Abstract
Approximately 40 - 50% of individuals affected by tuberous sclerosis (TSC) develop autism spectrum disorders (ASD). One possible explanation for this partial penetrance is an interaction between TSC gene mutations and other risk factors such as gestational immune activation. Here, we report interactive effects of these two ASD risk factors in a mouse model of TSC. Combined, but not single exposure had adverse effects on intrauterine survival. Additionally, provisional results suggest that these factors synergize to disrupt social approach behavior in adult mice. Moreover, studies in human populations are consistent with an interaction between high seasonal flu activity in late gestation and TSC mutations in ASD. Taken together, our studies raise the possibility of a gene × environment interaction between heterozygous TSC gene mutations and gestational immune activation in the pathogenesis of tuberous sclerosis-related ASD.
Introduction
An emerging theme in the biology of ASD is the involvement of signaling mechanisms that regulate protein synthesis, such as those involving mammalian target of rapamycin (mTOR)1, 2. For example, heterozygous mutations in the TSC1 or TSC2 genes, key regulators of mTOR signaling, cause tuberous sclerosis and elevate the risk for autism 100-fold compared to the general population3-5. In addition, upstream regulators (PTEN, MET, NF1)6-9 and a downstream effector (eIF4E)10 of the TSC-mTOR pathway have been implicated in ASD. Moreover, alterations in mTOR-dependent translational control are also associated with fragile X syndrome11, 12, a single-gene disorder caused by mutations in the FMR1 gene and associated with high rates of ASD. Collectively, these findings suggest an overlapping genetic signature in a subset of ASD-related disorders.
Prenatal viral infections also constitute a risk factor for neuropsychiatric disorders, including schizophrenia and ASD13-22. Prenatal rubella virus infections increase the risk of autism in the offspring >200-fold14-16 and available evidence indicates other viral infections confer risk as well19-21. Together with the presence of inflammatory brain changes13, 23, 24, these findings suggest a role of infections and/or immunological processes in at least a subset of ASD.
Gestational viral infections trigger a maternal immune response, which can perturb fetal brain development, at least in part due to effects of cytokines in the developing nervous system13, 25-27. In animals, gestational viral infections can be mimicked by systemic administration of Poly I:C, a synthetic double-stranded RNA, which elicits an innate immune response via activation of Toll-like receptor 3 (TLR3)13, 27, 28.
An interaction between TSC mutations and gestational immune activation could explain the partial penetrance of ASD phenotypes in TSC. TSC-mTOR signaling is downstream of Poly I:C/TLR3, Poly I:C-induced cytokines29, 30, and TSC-mTOR signaling modulates immune responses30-33. To test for possible interactive effects, we combined Tsc2 haploinsufficiency and the Poly I:C model of gestational immune activation in mice and assessed the impact on behavior in adult animals.
Material & Methods
Mice
To test for interactive effects between a Tsc2+/− mutation and immune activation during pregnancy, we first mated male Tsc2+/− mice34 with female wild-type mice (see below). Tsc2+/− male breeders were on a C57BL/6Ncrl genetic background. We used C57BL/6J females for breeding because prior immune activation studies were conducted, in the context of which Tsc2+/− males were crossed with a mutant line on a C57BL/6J genetic background. Female breeders with a vaginal plug subsequent to over-night mating (designated E0) were single-housed and were left undisturbed, except for weekly cage change. The establishment of pregnancy was determined by checking for abdominal distension at E12. Pregnant females were injected (i.p.; injected volume: 10 μl/g body weight) with either 20 mg/kg Poly I:C (Sigma, potassium salt; Poly I:C is supplied at 10% of the total weight of the salt; dosage was based on the weight of Poly I:C itself) or vehicle only (0.9% sterile saline) at E12.5.
Litter composition with respect to numbers and genotypes was assessed in a subset of surviving litters (i.e., live births). In these litters, animals were closely monitored from birth to weaning. Tail biopsies for genotyping were taken at weaning (P21) or whenever individual pups were lost postnatally (no group differences in terms of postnatal loss of pups were observed). The litter composition data provided refer to the observations carried out at birth.
To assess potential interactive effects between a heterozygous Tsc2 mutation and advanced paternal age, we generated Tsc2+/− and WT offspring derived from old (18-22 months at conception) or young (3-6 months at conception) fathers, by mating male Tsc2+/− mice (C57BL/6Ncrl) with female WT animals (C57BL/6Ncrl; 3-4 months at conception). Litters of each paternal age group were derived from at least 4 different fathers.
All experiments were conducted blind to genotype and treatment. The Chancellor’s Animal Research Committee at the University of California – Los Angeles approved the research protocols used here.
Behavior
Behavioral experiments were conducted in 3-6 months old, male mice. Social approach behavior was studied in a 3-compartment apparatus and the procedure was similar to previously reported protocols35, 36. Initially, test animals were allowed to explore the 3-compartment apparatus freely for a 20 min period (habituation). At the next day, animals were briefly (5 min) re-habituated to the apparatus. For the social behavior phase of the task, an unfamiliar conspecific (same sex, age and genetic background as the test subject) was placed into one of the side compartments and restrained by a small wire object (“social cage”). The other side compartment contained an empty wire object (“empty cage”). The test subject was then released into the center compartment and allowed to explore the 3-compartment apparatus freely for 10 min. Behavior was videotaped and scored offline by an experienced observer; we measured the time (s) that the test subject spent exploring (sniffing, rearing) the social cage and the empty cage. In addition, we used commercially available software (TopScan) for automated analyses of social exploration. To this end, we defined an annular zone around the social and empty cage and used the software to track nose entries of the subject mouse into this annular zone. These automated analyses yielded estimates of total sniff time, number of sniff episodes and average duration of sniff episodes.
Activity was assessed in an open field assay as previously described36. In brief, mice were placed in a square open field made of acrylic and activity was recorded by an automated system (Med Associates) for 10 min. Total distance moved (cm) was the primary measure of interest for the assessment of activity levels.
For the olfactory sensitivity test, an unfamiliar food item high in carbohydrate content (one froot loop from Kellogg’s® cereal) was placed into the home cages of test subjects for two days prior to testing. Testing was performed in novel standard cages containing a 3 cm deep layer of bedding. Test subjects were initially habituated to the novel cages for 5 min. After habituation, we buried one froot loop in the bedding of the test cages. For retrieval of the froot loop, test subjects were placed into the test cages for 15 min. Behavior was videotaped for offline analysis. We measured the latency to retrieve the froot loop.
Human TSC and ASD data
Birth dates and clinical information of TSC individuals was contributed by 4 sources: the TSC Natural History Database (TS Alliance) and the patient records of Drs. Petrus de Vries, David Franz and Mustafa Sahin. All individuals included in the analysis were born in the US or the UK. The following clinical information was available: intellectual disability (Yes/No), ASD (Yes/No), lifetime history of infantile spasms (Yes/No). We analyzed data of TSC individuals affected by ASD (TSC-ASD individuals; n = 230) and TSC individuals who were not affected by ASD, intellectual disability or infantile spasms (n = 265). Details regarding year of birth, gender composition, clinical features and mutational status of these clinical samples are summarized in Supplementary Table 1.
Date of conception was estimated for each individual by subtracting 267 days (average duration of a pregnancy) from the birth date. Seasonal influenza infections typically peak between January and March (average mid February). To estimate gestational age during peak influenza activity for each individual, we calculated gestational age at February 14th following conception. Subsequently, we assigned each individual to one of 4 groups (all covering 13 week periods): individuals for whom peak seasonal flue activity coincided with the first trimester (weeks 1 – 13), second trimester (weeks 14 – 26) or third trimester (weeks 27 – 39) of pregnancy or for whom peak seasonal flue activity was outside of gestational periods. Using chi square analyses, we determined, for each population (TSC-ASD; TSC-no ASD or other neurodevelopmental phenotypes), if the observed frequency of cases in the 4 different groups (peak seasonal flu activity: coinciding either with the first trimester, second trimester, third trimester or outside of gestation) differed significantly from the expected frequencies (i.e, from an even distribution of cases across groups).
For ASD individuals unaffected by TSC, obtained through the Autism Genetic Resource Exchange (www.agre.org), birth month information (rather than precise birth dates) was available. Individuals born between August and October were considered to be in first trimester of gestation during peak seasonal flu activity; births in May, June or July were considered to correspond to second trimester gestation during peak seasonal flu activity; births between February and April were considered third trimester gestation during peak seasonal flu activity. In November, December and January births, gestation was assumed to not overlap with peak seasonal flu activity. Chi square analysis was performed as described above.
Statistics
All statistical tests (ANOVAs, posthoc analyses, t-tests, chi square analyses) were chosen a priori and are described in more detail in the main text and figure legends.
Results
To test for an interaction between the Tsc2+/− mutation in mice and gestational Poly I:C (a model of gestational immune activation), we performed timed matings of Tsc2+/− males in the C57BL/6Ncrl genetic background and C57BL/6J wild-type (WT) females, and injected pregnant females at E12.5 with either Poly I:C (20 mg/kg)27 or saline control.
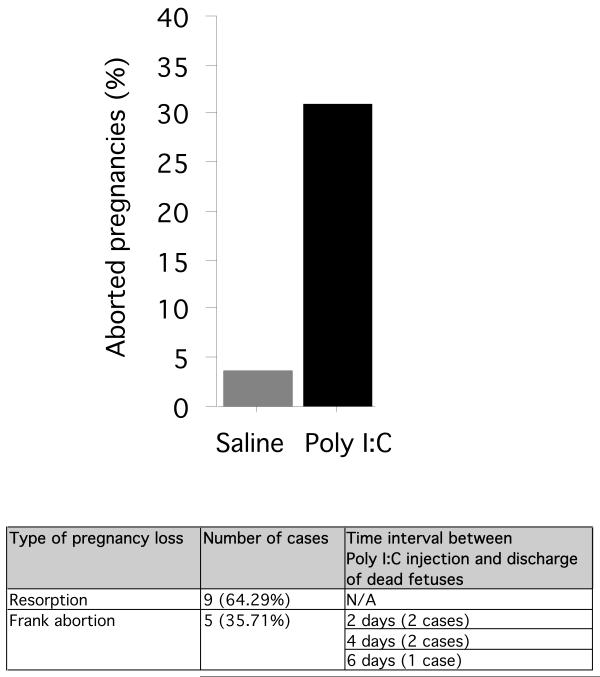
While established pregnancies were successfully carried to term in the vast majority of saline-injected breeders (96.43% survived; 3.57% lost; n = 28 pregnancies), abdominal collapse or frank abortion of dead fetuses occurred in a considerable portion of Poly I:C-injected mice (68.89% survived; 31.11% lost; n = 45 pregnancies) (Fig. 1; Table 1).
Fig. 1.
Percentage of pregnancies lost in Poly I:C-injected and saline-treated animals. The vast majority of saline-injected females carried pregnancy to term; in contrast, a considerable portion of pregnancies was aborted as a consequence of Poly I:C injection. The table shows the proportion of Poly I:C-related lost pregnancies either associated with fetal resorption (abdominal collapse without abortion of dead fetuses) or frank abortion (premature discharge of dead fetuses).
Table 1.
The table shows the number of cases (n) in which peak seasonal flu activity coincided with the first, second or third trimester of gestation or was outside gestational periods. Data are shown for TSC individuals affected by ASD (tuberous sclerosis and ASD), TSC individuals unaffected by ASD or other neurodevelopmental phenotypes (tuberous sclerosis, no ASD or other neurodevelopmental phenotypes) and ASD cases unrelated to tuberous sclerosis (ASD, no tuberous sclerosis), along with results from the corresponding chi square analyses
| First trimester (n) |
Second trimester (n) |
Third trimester (n) |
Outside gestation (n) |
χ 2 | P |
|---|---|---|---|---|---|
| Tuberous sclerosis and ASD | |||||
| 47 | 57 | 75 | 51 | 7.983 | 0.0463* |
| Tuberous sclerosis, no ASD or other neurodevelopmental phenotypes | |||||
| 67 | 74 | 58 | 66 | 1.943 | 0.5843 |
| ASD, no tuberous sclerosis | |||||
| 1741 | 1687 | 1606 | 1700 | 5.701 | 0.1271 |
P < 0.05
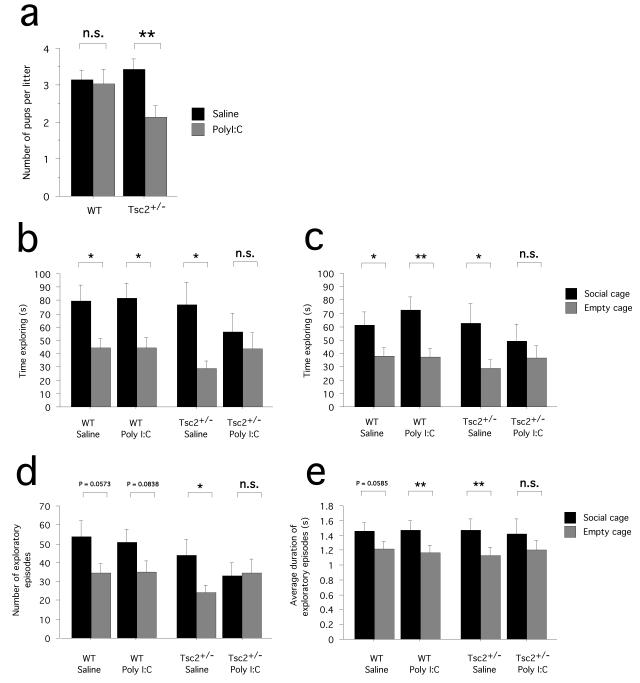
We compared the number of Tsc2+/− and WT pups in litters carried to term. Analyses revealed that fewer Tsc2+/− pups per litter were born to Poly I:C-injected mice than to saline controls, while the number of WT pups per litter did not differ between the Poly I:C and saline control group (two-way ANOVA with treatment and genotype as between-subjects factors: effect of treatment F(1,80) = 4.968; P = 0.0286; Poly I:C: n = 21 litters; saline: n = 21 litters; Bonferroni/Dunn posthoc analysis: Tsc2+/−/Poly I:C vs. Tsc2+/−/saline, P = 0.0038; WT/Poly I:C vs. WT/saline, P = 0.8358) (Fig. 2a). These findings suggest that Tsc2+/− fetuses are more vulnerable to Poly I:C-induced intrauterine death than their wild-type counterparts. Indeed, when we isolated fetuses at 6.5 h after Poly I:C injection, we found signs of fetal resorption. Our observations indicated that, at 6.5h after Poly I:C, 3 out of 21 Tsc2+/− fetuses (14.29%) appeared to be affected by resorption (pale, deformed, mushy consistency) compared to 1 out of 18 WT control fetus (5.56%) tested.
Fig. 2.
Interactive effects of Tsc2 haploinsufficiency and gestational Poly I:C on intrauterine survival and adult social approach behavior. (a) Graph shows the number of pups born in surviving litters, plotted by genotype (Tsc2+/− and WT) and treatment (Poly I:C and saline). Fewer Tsc2+/− pups were born to Poly I:C-injected females compared to saline-injected controls. (b) Graph shows the time (s) spent actively exploring the social cage and the empty cage (sniffing, rearing), as determined by an experienced observer. WT/saline, Tsc2+/−/saline and WT/Poly I:C mice spent significantly more time exploring the social cage than the empty cage, demonstrating normal social approach behavior. Tsc2+/−/Poly I:C mice, in contrast, did not spent more time exploring the social cage than the empty cage. (c,d,e) These graphs show the time spent exploring (c), the number of exploratory episodes (d) and the average duration of exploratory episodes (e) during the social approach behavioral task as quantified with an automated system. While WT/saline, Tsc2+/−/saline and WT/Poly I:C mice tended to show higher values for the social cage than the empty cage, this was not the case for Tsc2+/−/Poly I:C mice. ** P < 0.01, * P < 0.05, n.s. P > 0.05. Data represent means +/− S.E.M.
Next, we wanted to determine if Tsc2 haploinsufficiency and Poly I:C-related gestational immune activation interacted to affect mouse behavioral models of autistic phenotypes. Since abnormalities in social behaviors represent a core feature of ASD, we assayed social approach behavior in mice, using a 3-compartment apparatus35. In brief, the 3-compartment apparatus comprised one side compartment containing an empty wire object (“empty cage”) and one side compartment containing a small wire object constraining a mouse (“social cage”). To quantify social approach behavior, we let test mice freely explore the 3-compartment apparatus and measured the time these mice spent exploring the social cage versus the empty cage.
WT and Tsc2+/− mice born to saline-injected mothers and WT mice born to Poly I:C-injected females spent significantly more time exploring the social cage than the empty cage (WT/saline: planned t-test, P = 0.0140, n = 16 mice; Tsc2+/−/saline: planned t-test: P = 0.0116, n = 13 mice; WT/Poly I:C: planned t-test, P = 0.0101, n = 16 mice) (Fig. 2b), demonstrating robust social approach behavior. In contrast, mice exposed to both factors, the Tsc2+/− mutation and gestational Poly I:C (Tsc2+/−/Poly I:C mice), did not spend significantly more time exploring the social cage than the empty cage (planned t-test: P = 0.5062, n = 13 mice) (Fig. 2b), demonstrating a lack of normal social approach behavior. To explore this further, we performed automated analyses of social exploration in Tsc2+/−/Poly I:C mice and the other experimental groups (for details, see Material & Methods). These analyses also showed that all groups except the Tsc2+/−/Poly I:C group spent significantly more time exploring the social cage than the empty cage (WT/saline: planned t-test, P = 0.0232, n = 16 mice; Tsc2+/−/saline: planned t-test: P = 0.0405, n = 13 mice; WT/Poly I:C: planned t-test, P = 0.0025, n = 16 mice; Tsc2+/−/Poly I:C: planned t-test: P = 0.2070, n = 13 mice) (Fig. 2c). Next, we determined the number of exploratory episodes and the average duration of an exploratory episode for each animal. With respect to these measures, WT/saline, Tsc2+/−/saline and WT/Poly I:C animals tended to show higher values for the social cage than the empty cage, even though not all comparisons yielded statistical significance (number of exploratory episodes: WT/saline, planned t-test, P = 0.0573, n = 16 mice; Tsc2+/−/saline, planned t-test: P = 0.0204, n = 13 mice; WT/Poly I:C, planned t-test, P = 0.0838, n = 16 mice; average duration of exploratory episodes: WT/saline, planned t-test, P = 0.0585, n = 16 mice; Tsc2+/−/saline, planned t-test: P = 0.0089, n = 13 mice; WT/Poly I:C, planned t-test, P = 0.0029, n = 16 mice) (Fig. 2d,e). Tsc2+/−/Poly I:C mice, in contrast, did not show more exploratory episodes and longer exploration of the social cage than the empty cage (number of exploratory episodes: planned t-test, P = 0.8351, average duration of exploratory episodes: planned t-test, P = 0.2135; n = 13 mice) (Fig. 2d,e).
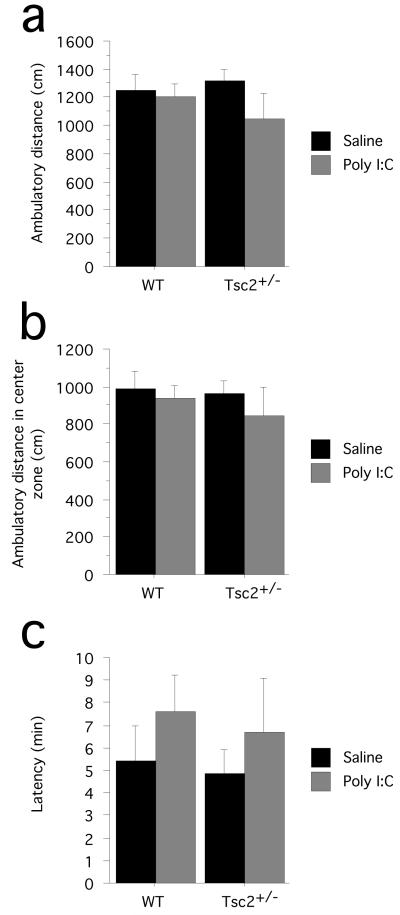
Additional behavioral testing with the open field revealed no group difference with respect to general exploratory behavior (ambulatory distance; two-way ANOVA with genotype and treatments as between-subjects factors: genotype effect, F(1,45) = 0.154, P = 0.6964; treatment effect: F(1,45) = 1.822, P = 0.1839; genotype × treatment interaction: F(1,45) = 0.846, P = 0.3625; WT/saline, n = 13 mice; WT/Poly I:C, n = 18 mice; Tsc2+/−/saline, n = 11 mice; Tsc2+/−/Poly I:C, n = 7 mice) (Fig. 3a). The open field assay also revealed that ambulatory distance in the center zone did not differ between groups (ambulatory distance in center zone of the open field; two-way ANOVA with genotype and treatments as between-subjects factors: genotype effect, F(1,45) = 0.448, P = 0.5067; treatment effect: F(1,45) = 0.949, P = 0.3351; genotype × treatment interaction: F(1,45) = 0.129, P = 0.7213; WT/saline, n = 13 mice; WT/Poly I:C, n = 18 mice; Tsc2+/−/saline, n = 11 mice; Tsc2+/−/Poly I:C, n = 7 mice) (Fig. 3b), suggesting normal levels of anxiety-related behaviors in Tsc2+/−/Poly I:C mice. To evaluate general olfactory function, animals were tested with an olfactory sensitivity test that uses a food (froot loop) retrieval assay. There was no significant difference with respect to latencies to retrieve the food item across the experimental groups (two-way ANOVA with genotype and treatment as between-subjects factors, latency to retrieve food item: genotype effect, F(1,39) = 0.187, P = 0.6681; treatment effect, F(1,39) = 1.401, P = 0.2437; genotype × treatment interaction, F(1,39) = 0.011, P = 0.9182) (Fig. 3c), suggesting normal general olfaction in Tsc2+/−/Poly I:C mice. Taken together, these findings suggest that abnormalities in social approach behavior described above for the Tsc2+/−/Poly I:C mice were not simply due to altered activity levels or perturbations of general olfactory function.
Fig. 3.
(a) Behavioral testing, using the open field, revealed no significant effect of Tsc2 haploinsufficiency or gestational Poly I:C on ambulatory behavior, demonstrating normal locomotion in Tsc2+/−/Poly I:C mice. (b) Ambulatory distance in the center zone did also not differ between groups, suggesting normal levels of anxiety-related behaviors in Tsc2+/−/Poly I:C mice. (c) The graph shows the latency (min) to retrieve a buried food item in an olfactory sensitivity test. There was no significant difference with respect to latencies across the experimental groups, suggesting that olfactory dysfunction did not account for the behavioral impairments in Tsc2+/−/Poly I:C mice. Data represent means +/− S.E.M.
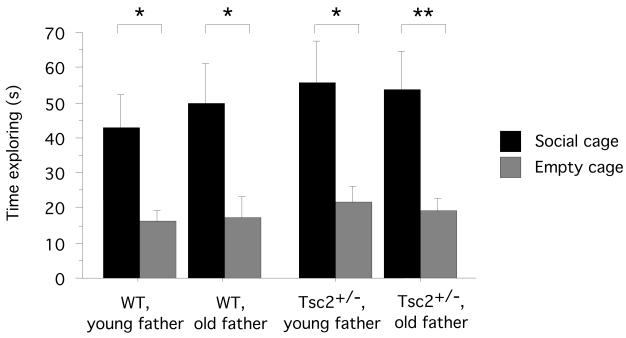
Next, we determined if Tsc2 haploinsufficiency also interacts with other ASD risk factors. Advanced paternal age confers ASD risk37, but has no obvious links to TSC-mTOR signaling. To combine Tsc2 haploinsufficiency with advanced paternal age in mice, we bred old Tsc2+/− males with WT females (for details, see Material & Methods); we generated controls by crossing young Tsc2+/− males with WT females and assessed social behavior in the adult offspring. Adult Tsc2+/− and WT mice conceived by either young or old fathers showed normal social approach behavior (WT/young father: planned t-test, time exploring social cage vs. time exploring empty cage, P = 0.0129, n = 14 mice; WT/old father: planned t-test, time exploring social cage vs. time exploring empty cage, P = 0.0229, n = 9 mice; Tsc2+/−/young father, planned t-test, time exploring social cage vs. time exploring empty cage, P = 0.0177, n = 9 mice; Tsc2+/−/old father, planned t-test, time exploring social cage vs. time exploring empty cage: P = 0.0042, n = 16 mice) (Fig. 4). These data show that our studies did not detect an interaction between the Tsc2+/− mutation and advanced paternal age as we had uncovered in the Poly I:C studies. These findings attest to the selectivity of the interactive effects between Tsc2 haploinsufficiency and gestational Poly I:C.
Fig. 4.
Advanced paternal age confers autism susceptibility and also is a risk factor for other neurodevelopmental disorders. To combine Tsc2 haploinsufficiency with advanced paternal age in mice, we bred old Tsc2+/− males with WT females and generated controls by crossing young Tsc2+/− males with WT females and assessed social behavior in the adult offspring. Tsc2+/− and WT offspring of both young and old fathers showed normal social approach behavior. Plotted is the time (s) test subjects spent exploring either a cage containing a conspecific (social cage) or an empty cage. * P < 0.05, ** P > 0.01. Data represent means +/− S.E.M.
The animal model data described above suggests that gestational viral infections might contribute to autistic phenotypes in human TSC populations. Seasonal influenza infections typically peak between the beginning of January and late March each year (www.cdc.gov/flu/weekly/fluactivity.htm) and they are a common source of gestational immune activation. To test if seasonal influenza infections interact with TSC gene mutations in the pathogenesis of TSC-related ASD, we obtained birth date and clinical information from human TSC populations (clinical samples from the US and the UK, for details see Material & Methods) and estimated gestational age during peak seasonal flu activity for TSC-ASD individuals (n = 230). As a reference group, we used TSC individuals without major neurodevelopmental phenotypes (i.e., without intellectual disability, ASD or a history of infantile spasms; n = 265) (for details, see Material & Methods).
Our analyses revealed an uneven distribution of peak seasonal flu activity across gestational periods in TSC-ASD individuals (peak seasonal flu activity: coinciding with the first trimester, n = 47 individuals; second trimester, n = 57 individuals; third trimester, n = 75 individuals; outside gestational periods, n = 51 individuals; χ2 = 7.983, P = 0.0463) (Table 1), suggesting an excess of TSC-ASD individuals, for whom late stages of pregnancy coincided with peak seasonal flu activity. In contrast, TSC controls without ASD or other neurodevelopmental phenotypes showed no obvious association between gestational periods and seasonal flu activity (peak seasonal flu activity: coinciding with the first trimester, n = 67 individuals; second trimester, n = 74 individuals; third trimester, n = 58 individuals; outside gestational periods, n = 66 individuals; χ2 = 1.943, P = 0.5843) (Table 1).
We also obtained birth date information of ASD individuals unaffected by TSC from the Autism Genetic Resource Exchange database (www.agre.org; n = 6,734 individuals). There was no obvious association between gestational periods and peak seasonal flu activity in ASD individuals unaffected by TSC (peak seasonal flu activity: coinciding with the first trimester, n = 1741 individuals; second trimester, n = 1687 individuals; third trimester, n = 1606 individuals; outside gestational periods, n = 1700 individuals; χ2 = 5.701, P = 0.1271) (Table 1). These findings suggest that the association of late gestation and peak seasonal flu activity was specific for TSC-related ASD.
Discussion
Here, we presented evidence indicating that Tsc2 haploinsufficiency and the Poly I:C model of gestational immune activation cooperate to disrupt intrauterine survival and adult social approach behavior in mice. In addition, studies in human populations were consistent with an interaction between high seasonal flu activity in late gestation and TSC mutations in ASD.
Dysfunctional social behaviors represent a core feature of ASD. Social approach behavior in the 3-compartment apparatus is used as a model to study social interactions in mice35, 38. Our provisional results suggest abnormal social approach behavior in Tsc2+/−/Poly I:C mice, which could not be explained by altered levels of general exploratory behavior (activity in the open field was normal) or by general olfactory dysfunction (which was normal in an olfactory sensitivity test). Alterations in higher-order circuits could form the basis of dysfunctional social approach behavior in Tsc2+/−/Poly I:C mice. Nevertheless, it is also conceivable that altered social behavior in the Tsc2+/−/Poly I:C mice is related to perturbations in the accessory olfactory system, which plays an important role in detecting socially relevant odors39.
For the Poly I:C experiments we used a single injection of 20 mg/kg at E12.5 (for details, see Material & Methods). Pilot experiments had established that this Poly I:C dose does not adversely affect social approach behavior in C57BL/6Ncrl × C57BL/6J F1 wild-type animals (data not shown; see also Fig. 2b-e). This Poly I:C dose did also not have an effect on behavior in the open field assay, neither in WT mice nor in Tsc2+/− mutants (Fig. 3a,b). A prior report27 indicated effects of this dose in pure C57BL/6J WT animals. One possibility is that differences in the genetic backgrounds used in the two studies modulated behavioral phenotypes, although we have not formally addressed this possibility.
As our behavioral analyses were naturally performed in only those Tsc2+/− mice that survived Poly I:C treatment, it is in principle possible that this analysis inevitably focused on a specialized subset of unique individuals (e.g., selected for certain genetic traits by the Poly I:C treatment). The possibility that the surviving mice are genetically distinct from those that died during gestation is unlikely since in our experiments we used isogenic mice derived from a cross between C57BL/6NCrl and C57BL/6J mice (homogeneous with respect to genetic background). Given the lack of an assortment of background genes in the tested offspring, it appears unlikely that the synergistic effect between the Tsc2+/− mutation and Poly I:C is due to an interaction between the Tsc2+/− mutation and modifier genes segregating in the genetic background of the mice studied. We conclude that an interaction between the Tsc2+/− mutation and gestational immune activation represents the most parsimonious explanation for the synergistic effect on adult social behavior in Tsc2+/−/Poly I:C mice.
There are several ways, in which the Tsc2+/− mutation could interact with gestational immune activation to result in neurological damage. Gestational immune activation is thought to perturb fetal brain development, at least in part due to effects of cytokines in the developing nervous system13, 25-27. Importantly, TSC/mTOR signaling is downstream of multiple factors implicated in gestational immune activation, including various cytokines and growth factors29, 30. Accordingly, it is possible that disinhibited TSC/mTOR signaling downstream of mediators of gestational immune activation effects (i.e., cytokines, growth factors) amplifies their impact on the Tsc2+/− fetal brain.
Additionally, TSC/mTOR signaling also plays an important role in the modulation of immune responses. The mTOR pathway promotes the activation of the innate immune system in response to Toll-like receptor engagement or viral infections30, 32. At least in part via its effect on the innate immune system, TSC/mTOR signaling is also involved in the regulation of the adaptive immune response, including T cell proliferation and cytokine production. For instance, mTOR inhibition dampened the T cell response to virus vaccination32 and favored the stimulation of tolerogenic regulatory T cells relative to that of effector T cells33. Taken together, the Poly I:C-related immune activation may be more pronounced in Tsc2+/− mutants, which could explain why the ensuing damage is larger in Tsc2+/− mice than in wild-type controls.
Interestingly, deletion of Pten, an upstream regulator of the TSC/mTOR pathway, led to severe autoimmune disease in mice due to deficient removal of autoreactive lymphocytes40. Consistent with these results in Pten haploinsufficient mutants, increased PI3K activity also caused lymphoproliferative autoimmune disorder in mice41. In another mouse model of autoimmune lymphoproliferative syndrome, also associated with disinhibited PI3K/AKT/mTOR signaling, pharmacological mTOR inhibition ameliorated lymphoproliferation and modulated levels of serum IgG nuclear auto-antibodies42. Collectively, these findings suggest that disinhibition of PI3K/AKT signaling is sufficient to generate an autoimmune phenotype, which depends, at least in part, on disinhibited mTOR signaling. Tsc2+/− mice do not show obvious signs of autoimmune disease but, conceivably, the Poly I:C-related immune challenge may uncover a latent predisposition for autoimmunity in Tsc2+/− mutants43-46, a possibility which has to be addressed in future studies. Additionally, it will be of interest to determine if autoimmune phenomena are present in human TSC individuals affected by ASD.
Of note, preliminary observations suggest that immune responses may be significantly altered in human TSC subjects47. Additionally, findings consistent with inflammatory changes in tubers have been reported with respect to brain tissue from TSC individuals48. These observations, together with the data discussed above, should prompt further research into the relationship of immunological and neurological phenotypes in tuberous sclerosis.
To determine if gestational immune activation may interact with TSC mutations in generating ASD-related phenotypes in human populations, we analyzed the temporal relationship of peak seasonal flu activity and gestational periods in TSC individuals affected by ASD. As a comparison, we carried out similar studies in TSC individuals unaffected by ASD or other neurodevelopmental disorders. An excess of TSC-ASD individuals had late trimester pregnancy coincide with peak seasonal flu activity, while this was not the case on TSC individuals without either ASD or other neurodevelopmental disorders. In contrast to the data in TSC populations, peak seasonal flu activity was equally distributed across gestational and extra-gestational periods in a general ASD population, suggesting that the association of late gestation with peak seasonal flu activity was specific for TSC-ASD individuals.
Several studies in general ASD populations have examined the relationship of ASD and seasonality of birth and have yielded mixed results49-55. Our findings suggest that certain ASD subpopulations (i.e., ASD associated with TSC) may be prone to an interaction with seasonal infections, while this may not be the case for ASD of other etiologies. In the future, it may be possible to stratify ASD populations according to genetic risk factors and more specifically test which ASD subpopulations show interaction with seasonal infections. Genetic factors might interact with gestational viral infections in the pathogenesis of ASD by influencing the susceptibility for infection, through the qualitative and/or quantitative modulation of the host immune response or by influencing the extent to which the immune response interacts with other downstream processes (e.g., cross-talk with neurodevelopmental processes).
Direct evidence for a role of gestational viral infections in ASD comes from studies that show an elevated risk in subjects that were gestationally exposed to specific viral infections14-16, 19, 20, such as rubella, or in subjects that were hospitalized due to viral infections21. Further study of the relationship of TSC-related ASD and gestational viral infections in populations with proven maternal viral infection would be useful but are difficult to perform due to the relatively low birth incidence of TSC.
Taken together, the data described above show that a heterozygous Tsc2 mutation and the Poly I:C model of gestational viral infections display interactive effects on intrauterine survival and may also have a synergistic impact on social behavior in adult mice. Studies in human TSC populations indicated an association between high seasonal flu activity in late gestation and ASD. Collectively, our data raise the possibility that TSC gene mutations interact with gestational viral infections in the pathogenesis of TSC-related ASD.
Supplementary Material
Acknowledgments
This work was supported by funds of the German Centre for Neurodegenerative Diseases to D.E., NIH R01 MH084315 to A.J.S. and a grant from the Children’s Hospital Boston Translational Research Program to M.S.
References
- 1.Kelleher RJ, 3rd, Bear MF. The autistic neuron: troubled translation? Cell. 2008;135(3):401–406. doi: 10.1016/j.cell.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Ehninger D, Silva AJ. Genetics and neuropsychiatric disorders: treatment during adulthood. Nat Med. 2009;15(8):849–850. doi: 10.1038/nm0809-849. [DOI] [PubMed] [Google Scholar]
- 3.Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: an update. J Autism Dev Disord. 2003;33(4):365–382. doi: 10.1023/a:1025054610557. [DOI] [PubMed] [Google Scholar]
- 4.Smalley SL. Autism and tuberous sclerosis. J Autism Dev Disord. 1998;28(5):407–414. doi: 10.1023/a:1026052421693. [DOI] [PubMed] [Google Scholar]
- 5.Smalley SL, Tanguay PE, Smith M, Gutierrez G. Autism and tuberous sclerosis. J Autism Dev Disord. 1992;22(3):339–355. doi: 10.1007/BF01048239. [DOI] [PubMed] [Google Scholar]
- 6.Campbell DB, Sutcliffe JS, Ebert PJ, Militerni R, Bravaccio C, Trillo S, et al. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci U S A. 2006;103(45):16834–16839. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, Takahashi TN, et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005;42(4):318–321. doi: 10.1136/jmg.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams PG, Hersh JH. Brief report: the association of neurofibromatosis type 1 and autism. J Autism Dev Disord. 1998;28(6):567–571. doi: 10.1023/a:1026012414193. [DOI] [PubMed] [Google Scholar]
- 9.Zafeiriou DI, Ververi A, Vargiami E. Childhood autism and associated comorbidities. Brain Dev. 2007;29(5):257–272. doi: 10.1016/j.braindev.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Neves-Pereira M, Muller B, Massie D, Williams JH, O’Brien PC, Hughes A, et al. Deregulation of EIF4E: a novel mechanism for autism. J Med Genet. 2009;46(11):759–765. doi: 10.1136/jmg.2009.066852. [DOI] [PubMed] [Google Scholar]
- 11.Vanderklish PW, Edelman GM. Differential translation and fragile X syndrome. Genes Brain Behav. 2005;4(6):360–384. doi: 10.1111/j.1601-183X.2005.00134.x. [DOI] [PubMed] [Google Scholar]
- 12.Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, et al. Dysregulation of mTOR signaling in fragile X syndrome. J Neurosci. 2010;30(2):694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson PH. Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Chess S. Autism in children with congenital rubella. J Autism Child Schizophr. 1971;1(1):33–47. doi: 10.1007/BF01537741. [DOI] [PubMed] [Google Scholar]
- 15.Chess S. Follow-up report on autism in congenital rubella. J Autism Child Schizophr. 1977;7(1):69–81. doi: 10.1007/BF01531116. [DOI] [PubMed] [Google Scholar]
- 16.Chess S, Fernandez P, Korn S. Behavioral consequences of congenital rubella. J Pediatr. 1978;93(4):699–703. doi: 10.1016/s0022-3476(78)80921-4. [DOI] [PubMed] [Google Scholar]
- 17.Libbey JE, Sweeten TL, McMahon WM, Fujinami RS. Autistic disorder and viral infections. J Neurovirol. 2005;11(1):1–10. doi: 10.1080/13550280590900553. [DOI] [PubMed] [Google Scholar]
- 18.Deykin EY, MacMahon B. Viral exposure and autism. Am J Epidemiol. 1979;109(6):628–638. doi: 10.1093/oxfordjournals.aje.a112726. [DOI] [PubMed] [Google Scholar]
- 19.Sweeten TL, Posey DJ, McDougle CJ. Brief report: autistic disorder in three children with cytomegalovirus infection. J Autism Dev Disord. 2004;34(5):583–586. doi: 10.1007/s10803-004-2552-y. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita Y, Fujimoto C, Nakajima E, Isagai T, Matsuishi T. Possible association between congenital cytomegalovirus infection and autistic disorder. J Autism Dev Disord. 2003;33(4):455–459. doi: 10.1023/a:1025023131029. [DOI] [PubMed] [Google Scholar]
- 21.Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, et al. Maternal Infection Requiring Hospitalization During Pregnancy and Autism Spectrum Disorders. J Autism Dev Disord. 2010 doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- 22.Ciaranello AL, Ciaranello RD. The neurobiology of infantile autism. Annu Rev Neurosci. 1995;18:101–128. doi: 10.1146/annurev.ne.18.030195.000533. [DOI] [PubMed] [Google Scholar]
- 23.Chez MG, Dowling T, Patel PB, Khanna P, Kominsky M. Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr Neurol. 2007;36(6):361–365. doi: 10.1016/j.pediatrneurol.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57(1):67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 25.Nawa H, Takahashi M, Patterson PH. Cytokine and growth factor involvement in schizophrenia--support for the developmental model. Mol Psychiatry. 2000;5(6):594–603. doi: 10.1038/sj.mp.4000730. [DOI] [PubMed] [Google Scholar]
- 26.Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. Int J Dev Neurosci. 2005;23(2-3):299–305. doi: 10.1016/j.ijdevneu.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27(40):10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuckerman L, Weiner I. Post-pubertal emergence of disrupted latent inhibition following prenatal immune activation. Psychopharmacology (Berl) 2003;169(3-4):308–313. doi: 10.1007/s00213-003-1461-7. [DOI] [PubMed] [Google Scholar]
- 29.Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130(3):440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 30.Colina R, Costa-Mattioli M, Dowling RJ, Jaramillo M, Tai LH, Breitbach CJ, et al. Translational control of the innate immune response through IRF-7. Nature. 2008;452(7185):323–328. doi: 10.1038/nature06730. [DOI] [PubMed] [Google Scholar]
- 31.Lorne E, Zhao X, Zmijewski JW, Liu G, Park YJ, Tsuruta Y, et al. Participation of mammalian target of rapamycin complex 1 in Toll-like receptor 2- and 4-induced neutrophil activation and acute lung injury. Am J Respir Cell Mol Biol. 2009;41(2):237–245. doi: 10.1165/rcmb.2008-0290OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9(10):1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178(11):7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 34.Onda H, Lueck A, Marks PW, Warren HB, Kwiatkowski DJ. Tsc2(+/−) mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J Clin Invest. 1999;104(6):687–695. doi: 10.1172/JCI7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3(5):287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 36.Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, et al. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008;14(8):843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reichenberg A, Gross R, Weiser M, Bresnahan M, Silverman J, Harlap S, et al. Advancing paternal age and autism. Arch Gen Psychiatry. 2006;63(9):1026–1032. doi: 10.1001/archpsyc.63.9.1026. [DOI] [PubMed] [Google Scholar]
- 38.Moy SS, Nadler JJ, Magnuson TR, Crawley JN. Mouse models of autism spectrum disorders: The challenge for behavioral genetics. Am J Med Genet C Semin Med Genet. 2006;142(1):40–51. doi: 10.1002/ajmg.c.30081. [DOI] [PubMed] [Google Scholar]
- 39.Wysocki CJ, Lepri JJ. Consequences of removing the vomeronasal organ. J Steroid Biochem Mol Biol. 1991;39(4B):661–669. doi: 10.1016/0960-0760(91)90265-7. [DOI] [PubMed] [Google Scholar]
- 40.Di Cristofano A, Kotsi P, Peng YF, Cordon-Cardo C, Elkon KB, Pandolfi PP. Impaired Fas response and autoimmunity in Pten+/− mice. Science. 1999;285(5436):2122–2125. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- 41.Borlado LR, Redondo C, Alvarez B, Jimenez C, Criado LM, Flores J, et al. Increased phosphoinositide 3-kinase activity induces a lymphoproliferative disorder and contributes to tumor generation in vivo. FASEB J. 2000;14(7):895–903. doi: 10.1096/fasebj.14.7.895. [DOI] [PubMed] [Google Scholar]
- 42.Xie C, Patel R, Wu T, Zhu J, Henry T, Bhaskarabhatla M, et al. PI3K/AKT/mTOR hypersignaling in autoimmune lymphoproliferative disease engendered by the epistatic interplay of Sle1b and FASlpr. Int Immunol. 2007;19(4):509–522. doi: 10.1093/intimm/dxm017. [DOI] [PubMed] [Google Scholar]
- 43.Okada C, Akbar SM, Horiike N, Onji M. Early development of primary biliary cirrhosis in female C57BL/6 mice because of poly I:C administration. Liver Int. 2005;25(3):595–603. doi: 10.1111/j.1478-3231.2005.01043.x. [DOI] [PubMed] [Google Scholar]
- 44.Paronen J, Liu E, Moriyama H, Devendra D, Ide A, Taylor R, et al. Genetic differentiation of poly I:C from B:9-23 peptide induced experimental autoimmune diabetes. J Autoimmun. 2004;22(4):307–313. doi: 10.1016/j.jaut.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Wen L, Peng J, Li Z, Wong FS. The effect of innate immunity on autoimmune diabetes and the expression of Toll-like receptors on pancreatic islets. J Immunol. 2004;172(5):3173–3180. doi: 10.4049/jimmunol.172.5.3173. [DOI] [PubMed] [Google Scholar]
- 46.Moriyama H, Wen L, Abiru N, Liu E, Yu L, Miao D, et al. Induction and acceleration of insulitis/diabetes in mice with a viral mimic (polyinosinic-polycytidylic acid) and an insulin self-peptide. Proc Natl Acad Sci U S A. 2002;99(8):5539–5544. doi: 10.1073/pnas.082120099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haidinger M, Hecking M, Weichhart T, Poglitsch M, Enkner W, Vonbank K, et al. Sirolimus in renal transplant recipients with tuberous sclerosis complex: clinical effectiveness and implications for innate immunity. Transpl Int. 2010 doi: 10.1111/j.1432-2277.2009.01041.x. [DOI] [PubMed] [Google Scholar]
- 48.Maldonado M, Baybis M, Newman D, Kolson DL, Chen W, McKhann G, 2nd, et al. Expression of ICAM-1, TNF-alpha, NF kappa B, and MAP kinase in tubers of the tuberous sclerosis complex. Neurobiol Dis. 2003;14(2):279–290. doi: 10.1016/s0969-9961(03)00127-x. [DOI] [PubMed] [Google Scholar]
- 49.Lee LC, Newschaffer CJ, Lessler JT, Lee BK, Shah R, Zimmerman AW. Variation in season of birth in singleton and multiple births concordant for autism spectrum disorders. Paediatr Perinat Epidemiol. 2008;22(2):172–179. doi: 10.1111/j.1365-3016.2007.00919.x. [DOI] [PubMed] [Google Scholar]
- 50.Atladottir HO, Parner ET, Schendel D, Dalsgaard S, Thomsen PH, Thorsen P. Variation in incidence of neurodevelopmental disorders with season of birth. Epidemiology. 2007;18(2):240–245. doi: 10.1097/01.ede.0000254064.92806.13. [DOI] [PubMed] [Google Scholar]
- 51.Stevens MC, Fein DH, Waterhouse LH. Season of birth effects in autism. J Clin Exp Neuropsychol. 2000;22(3):399–407. doi: 10.1076/1380-3395(200006)22:3;1-V;FT399. [DOI] [PubMed] [Google Scholar]
- 52.Bolton P, Pickles A, Harrington R, Macdonald H, Rutter M. Season of birth: issues, approaches and findings for autism. J Child Psychol Psychiatry. 1992;33(3):509–530. doi: 10.1111/j.1469-7610.1992.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 53.Barak Y, Ring A, Sulkes J, Gabbay U, Elizur A. Season of birth and autistic disorder in Israel. Am J Psychiatry. 1995;152(5):798–800. doi: 10.1176/ajp.152.5.798. [DOI] [PubMed] [Google Scholar]
- 54.Gillberg C. Do children with autism have March birthdays? Acta Psychiatr Scand. 1990;82(2):152–156. doi: 10.1111/j.1600-0447.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 55.Landau EC, Cicchetti DV, Klin A, Volkmar FR. Season of birth in autism: a fiction revisited. J Autism Dev Disord. 1999;29(5):385–393. doi: 10.1023/a:1023030911527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.