IL-35 reduced the severity of CNS demyelination with IL-12 and IL-35 playing a more important role in reducing or eliminating demyelination, respectively. IL-12 reduced the severity of demyelination in a dose-dependent manner, while reduction in demyelination in IL-23 and IL-35 injected mice was dose-independent as was the increase in the severity of demyelination in IL-27 DNA injected mice.
Fig. 5. Severity of CNS demyelination in mice injected with IL-12 family DNAs and infected with HSV-IL-2.
The spinal cords of each of the 5 animals in each treatment group described in Figure 5 were sectioned every fourth section was stained, and the size of the demyelination plaques in the entire sections of the spinal cords was determined as described in Materials and Methods. Data are presented as the percentage of the demyelination area in each section per total sections stained using a total of 150 sections from 5 mice per group.
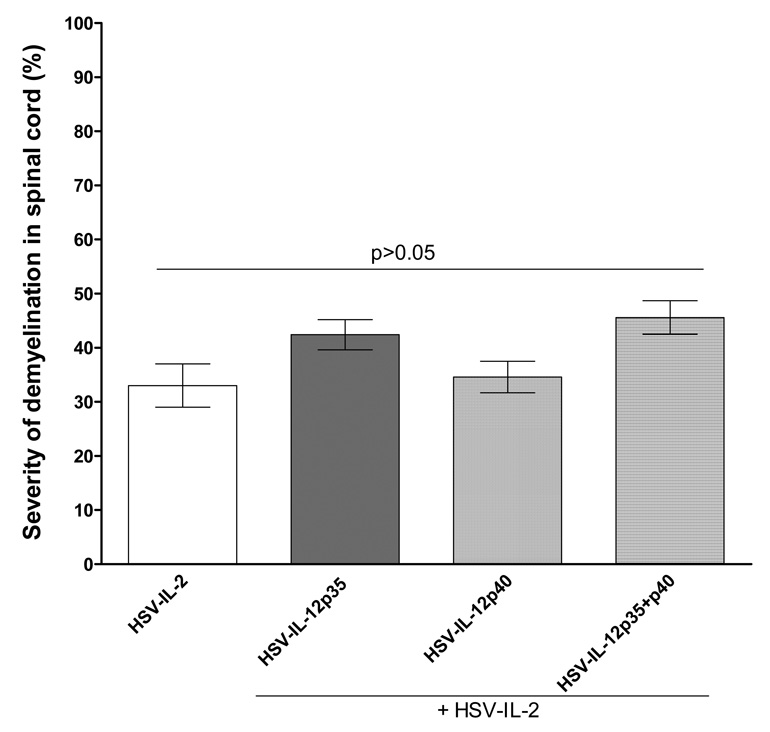
Our Co-infection results with HSV-IL-12p70 and DNA injection with IL-12 DNA, IL-23 DNA, and IL-35 DNA showed significant reduction or elimination of HSV-IL-2 induced CNS demyelination in infected mice. Since IL-12p35 and/or IL-12p40 are components of IL-12p70, IL-23, IL-27, and IL-35, to determine if IL-12p35 or IL-12p40 individually or in combination would alter the severity of HSV-IL-2 induced demyelination, mice were co-infected with HSV-IL-2 + HSV-IL12p35, HSV-IL-2 + HSV-IL12p40, or HSV-IL-2 + HSV-IL12p35 + HSV-IL-12p40. Similarly, some mice were injected with vector DNA, IL-12p35 DNA, IL-12p40 DNA, or IL-12p35 + IL-12p40 DNA and injected mice were ocularly infected with HSV-IL-2. We counted the number and size of the observed plaques in the spinal cords of infected mice. The data are shown as the area of demyelination per total stained sections in Figure 6. The area of demyelination in HSV-IL-2 + HSV-IL-12p35, HSV-IL-2 + HSV-IL12p40, or HSV-IL-2 + HSV-IL-12p35 + HSV-IL-12p40 infected mice was similar to mice that were infected with HSV-IL-2 alone (Fig. 6, Left Panel). Similarly, no increase in demyelination was observed in mice injected with IL-12p35 DNA, IL-12p40 DNA, or IL-12p35 + IL-12p40 DNA and infected with HSV-IL-2 compared with vector DNA (not shown). Thus, our results suggest that in contrast to heterodimer forms of IL-12, IL-23, or IL-35, the individual forms of IL-12p35 or IL-12p40 do not affect the HSV-IL-2-induced demyelination, neither increasing nor decreasing its severity.
Fig. 6. Severity of CNS demyelination in mice infected with recombinant viruses expressing IL-12 subunits or injected with their DNAs and infected with HSV-IL-2.
The spinal cords of each of the 5 animals in each treatment group co-infected with HSV-IL-2 + HSV-IL-12p35, HSV-IL-2 + HSV-IL-12p40, or HSV-IL-2 + HSV-IL-12p35 + HSV-IL-12p40 were sectioned every fourth section was stained, and the size of the demyelination plaques in the entire sections of the spinal cords was determined as described in Materials and Methods. Data are presented as the percentage of the demyelination area in each section per total sections stained using a total of 150 sections from 5 mice per group.
DISCUSSION
We have reported previously that HSV-IL-2 causes demyelination in periventricular white matter, brain stem, and spinal cord white matter infected mice, whereas wild-type HSV-1 strain McKrae, HSV-1 strain KOS, parental dLAT2903, or recombinants HSV-1 expressing IL-4 or IFN-γ do not 19, 20. To further confirm the importance of IL-2 in our HSV-IL-2 model of MS, we looked at the possibility of blocking MS-like symptoms in HSV-IL-2 infected mice by co-infection of HSV-IL-2 infected mice with recombinant viruses expressing TH1 or TH2 cytokines. The results of these co-infection experiments were then confirmed by injecting mice with plasmid DNAs expressing the genes of interest.
In contrast to IL-2 9–12, the levels of IL-4 have been reported to be lower than normal in the sera of patients with MS 32, 33. Recombinant IL-4 (rIL-4) has been shown to diminish demyelination and improve the clinical course of mice with EAE, apparently by influencing the development of the T cells to favor protective, rather than harmful, effects 27. When fed to mice, retinoids, which increase the levels of IL-4 and TGF-α, also improve the course of EAE 34. It also has been shown that CNS gene therapy with HSV-1 vectors expressing IL-4 protected rhesus monkeys 26 and mice 29, 30 from autoimmune encephalomyelitis, suggesting that vectors containing anti-inflammatory cytokine genes may have a therapeutic potential for MS. Similar to these previous reports, we significantly reduced but could not block HSV-IL-2 CNS demyelination in mice co-infected with HSV-IL-4, independent of dose of HSV-IL-4 relative to that of HSV-IL-2.
In MS, a clinical trial of rIFN-γ was discontinued prematurely due to worsening of disease 29. Similarly, in this study we have shown that the CNS demyelination observed in HSV-IL-2-infected mice was exacerbated on co-infection with HSV-IFN-γ recombinant virus in dose-independent manner even though mice infected with HSV-IFN-γ recombinant virus alone do not develop CNS demyelination 19, 20. It has been reported that in EAE induced by CD8+ T cells, administration of anti–IFN-γ antibody reduces the severity of the disease, whereas in EAE induced by CD4+ T cells, administration of anti–IFN-γ actually aggravates the disease 35. In our model, both CD4+ and CD8+ T cells contribute to CNS demyelination with CD8+ T cells playing a more prominent role then the CD4+ T cells (Manuscript in preparation). Thus, the greater demyelination we observed in the presence of HSV-IFN-γ recombinant virus could be due in a shift in the TH1 arm of the immune response in the infected mice.
Previously, we have shown that the infection of mice with HSV-IL-2 induces a TH1 + TH2 response 19. IL-12 is known to play a major role in regulating the balance of the TH1 and TH2 responses 36, 37. It has been reported that IL-12 is a critical cytokine in the pathogenesis of EAE 38 and subsequent studies showed that the IL-12p40 component of IL-12 is involved in EAE-induced CNS pathology but this effect is mediated by the binding of the IL-12p40 to IL-23p19 rather than its binding to IL-12p35 39, 40. Thus, an imbalance of IL-12p40 and IL-12p35 in the HSV-IL-2-infected mice may be responsible for a shift to a TH1 + TH2 pattern of cytokine responses as we reported previously 19. Recently, we have shown that HSV-IL-2 infection of macrophages alters the balance of IL-12p35 and IL12p40 transcripts, which could alter the development of the TH1 response in the HSV-IL-2 infected mice. This possibility is supported by our current results that showed that co-infection of mice with a recombinant HSV-1 expressing IL-12p70 blocked HSV-IL-2-induced demyelination in a dose-dependent manner. At a 1:1 or 1:10 ratio of HSV-IL-2 to HSV-IL-12p70, 80% of the co-infected mice were protected from demyelination, and at a ratio of 1:100, none of the infected mice developed CNS demyelination. Similarly, when mice were injected either one or twice with IL-12p70 DNA and then infected with HSV-IL-2, 80% of the mice were protected from CNS demyelination, while 100% of the mice injected three times with IL-12p70 DNA were protected from demyelination. However, co-infection of mice with HSV-IL-2 + HSV-IL-12p35, HSV-IL-2 + IL-12p40, or HSV-IL-2 + HSV-IL-12p35 + IL-12p40 did not block demyelination. Similarly injection of IL-12p35 DNA alone, IL-12p40 DNA alone, or a mixture did not block demyelination in HSV-IL-2 infected mice.
The IL-12 family members are heterodimeric complexes, composed of an α-subunit (p35, p19, or p28) and a β-subunit {p40 and Epstein–Barr virus-induced gene 3 (EBI3)} 41. The p40 subunit covalently binds to the p35 and p19 chains to form IL-12p70 and IL-23, respectively, while EBI3 binds noncovalently to p28 and p35 to form IL-27 and IL-35, respectively 42. In this study, we have shown that in contrast to IL-12p70, injection of mice with IL-23 or IL-27 did not protect injected mice from HSV-IL-2-induced demyelination, while IL-35 significantly reduced CNS demyelination. In this study, we found that injection of mice with IL-27, but not IL-23 or IL-35, exacerbated the severity of demyelination in HSV-IL-2 infected mice. IL-27 is a pleiotropic cytokine with divergent effects. It has been reported that IL-27 negatively regulates TH2 responses following intestinal nematode infection 43 and asthma 44 and that IL-27 neutralization protects mice against lethal septic peritonitis by enhancing the neutrophil function 45. IL-27 also was shown to inhibit peripheral conversion of T cells into inducible Tregs 46. The IL-27 inhibitory effect on TH2 and cytokine production is due to downregulation of GATA-3 and upregulation of T-bet expression 47. Thus, in contrast to other members of IL-12 family, IL-27 seems to have a negative effect on TH-development and cytokine production. Therefore, the higher demyelination in IL-27 injected mice could be due to a shift in TH1/TH2 responses. Some studies suggested that the IL-23/TH17 pathway plays a crucial role in the development of autoimmunity 48. However, our results rule out the involvement of IL-23 in HSV-IL-2-induced demyelination. In this study, we have shown that IL-35 blocks demyelination in optic nerves and brain of HSV-IL-2-infected mice and significantly reduces demyelination in SC of infected mice. Previously it was shown that IL-35 displays immunosuppressive properties 49, 50.
The results indicate that: (1) The development of the demyelinated lesions observed after infection of mice with HSV-IL-2 is blocked in a dose-dependent manner on co-infection with IL-12p70 recombinant virus or injection of IL-12p70 DNA. Demyelination also was significantly reduced on injection of IL-35 DNA injection; (2) The HSV-IL-2-induced demyelination was reduced by co-infection with recombinant virus expressing IL-4 or injection of IL-23 DNA; (3) The HSV-IL-2-induced demyelination was not altered by co-infection with recombinant viruses expressing IL-12p35, IL-12p40 or both; and (4) The HSV-IL-2-induced demyelination was enhanced by co-infection with IFN-γ recombinant virus or injection of IL-27 DNA. In summary, our results suggest a potential role for IL-12p70 and IL-35 in the inhibition of HSV-IL-2-induced immunopathology.
MATERIALS AND METHODS
Mice, viruses, and cells
Six-week old female BALB/c or C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were handled in accordance with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research under an approved IACUC protocol.
Plaque-purified HSV-1 strains, McKrae or KOS (wild type) and HSV-1 recombinant viruses expressing IL-2, IL-4, IFN-γ, IL-12p35, IL-12p40, or IL-12p70 (HSV-IL-2, HSV-IL-4, HSV-IFN-γ, HSV-IL-12p35, HSV-IL-12p40, and HSV-IL-12p70) were grown in rabbit skin (RS) cell monolayers in minimal essential medium (MEM) containing 5% fetal calf serum (FCS), as described previously 14, 15, 17. McKrae and HSV-IFN-γ viruses are virulent at an infectious dose of 2 × 105 plaque forming units (PFU)/eye, whereas the other viruses used in this study are attenuated. Previously we have shown that in tissue culture, the replication of the HSV-IL-2 was two-logs lower than the wild-type virus at a low MOI 14, 15, 17. Addition of recombinant anti-IL-2 polyclonal antibody markedly enhanced HSV-IL-2 replication in tissue culture. In the 7-day period after ocular infection of BALB/c mice, the replication of HSV-IL-2 was significantly lower than that of wild-type virus in tear cultures, whole eyes, and brain, but was equivalent to wild-type replication in the trigeminal ganglia.
Ocular infection
Mice were infected ocularly with 2 × 105 PFU of McKrae, KOS, recombinant HSV-IL-2, HSV-IL-4, HSV-IFN-γ, HSV-IL-12p35, or HSV-IL-12p40 per eye. Some mice were co-infected with 2 × 105 PFU/eye of HSV-IL-2 and 2 × 105, 2 × 106, or 2 × 107 PFU of HSV-IL-4, HSV-IFN-γ, HSV-IL-12p70, HSV-IL-12p35, HSV-12p40, or HSV-IL-12p35 + HSV-IL-12p40 virus. Each virus was suspended in 5 µl of tissue culture media and administered as an eye drop.
Preparation of optic nerve, spinal cord, and brain for pathologic analysis
The optic nerves, spinal cords, and brains of the experimental and control mice were removed at necropsy on day 14 post-infection. They were then snap-frozen in an isopentane-liquid nitrogen bath and stored at −80°C. Transverse sections of each tissue, 8–10 µm thick, were cut, air-dried overnight, and fixed in acetone for 3 min at 25°C 51. Demyelination in each section was confirmed by monitoring adjacent sections.
Analysis of demyelination using Luxol Fast Blue (LFB) staining
The presence or absence of demyelination in the optic nerves, spinal cords, and brains of infected mice was evaluated using LFB staining of formalin-fixed sections as we described previously 19. The number of plaques, size of plaques, and shape of plaques on multiple fields were evaluated by investigators, who were blinded to the treatment groups, using serial sections of CNS tissues. The amount of myelin loss in the stained sections of spinal cords, optic nerves, and brains also was measured using an image analysis system coupled to a microscope. The area of demylinated lesion (clear) to the blue (normal) were measured using 150 random sections from brain, spinal cords, and optic nerves of each animal. The percentage of myelin loss was calculated by dividing the lesion size into the total area for each section.
DNA injection
The complete open-reading frames (ORF) for IL-12p70, IL-23, IL-27, and IL-35 (pORF-mIL12, pORF-mIL23, pORF-mIL27, pORF-mIL35) were purchased from InvivoGen (San Diego, CA), while the ORF for IL-12p35 and IL-12p40 was inserted into pVR1055 as we described previously 52. Plasmids DNA were purified using a cesium chloride gradient. In each experiment, five mice per group were injected intramuscularly (in the quadriceps) using a 27 gauge needle with 100 µg of cesium chloride-purified DNA in a total volume of 50 µl of PBS once (4 h before ocular infection), twice (7 days and 4 h before ocular infection), or three times (14, days, 7 days and 4 hr before ocular infection). As a negative control, we used mock-treated mice that were similarly injected with vector DNA alone.
Statistical analysis
Fisher’s exact tests were performed using the computer program Instat (GraphPad, San Diego) to compare demyelination in infected mice and in control groups. Results were considered statistically significant when the P value was <0.05.
ACKNOWLEDGEMENTS
This work was supported by Public Health Service grant EY15557 from the National Eye Institute. We thanks Dr. James M. Markert Division of Neurosurgery, Department of Surgery, University of Alabama at Birmingham for providing the M002 (HSV-IL-12p70) virus.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 2.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343(13):938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 3.Hunter SF, Weinshenker BG, Carter JL, Noseworthy JH. Rational clinical immunotherapy for multiple sclerosis. Mayo Clin Proc. 1997;72(8):765–780. doi: 10.1016/S0025-6196(11)63598-2. [DOI] [PubMed] [Google Scholar]
- 4.Soderstrom M, Ya-Ping J, Hillert J, Link H. Optic neuritis: prognosis for multiple sclerosis from MRI, CSF, and HLA findings. Neurology. 1998;50(3):708–714. doi: 10.1212/wnl.50.3.708. [DOI] [PubMed] [Google Scholar]
- 5.Sandberg-Wollheim M, Bynke H, Cronqvist S, Holtas S, Platz P, Ryder LP. A long-term prospective study of optic neuritis: evaluation of risk factors. Ann Neurol. 1990;27(4):386–393. doi: 10.1002/ana.410270406. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez M, Siva A, Cross SA, O'Brien PC, Kurland LT. Optic neuritis: a population-based study in Olmsted County, Minnesota. Neurology. 1995;45(2):244–250. doi: 10.1212/wnl.45.2.244. [DOI] [PubMed] [Google Scholar]
- 7.O'Riordan JI, Losseff NA, Phatouros C, Thompson AJ, Moseley IF, MacManus DG, et al. Asymptomatic spinal cord lesions in clinically isolated optic nerve, brain stem, and spinal cord syndromes suggestive of demyelination. J Neurol Neurosurg Psychiatry. 1998;64(3):353–357. doi: 10.1136/jnnp.64.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghezzi A, Martinelli V, Torri V, Zaffaroni M, Rodegher M, Comi G, et al. Long-term follow-up of isolated optic neuritis: the risk of developing multiple sclerosis, its outcome, and the prognostic role of paraclinical tests. J Neurol. 1999;246(9):770–775. doi: 10.1007/s004150050453. [DOI] [PubMed] [Google Scholar]
- 9.Lu CZ, Fredrikson S, Xiao BG, Link H. Interleukin-2 secreting cells in multiple sclerosis and controls. J Neurol Sci. 1993;120(1):99–106. doi: 10.1016/0022-510x(93)90032-t. [DOI] [PubMed] [Google Scholar]
- 10.Gallo P, Piccinno M, Pagni S, Tavolato B. Interleukin-2 levels in serum and cerebrospinal fluid of multiple sclerosis patients. Ann Neurol. 1988;24(6):795–797. doi: 10.1002/ana.410240618. [DOI] [PubMed] [Google Scholar]
- 11.Gallo P, Piccinno MG, Pagni S, Argentiero V, Giometto B, Bozza F, et al. Immune activation in multiple sclerosis: study of IL-2, sIL-2R, and gamma-IFN levels in serum and cerebrospinal fluid. J Neurol Sci. 1989;92(1):9–15. doi: 10.1016/0022-510x(89)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Trotter JL, Clifford DB, McInnis JE, Griffeth RC, Bruns KA, Perlmutter MS, et al. Correlation of immunological studies and disease progression in chronic progressive multiple sclerosis. Ann Neurol. 1989;25(2):172–178. doi: 10.1002/ana.410250211. [DOI] [PubMed] [Google Scholar]
- 13.Petitto JM, Streit WJ, Huang Z, Butfiloski E, Schiffenbauer J. Interleukin-2 gene deletion produces a robust reduction in susceptibility to experimental autoimmune encephalomyelitis in C57BL/6 mice. Neurosci Lett. 2000;285(1):66–70. doi: 10.1016/s0304-3940(00)00996-4. [DOI] [PubMed] [Google Scholar]
- 14.Ghiasi H, Osorio Y, Perng GC, Nesburn AB, Wechsler SL. Overexpression of interleukin-2 by a recombinant herpes simplex virus type 1 attenuates pathogenicity and enhances antiviral immunity. J Virol. 2002;76(18):9069–9078. doi: 10.1128/JVI.76.18.9069-9078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghiasi H, Osorio Y, Hedvat Y, Perng GC, Nesburn AB, Wechsler SL. Infection of BALB/c mice with a herpes simplex virus type 1 recombinant virus expressing IFN-g driven by the LAT promoter. Virology. 2002;302(2):144–154. doi: 10.1006/viro.2002.1609. [DOI] [PubMed] [Google Scholar]
- 16.Osorio Y, Sharifi BG, Perng G, Ghiasi NS, Ghiasi H. The role of T(H)1 and T(H)2 cytokines in HSV-1-induced corneal scarring. Ocular Immunology & Inflammation. 2002;10(2):105–116. doi: 10.1076/ocii.10.2.105.13982. [DOI] [PubMed] [Google Scholar]
- 17.Ghiasi H, Osorio Y, Perng GC, Nesburn AB, Wechsler SL. Recombinant herpes simplex virus type 1 expressing murine interleukin-4 is less virulent than wild-type virus in mice. J Virol. 2001;75(19):9029–9036. doi: 10.1128/JVI.75.19.9029-9036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker JN, Pfister LA, Quenelle D, Gillespie GY, Markert JM, Kern ER, et al. Genetically engineered herpes simplex viruses that express IL-12 or GM-CSF as vaccine candidates. Vaccine. 2006;24(10):1644–1652. doi: 10.1016/j.vaccine.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 19.Osorio Y, La Point SF, Nusinowitz S, Hofman FM, Ghiasi H. CD8+-dependent CNS demyelination following ocular infection of mice with a recombinant HSV-1 expressing murine IL-2. Exp Neurol. 2005;193(1):1–18. doi: 10.1016/j.expneurol.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Zandian M, Belisle R, Mott KR, Nusinowitz S, Hofman FM, Ghiasi H. Optic neuritis in different strains of mice by a recombinant HSV-1 expressing murine interleukin-2. Invest Ophthalmol Vis Sci. 2009;50(7):3275–3282. doi: 10.1167/iovs.08-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 22.Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154(10):5071–5079. [PubMed] [Google Scholar]
- 23.Guler ML, Gorham JD, Hsieh CS, Mackey AJ, Steen RG, Dietrich WF, et al. Genetic susceptibility to Leishmania: IL-12 responsiveness in TH1 cell development. Science. 1996;271(5251):984–987. doi: 10.1126/science.271.5251.984. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh CS, Heimberger AB, Gold JS, O'Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc Natl Acad Sci U S A. 1992;89(13):6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abehsira-Amar O, Gibert M, Joliy M, Theze J, Jankovic DL. IL-4 plays a dominant role in the differential development of Tho into Th1 and Th2 cells. J Immunol. 1992;148(12):3820–3829. [PubMed] [Google Scholar]
- 26.Poliani PL, Brok H, Furlan R, Ruffini F, Bergami A, Desina G, et al. Delivery to the central nervous system of a nonreplicative herpes simplex type 1 vector engineered with the interleukin 4 gene protects rhesus monkeys from hyperacute autoimmune encephalomyelitis. Hum Gene Ther. 2001;12(8):905–920. doi: 10.1089/104303401750195872. [DOI] [PubMed] [Google Scholar]
- 27.Furlan R, Poliani PL, Marconi PC, Bergami A, Ruffini F, Adorini L, et al. Central nervous system gene therapy with interleukin-4 inhibits progression of ongoing relapsing-remitting autoimmune encephalomyelitis in Biozzi AB/H mice. Gene Ther. 2001;8(1):13–19. doi: 10.1038/sj.gt.3301357. [DOI] [PubMed] [Google Scholar]
- 28.Broberg E, Setala N, Roytta M, Salmi A, Eralinna JP, He B, et al. Expression of interleukin-4 but not of interleukin-10 from a replicative herpes simplex virus type 1 viral vector precludes experimental allergic encephalomyelitis. Gene Ther. 2001;8(10):769–777. doi: 10.1038/sj.gt.3301465. [DOI] [PubMed] [Google Scholar]
- 29.Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 1987;37(7):1097–1102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- 30.Osorio Y, Ghiasi H. Recombinant herpes simplex virus type 1 (HSV-1) codelivering interleukin-12p35 as a molecular adjuvant enhances the protective immune response against ocular HSV-1 challenge. J Virol. 2005;79(6):3297–3308. doi: 10.1128/JVI.79.6.3297-3308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giri M, Ugen KE, Weiner DB. DNA vaccines against human immunodeficiency virus type 1 in the past decade. Clin Microbiol Rev. 2004;17(2):370–389. doi: 10.1128/CMR.17.2.370-389.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koehler NK, Genain CP, Giesser B, Hauser SL. The human T cell response to myelin oligodendrocyte glycoprotein: a multiple sclerosis family-based study. J Immunol. 2002;168(11):5920–5927. doi: 10.4049/jimmunol.168.11.5920. [DOI] [PubMed] [Google Scholar]
- 33.Kahl KG, Kruse N, Toyka KV, Rieckmann P. Serial analysis of cytokine mRNA profiles in whole blood samples from patients with early multiple sclerosis. J Neurol Sci. 2002;200(1–2):53–55. doi: 10.1016/s0022-510x(02)00136-3. [DOI] [PubMed] [Google Scholar]
- 34.Racke MK, Burnett D, Pak SH, Albert PS, Cannella B, Raine CS, et al. Retinoid treatment of experimental allergic encephalomyelitis. IL-4 production correlates with improved disease course. J Immunol. 1995;154(1):450–458. [PubMed] [Google Scholar]
- 35.Huseby ES, Liggitt D, Brabb T, Schnabel B, Ohlen C, Goverman J. A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis. J Exp Med. 2001;194(5):669–676. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D'Andrea A, Rengaraju M, Valiante NM, Chehimi J, Kubin M, Aste M, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176(5):1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwarz T. Interleukin-12 and its role in cutaneous sensitization. Res Immunol. 1995;146(7–8):494–499. doi: 10.1016/0923-2494(96)83022-7. [DOI] [PubMed] [Google Scholar]
- 38.Karp CL, van Boxel-Dezaire AH, Byrnes AA, Nagelkerken L. Interferon-beta in multiple sclerosis: altering the balance of interleukin-12 and interleukin-10? Curr Opin Neurol. 2001;14(3):361–368. doi: 10.1097/00019052-200106000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, et al. IL-12p35-Deficient Mice Are Susceptible to Experimental Autoimmune Encephalomyelitis: Evidence for Redundancy in the IL-12 System in the Induction of Central Nervous System Autoimmune Demyelination. J Immunol. 2002;169(12):7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 40.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 41.Boulay JL, O'Shea JJ, Paul WE. Molecular phylogeny within type I cytokines and their cognate receptors. Immunity. 2003;19(2):159–163. doi: 10.1016/s1074-7613(03)00211-5. [DOI] [PubMed] [Google Scholar]
- 42.Goriely S, Goldman M. Interleukin-12 family members and the balance between rejection and tolerance. Curr Opin Organ Transplant. 2008;13(1):4–9. doi: 10.1097/MOT.0b013e3282f406c4. [DOI] [PubMed] [Google Scholar]
- 43.Bancroft AJ, Humphreys NE, Worthington JJ, Yoshida H, Grencis RK. WSX-1: a key role in induction of chronic intestinal nematode infection. J Immunol. 2004;172(12):7635–7641. doi: 10.4049/jimmunol.172.12.7635. [DOI] [PubMed] [Google Scholar]
- 44.Miyazaki Y, Inoue H, Matsumura M, Matsumoto K, Nakano T, Tsuda M, et al. Exacerbation of experimental allergic asthma by augmented Th2 responses in WSX-1-deficient mice. J Immunol. 2005;175(4):2401–2407. doi: 10.4049/jimmunol.175.4.2401. [DOI] [PubMed] [Google Scholar]
- 45.Wirtz S, Tubbe I, Galle PR, Schild HJ, Birkenbach M, Blumberg RS, et al. Protection from lethal septic peritonitis by neutralizing the biological function of interleukin 27. J Exp Med. 2006;203(8):1875–1881. doi: 10.1084/jem.20060471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neufert C, Becker C, Wirtz S, Fantini MC, Weigmann B, Galle PR, et al. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol. 2007;37(7):1809–1816. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- 47.Yoshimoto T, Yasuda K, Mizuguchi J, Nakanishi K. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: a novel therapeutic way for Th2-mediated allergic inflammation. J Immunol. 2007;179(7):4415–4423. doi: 10.4049/jimmunol.179.7.4415. [DOI] [PubMed] [Google Scholar]
- 48.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 49.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450(7169):566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 50.Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, et al. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37(11):3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 51.Ghiasi H, Wechsler SL, Kaiwar R, Nesburn AB, Hofman FM. Local expression of tumor necrosis factor alpha and interleukin-2 correlates with protection against corneal scarring after ocular challenge of vaccinated mice with herpes simplex virus type 1. J Virol. 1995;69(1):334–340. doi: 10.1128/jvi.69.1.334-340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Osorio Y, Cohen J, Ghiasi H. Improved Protection from Primary Ocular HSV-1 Infection and Establishment of Latency Using Multigenic DNA Vaccines. Invest Ophthalmol Vis Sci. 2004;45(2):506–514. doi: 10.1167/iovs.03-0828. [DOI] [PubMed] [Google Scholar]