Abstract
Background
Marijuana contains multiple cannabinoids. Most attention is given to delta-9-tetrahydrocannabinol (THC) which produces euphoria and in some cases anxiety and panic reactions. Research suggests that another cannabinoid, cannabidiol (CBD), may offset some of these effects. Thus, there is growing interest in the health consequences of the THC to CBD ratio for marijuana.
Methods
Using data from over 5,000 marijuana samples in California from 1996-2008, we examine changes in the median THC-level, median CBD-level, and median THC:CBD-ratio.
Results
The median THC-level and median THC:CBD-ratio has dramatically increased for seizures in California, particularly north of the Mexican border.
Conclusion
Research on the consequences of the THC:CBD ratio should continue, especially as more attention is devoted to thinking about how to regulate marijuana for medical and recreational use. Researchers should also consider the lack of uniformity in the chemical composition of marijuana when evaluating its health effects.
Keywords: marijuana, mental health, potency, tetrahydrocannabinol, cannabidiol, California
1. INTRODUCTION
Fifteen states and the District of Columbia have made allowances for medicinal marijuana, and debate continues about whether it should be regulated and taxed for non-medicinal purposes like alcohol.
Marijuana is a complicated substance containing multiple cannabinoids. Much of the focus has been on delta-9-tetrahydrocannabinol (THC), which is the primary psychoactive substance in marijuana (Iversen, 2007; Pertwee, 2008). There are concerns that marijuana with higher levels of THC may induce anxiety, panic, and psychosis, especially for new and vulnerable users (Di Forti et al., 2009; Hall and Degenhardt, 2009; Hall and Pacula, 2003).
Less attention is devoted to the effects and levels of the other cannabinoids. Cannabidiol (CBD) in particular is relevant as it is thought to reduce anxiety (Crippa et al., 2010; Crippa et al., 2009; Fusar-Poli et al., 2009; Musty, 2005; Karniol et al., 1974; Zuardi et al., 1982) and have antipsychotic properties (Leweke et al., 2007; Morgan and Curran, 2008; Zuardi et al., 1995; Zuardi et al., 2006). Some studies suggest an increasing ratio of THC to CBD may result in more adverse mental health consequences among users (Morgan and Curran, 2008; Potter et al., 2008; Sewell et al., 2009; Smith, 2005), but more research is needed (Room et al., 2010). Learning more about this ratio and how it varies across geography and time may help us better understand mental health and public health consequences of marijuana consumption.
2. METHODS
To shed light on the THC:CBD ratio, we analyze data from 5,083 marijuana samples that were seized by law enforcement in California between 1996 and 2008 (mean samples per year: 391; median: 361; minimum: 281; maximum: 540). Of 5,556 observations in the original dataset, 432 were removed prior to analysis due to missing values for THC or CBD with an additional 51 removed due to insufficient geographic information. Most samples are from state or local law enforcement, and stem from arrests for manufacturing, distribution, and weapons charges (Personal communication with T. Lanier, National Marijuana Initiative, August 2010). While this represents a fraction of all California seizures, it is the largest database with information on cannabinoid levels for California. Observations are described in terms of location and year of seizure, as well as by cannabinoid content. To reduce the influence of outliers, we summarize the rates and ratios with medians and assess whether differences are statistically significant using the Mann Whitney test. Because of the importance of the Mexican border in the marijuana market, for some analyses we separately consider observations from communities with border crossings (San Ysidro, Otay Mesa, Tecate, Calexico, and Andrade; N=2,207) and observations from elsewhere in the state (N=2,876).
3. RESULTS
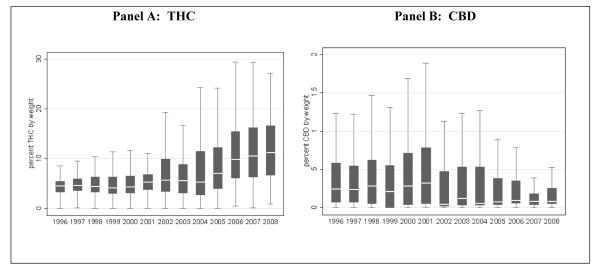
Panel A of Figure 1 tracks changes in THC levels in seized samples of marijuana in California and shows that median THC potency has increased from 4.56% in 1996 to 11.75% in 2008 (p<0.001). The increase in THC was far more dramatic in non-border areas (from 4.18% in 1996 to 13.95% in 2008) than in border areas (4.52% in 1996 to 6.84% in 2008). The variation in THC has also increased, with the interquartile range (represented by the size of the box) rising from 2.17 percentage points in 1996 to 9.93 percentage points in 2008. Interestingly, we see the opposite trend for CBD in Panel B. The median level of CBD dropped from 0.24% in 1996 to 0.08% in 2008 (p<0.001). The interquartile range has also decreased, from 0.51 percentage points in 1996 to 0.20 percentage points in 2008.
Figure 1. THC- and CBD-levels moving in opposite directions in California.
The line in the middle of the box is the median, the top and bottom of the box are the 75th and 25th percentiles, respectively, and the ends of the whiskers represent the 75th (or 25th) percentile ±1.5 x interquartile range. “Outside values” beyond this range are included in the calculations but are not displayed.
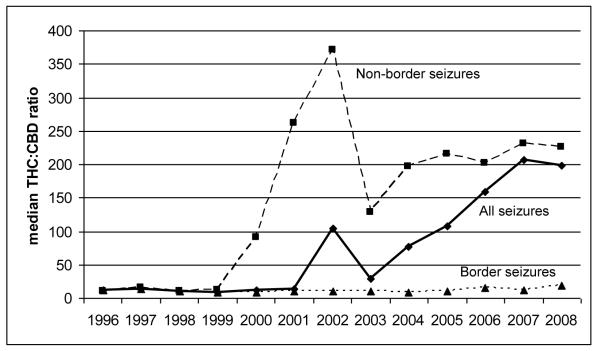
For Figure 2, we calculated the THC:CBD ratio for 4,561 observations with positive amounts of CBD and plotted the median ratio for all seizures and for our two geographic sub-regions. The median ratio for all seizures increased dramatically from 26.21 in 1996 to 187.99 in 2008 (p<0.001), largely driven by seizures away from the border communities (which tended to increase over time as a proportion of all seizures in the sample). While the median ratio for seizures near the border increased from 11.33 to 18.36 over the same period (p<0.001), away from the border the ratio skyrocketed from 11.03 to 226.05 (p<0.001). Note, however, that from 1996 to 1999 the THC:CBD ratio was similar in both regions.
Figure 2. Increasing THC:CBD ratio in California seizures is driven by non-border seizures.
Border seizures include those that took place at a Mexican border crossing or town: San Ysidro, Otay Mesa, Tecate, Calexico, and Andrade.
4. DISCUSSION
The composition of the seizures analyzed in this paper may not be representative of the marijuana produced, traded, or consumed in California—especially considering that law enforcement has discretion about what to seize and what to send to the lab. But given the possible health consequences of the THC:CBD ratio, the fact that the ratio is dramatically different for seizures made near the Mexican border versus those further from the border is noteworthy.
While the reasons behind the observed changes in THC and CBD levels fall outside of the scope of this study, one reason may be that growers are making greater use of plant strains that favor THC production over CBD production. Recent research suggests that sinsemilla marijuana tends to have higher levels of THC and lower levels of CBD (Hardwick and King, 2008; Mehmedic et al., 2010). Sinsemilla is typically grown indoors and accounts for an important share of domestic production in California (Kilmer et al., 2010; Room et al., 2010). Since Mexico is thought to be a major source of lower quality “commercial grade” marijuana (Kilmer et al., 2010), it is not surprising that the ratio is highest for seizures made away from the border.
Considering these trends, research on the consequences of the THC:CBD ratio should continue, especially as more attention is devoted to thinking about how to regulate marijuana for medical and recreational use. Epidemiological studies could exploit geographic variation in the average THC:CBD ratio to determine its relationship to local hospital or treatment admissions. In jurisdictions where experimental studies involving marijuana are permitted, researchers could vary THC:CBD ratios to assess effects on impairment, anxiety, and other outcomes. Also, qualitative research with medical marijuana providers and consumers who use or sell high-CBD strains may yield important insights. In the meantime, researchers studying marijuana should consider the lack of uniformity in the drug (across both time and geography) when evaluating its health effects.
Acknowledgements
We thank Tommy Lanier for helping us understand these data, but the views reflected here only reflect those of the authors.
Role of Funding Source This work was supported by NIDA grant R01DA019993, which covered the design and conduct of the study, the management, analysis, and interpretation of the data, and the preparation and review of the manuscript.
Footnotes
Conflicts of Interest No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
James Richard Burgdorf, Pardee RAND Graduate School RAND Corporation 1776 Main Street Santa Monica, CA 90401, USA.
Beau Kilmer, RAND Drug Policy Research Center RAND Corporation 1776 Main Street Santa Monica, CA 90401, USA.
Rosalie Liccardo Pacula, RAND Drug Policy Research Center RAND Corporation 1776 Main Street Santa Monica, CA 90401, USA.
5. REFERENCES
- Crippa JAS, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran F, Marti-n-Santos R, Simões MV, Bhattacharyya S, Fusar-Poli P, Atakan Z, Filho AS, Freitas-Ferrari MC, McGuire P, Zuardi AW, Busatto G, Hallak JEC. [accessed on October 19, 2010];Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2010 doi: 10.1177/0269881110379283. [Epub ahead of print]. Available from: http://jop.sagepub.com/content/early/2010/09/08/0269881110379283. [DOI] [PubMed] [Google Scholar]
- Crippa JA, Zuardi AW, Martín-Santos R, Bhattacharyya S, Atakan Z, McGuire P, Fusar-Poli P. Cannabis and anxiety: a critical review of the evidence. Hum. Psychopharmacol. 2009;24:515–523. doi: 10.1002/hup.1048. [DOI] [PubMed] [Google Scholar]
- Di Forti M, Morgan C, Dazzan P, Pariante C, Mondelli V, Marques TR, Handley R, Luzi S, Russo M, Paparelli A, Butt A, Stilo SA, Wiffen B, Powell J, Murray RM. High-potency cannabis and the risk of psychosis. Br. J. Psychiatry. 2009;195:488–491. doi: 10.1192/bjp.bp.109.064220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, Martin-Santos R, Seal M, Surguldadze SA, O’Carrol C, Atakan Z, Zuardi AW, McGuire PK. Distinct effects of D9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Ann. Gen. Psychiatry. 2009;66:95–105. doi: 10.1001/archgenpsychiatry.2008.519. [DOI] [PubMed] [Google Scholar]
- Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374:1383–1391. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- Hall W, Pacula RL. Cannabis Use and Dependence: Public Health and Public Policy. Cambridge University Press; Melbourne: 2003. [Google Scholar]
- Hardwick S, King L. Home Office Cannabis Potency Study 2008. Home Office Scientific Development Branch; London: 2008. [Google Scholar]
- Iversen L. The Science of Marijuana. Oxford University Press; Oxford: 2007. [Google Scholar]
- Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA. Cannabidiol interferes with the effects of Δ9-tetrahydrocannabidinol in man. Eur. J. Pharmacol. 1974;28:172–177. doi: 10.1016/0014-2999(74)90129-0. [DOI] [PubMed] [Google Scholar]
- Kilmer B, Caulkins JP, Bond BM, Reuter PH. Reducing drug trafficking revenues and violence in Mexico: Would legalizing marijuana in California help? RAND Corporation; Santa Monica: 2010. RAND Publication No. OP-325-RC. [Google Scholar]
- Leweke F, Koethe D, Gerth C, Nolden BM, Schreiber D, Gross S, Schultze-Lutter F, Hellmich M, Klosterkotter J. Cannabidiol as an antipsychotic agent. Eur. Psychiatry. 2007;22:S1–S82. [Google Scholar]
- Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, Ross SA, Khan IA, ElSohly MA. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J. Forensic Sci. 2010;55:1209–1217. doi: 10.1111/j.1556-4029.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- Morgan CJA, Curran HV. Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. Br. J. Psychiatry. 2008;192:306–307. doi: 10.1192/bjp.bp.107.046649. [DOI] [PubMed] [Google Scholar]
- Musty RE. Cannabinoids and anxiety. In: Mechoulam R, editor. Cannabinoids as Therapeutics. Birkhäuser Verlag; Basel: 2005. pp. 141–147. [Google Scholar]
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter DJ, Clark P, Brown MB. Potency of delta 9-THC and other cannabinoids in cannabis in England in 2005: implications for psychoactivity and pharmacology. J. Forensic Sci. 2008;53:90–94. doi: 10.1111/j.1556-4029.2007.00603.x. [DOI] [PubMed] [Google Scholar]
- Room R, Fischer B, Hall W, Lenton S, Reuter P. Cannabis Policy: Moving Beyond Stalemate. Oxford University Press; Oxford: 2010. [Google Scholar]
- Sewell RA, Ranganathan M, D’Souza DC. Cannabinoids and psychosis. Int. Rev. Psychiatry. 2009;21:152–162. doi: 10.1080/09540260902782802. [DOI] [PubMed] [Google Scholar]
- Smith N. High potency cannabis: the forgotten variable. Addiction. 2005;100:1558–1559. doi: 10.1111/j.1360-0443.2005.01295.x. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Crippa J, Hallak J, Moreira F, Guimaraes F. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz. J. Med Biol. Res. 2006;39:421–429. doi: 10.1590/s0100-879x2006000400001. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Morais S, Guimaraes F, Mechoulam R. Antipsychotic effect of cannabidiol. J. Clin. Psychiatry. 56:488–499. [PubMed] [Google Scholar]
- Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology (Berl) 1982;76:245–2. doi: 10.1007/BF00432554. [DOI] [PubMed] [Google Scholar]