Abstract
There is growing interest in use of progesterone receptor modulators (PRM) such as mifepristone for treatment of gynecological and other conditions, but interest in PRMs is dampened by effects of the agents on the endometrium. In this study, we examined the endometria of women exposed to mifepristone for treatment of leiomyomas in doses of 2.5 mg and 5 mg and compared them to unexposed endometria. We assessed the reliability of these features by comparing agreement in ratings between pathologists who were blinded to each other's readings. We assessed distinguishing features between exposed and unexposed groups by comparing frequency of features between groups. We found that key features could be reliably assessed by pathologists experienced in endometrial pathology. We observed several features (non synchronous endometrium, large fluid filled glands and abnormal blood vessels) that distinguished endometrial samples that were and were not exposed to the drug. These findings suggest several features that can be tracked during studies involving mifepristone and potentially other PRMs.
Keywords: Endometrium/Pathology, Endometrium/Drug Effects, Leiomyoma, Mifepristone
INTRODUCTION
There is growing evidence for the clinical application of progesterone receptor modulators (PRM) such as mifepristone. A number of randomized controlled trials have shown that use of mifepristone and similar agents are associated with significant shrinkage of leiomyomas, reductions in symptoms, and improvement in quality of life [1-7]. Mifepristone has also been shown to be an effective contraceptive agent [8] and there is emerging evidence for its use in psychotic depression [9].
Mifepristone binds strongly to endometrial progesterone receptors, minimally to estrogen receptors, and up-regulates androgen receptors [10]. It also displays anti-proliferative effects in non-human primates [11] and in low dose is associated with reduction in markers for proliferation in humans [12].
Characterization of endometrial morphology following exposure to mifepristone represents a first step towards improved understanding of its action. The general features associated with use of PRMs have been previously described [13]. However, there are relatively few data regarding the reliability of assessment of these endometrial changes and their relative frequency in samples exposed or not to mifepristone. Such data are relevant to tracking changes in the endometrium across doses, administration schedule, and following cessation of the drug.
In this study, we sought to identify the specific endometrial changes for mifepristone that: 1) could be reliably assessed by experienced pathologists; and 2) distinguished samples exposed or not to the drug.
MATERIALS AND METHODS
Endometrial samples were obtained from premenopausal women enrolled in clinical trials of mifepristone at two oral doses for symptomatic leiomyomas [2;4]. The mean age of the sample was 43 years (range 29-53 years). Most participants reported heavy, and not infrequently, irregular bleeding. No ovulation testing was conducted. Samples were obtained on all women prior to starting the drug and every six months thereafter for both women taking mifepristone and those taking a placebo. The study was reviewed by the University of Rochester Institutional Review Board and informed consent was obtained from all participants.
In the first study, 42 women with symptomatic leiomyomas were randomly assigned to mifepristone 5 mg daily or placebo for six months. Two participants assigned to the active drug and three participants assigned to placebo withdrew prior to study completion. None withdrew due to adverse effects. At the conclusion of the study, all participants, regardless of original treatment assignment, were invited to participate and were re-enrolled in an open label trial of the drug at the same dosage for up to an additional 12 months.
In the second trial, 23 women with symptomatic leiomyomas were enrolled in an open label trial of mifepristone 2.5 mg daily for six months, with an option to continue for additional six months. All leiomyomas were assessed by vaginal and/or abdominal ultrasound. Six women withdrew from this study for various reasons (i.e. headache, weight gain, and undisclosed reasons).
All participants were required to have endometrial sampling prior to being enrolled in the study and all but those who withdrew (and declined re-sampling at time of study withdrawal) were re-sampled every six-months and at study completion. Endometrial samples were collected using a Pipelle® (Unimar, Pipelle Cooper Surgical, Shelton, Conn) by an experienced gynecologist. Standard Pipelle technique was used. An effort was made to obtain as full a specimen as possible, with sampling from all four quadrants. Paracervical block was offered to all subjects as an option for pain relief. Sampling was done without regard to the menstrual cycle. At the six-month biopsy, most women taking the drug were amenorrheic. Specimens were fixed immediately in 10% buffered formalin at room temperature for 12-18 hrs, routinely processed and embedded in paraffin wax. Sections 5 microns thick were cut. All sections were then stained with hematoxylin and eosin (H+E).
Baseline and follow-up endometrial biopsies were read by a primary pathologist and by a second pathologist to assess inter-observer reliability. Both pathologists were experienced in gynecologic pathology and reading endometrial samples exposed to mifepristone from earlier studies. Both pathologists were blinded to each others’ reading and whether the patient had received mifepristone or not.
Samples were assessed for features previously described associated with PRM use. The entire sample was scanned at low power. The glandular component was viewed to record the size, and distribution of glands within the stroma and whether cysts were present. Glands were assessed based on an estimate of ratio of glands to stroma and whether they were cystically dilated. The epithelium of the glands was assessed for the presence of these features: stratification of the nuclei, cytological atypia, metaplasia and mitosis. Ki67 staining was performed on a subsample and interpreted based on subjective estimate of the proportion of nuclei staining in glands and stroma and the intensity of staining. Abnormal blood vessels, which included dilated thin walled vessels, anastomosing capillary proliferations, or increased thick-walled vessels, were recorded.
We assessed the inter-observer reliability of each feature using the Kappa statistic or inter-observer reliability coefficient for each pathology feature. Kappas assess the extent beyond chance that two values agree and account for differences in frequency. Kappa values range from “1.0” (perfect agreement) to “0” agreement (completely random) to -1.0 (perfect disagreement).
We compared the frequency of different features between different groups of women using chi square tests. Tests were adjusted for multiple comparisons using a modified Bonferroni-type method [14]. To assess independent association of specific pathology features with drug exposure, we entered each of the pathology features into a logistic regression model and examined the c statistic. The c statistic is analogous to the area under the receiver operating curve and indicates ability of a variable to discriminate or distinguish between two groups [15].
RESULTS
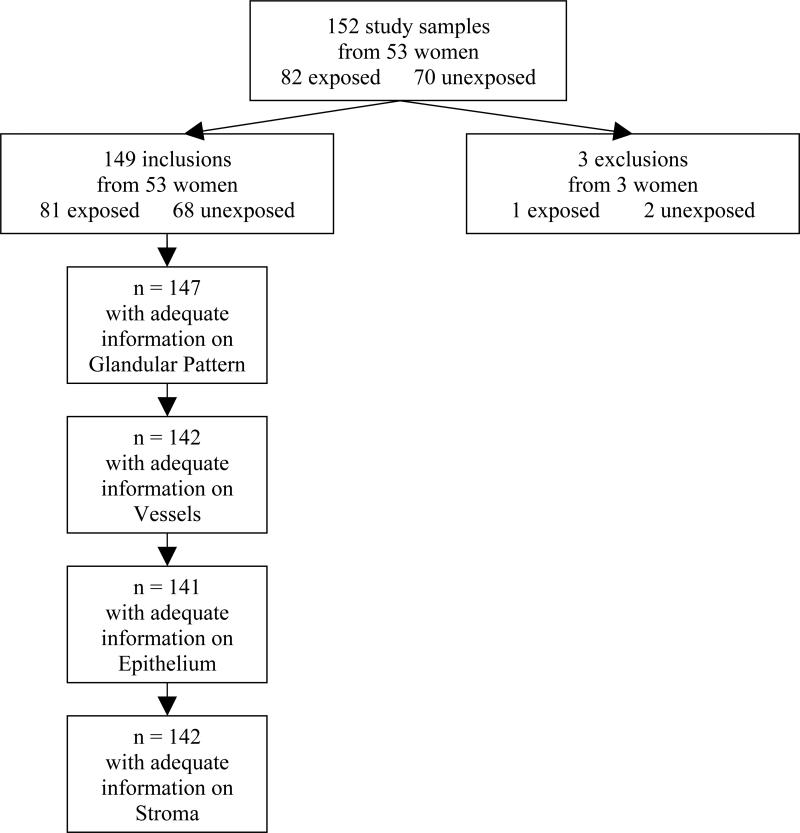
Our study sample included 152 samples from 53 women, including 82 exposed and 70 unexposed endometria (Figure 1). Three samples were insufficient for pathology reading and were excluded. The ages of participants ranged from 29 to 53 years. Further details regarding participants have been previously published [2;4].
Figure 1.
Study Sample
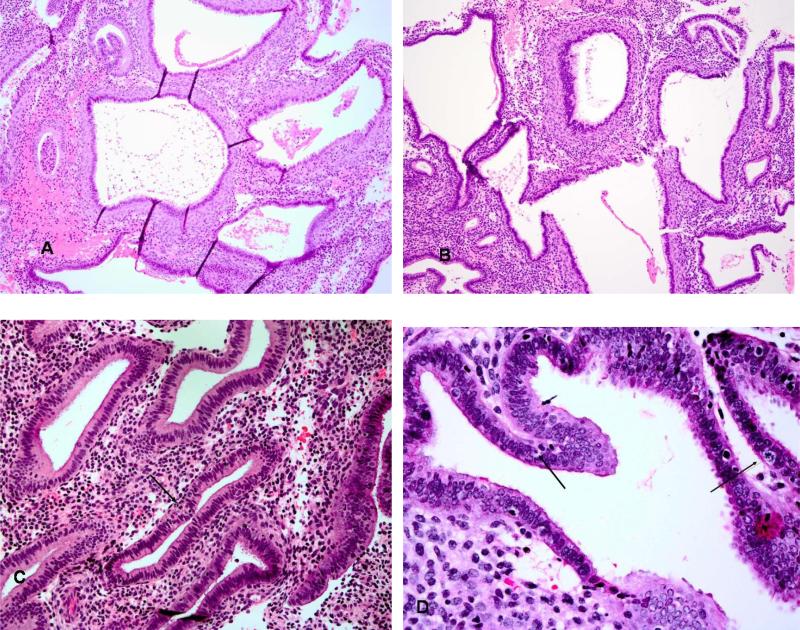
The pathology of the three groups is summarized in Table 1. In general, endometrial samples that were exposed and unexposed showed statistically significant differences across most characteristics. The exposed samples showed a variety of histologic changes that were different from the normal physiologic endometrium (Figure 2). Some of the changes included inactive glands with compact stroma without evidence of stromal breakdown. Other patterns consisted of cysts of varying sizes either scattered throughout a tightly packed non pre-decidualized stroma as seen in the normal cyclic endometria or patterns forming confluent structures of glands with little intervening stroma. Some cysts were lined by cells that showed secretory changes such as the presence of abortive glycogen- containing vacuoles. Apoptosis and mitotic changes were occasionally seen within the epithelium of the glands. The features of the cystically dilated glands were as described in detail by Mutter et al. [13]. The cysts were of varying sizes and appeared either scattered singly or throughout the biopsy (Figure 2). Other cysts formed large confluences with little intervening stroma. Cytologically, these glands did not have the features of atrophy, disordered proliferative endometrium or cystic hyperplasia, and showed only weak mitotic activity (Figure 2). Tubal metaplasia was commonly observed in glands.
Table 1.
Pathology features among samples by exposure to mifepristone
| Variable | Exposed | Unexposed | Chi-square p-value* |
|---|---|---|---|
| n=80 | n=67 | ||
| Glandular Pattern | |||
| Normal | 14% | 96% | <0.0001 |
| Cystically Dilated Glands | 86% | 4% | <0.0001 |
| Epithelium | n=78 | n=63 | |
| Pseudostratified | 67% | 0% | <0.0001 |
| Apoptosis | 47% | 0% | <0.0001 |
| Metaplasia | 70% | 3% | <0.0001 |
| Vessels | n=77 | n=65 | |
| Dilated Thin Wall | 16% | 0% | 0.0009 |
| Anastomosing Capillary Network | 5% | 0% | NS** |
| Plexiform Thick Wall | 13% | 0% | 0.0019** |
| Thick Wall | 35% | 0% | <0.0001 |
| Any of The Above (Abnormal Vessels) | 45% | 0% | <0.0001 |
| Stroma | n=77 | n=65 | |
| Pre-Decidua | 14% | 0% | 0.0015 |
Significant using modified Bonferonni adjustment for multiple comparisons unless marked NS
Fisher's exact test
Figure 2.
The hallmark of the glandular alterations was the presence of multiple cystic glands lined by a variable appearance of the epithelium.(A,B) The glands were frequently lined by a pseudostratified epithelium similar to the normal proliferative pattern but minimal mitotic activity was noted. Apoptotic bodies were often numerous (B). A rare mitotic figure in a gland with considerable apoptotic bodies is noted in (C). Similar glands sometimes displayed subnuclear secretory type vacuoles.
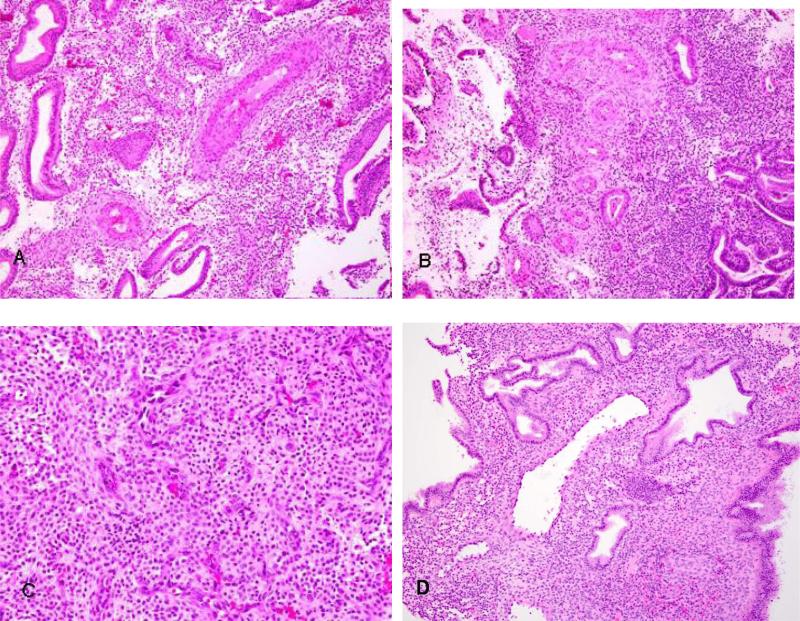
The glandular component of the endometrium was most consistently affected by the drug (Table 1). This change characterized by cystically dilated glands was seen in 86% of exposed samples and only 4% of unexposed samples. The vascular component of the endometrium showed changes that were highly specific to exposure to mifepristone among these samples. Abnormal vessels (dilated thin, anastomosing capillary network, or thick walled) were seen in 45% of exposed samples, but in none of the samples in the unexposed group (Figure 3).
Figure 3.
Vascular changes were a prominent feature in mifepristone exposed patients. A common pattern was the presence of thick walled vessels of the type usually associated with endometrial polyps or normally located in the basalis area distributed throughout the biopsies with no evidence of polyp formation. (A,B). Other vascular changes that were less common included the presence of an interlaced proliferation of fine capillary channels in the stroma (C) or dilated thin walled stromal vessels (D).
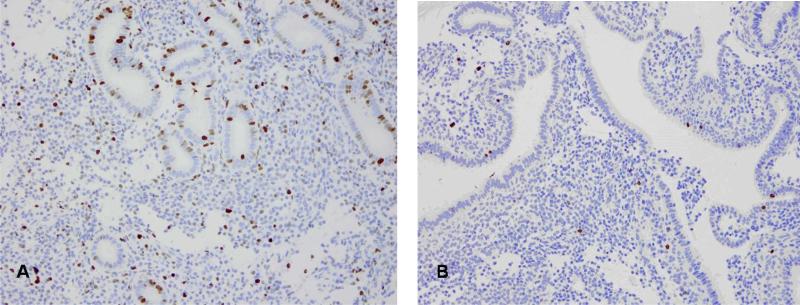
The stromal changes seen in endometria exposed to mifepristone were variable. They ranged from compacted cellular areas to edematous areas often within the same sample. Lymphocytes were identified in stroma. Plasma cells were rarely seen. Mitotic figures were decreased in exposed samples, but remained a constant presence. This was confirmed in the subsample with Ki67 staining (Figure 4 a,b). Pre-decidua changes were similar to normal cyclic endometrium. No endometrial polyps, complex hyperplasia, premalignant lesions (atypical hyperplasia, endometrial intraepithelial neoplasia, or carcinoma were noted in any of the samples.
Figure 4.
Ki-67 staining of endometrium biopsy prior to mifepristone exposure. (A) and example of staining pattern in biopsy taken after drug exposure (B) demonstrating minimal proliferative activity.
Vessel morphology within stroma was also variable in exposed samples. Some were thin and ectatic without fibrin thrombi. Others were dilated with a circumferential rim of fibrosis. There were collections of thick-walled vessels sometimes in plexiform-like patterns that were not localized to any part of the stroma. These areas were not associated with endometrial polyp formation. Another peculiar vascular pattern that was noted was anastomosing thin walled vessels in areas of loose edematous stroma (Figure 3c).
Agreement between the two pathologists in the identification of the features was high. The overall kappa for features was 0.88, but was higher for cystically dilated glands (0.93) and presence of abnormal vessels (0.92).
Among samples exposed to mifepristone, there was no statistically significant difference in any of the features between those exposed to 2.5 mg and 5 mg. Therefore, we combined these into a single exposed group. In a logistic regression model, the presence of cystically dilated glands alone showed a c statistic (measure of prediction) of 0.91. The addition of presence of abnormal vessels increased this to 0.94.
DISCUSSION
In this endometrial pathology study from two clinical trials of mifepristone, we observed features that could be reliably assessed and which distinguished between endometrial samples that were and were not exposed to the PRM, mifepristone. Consistent with other studies of mifepristone [7;8;16;17], and other PRMs [13], endometrial samples exposed to mifepristone were frequently disordered and contained large, fluid filled glands. In many instances, the epithelium of exposed endometrial samples showed metaplasia. Abnormal blood vessel morphology was not seen among any of the samples from unexposed endometrial samples.
An important negative finding as noted in other studies was the absence of any evidence of premalignant lesions including complex hyperplasia with or without atypia or endometrial intraepiphelial neoplazsia. This is a notable finding both in terms of drug safety over the period of observation, but also because endometrial changes associated with PRMs are sometimes mistaken for hyperplasia.
In general, these findings are consistent with known actions of mifepristone on the endometrium and blood vessel endothelium [18-20] and correspond to the spectrum of findings that Mutter et al, have termed progesterone receptor modulator associated changes (PAEC) [13].
These changes are generally similar to those reported for the PRM, CDB 4124 [21]. However, we observed rates of cystically dilated glands comparable to those seen with higher doses of CDB 4124 (50 mg) and much higher rates of abnormal vessels. In contrast to that study of CDB 4124, we did not observe any differences in features between samples exposed to a 5 mg or 2.5 mg dose. This could represent a threshold effect; perhaps differences in features would appear at doses lower than 2.5 mg.
Asoprisnil is also associated with similar findings although there may be subtle differences compared with mifepristone in terms of effects on blood vessel morphology. Specifically, thick walled vessels seem to be characteristic of that drug [22]. In contrast, blood vessel morphology was more variable with mifepristone. Potentially, effects on blood vessels may contribute to the significant reductions in bleeding seen with use of these drugs [1;2;6;23].
Our findings suggest several key histopathological characteristics that might be monitored during treatment with mifepristone or agents with very similar properties [21]. The most sensitive indicator of drug exposure was presence of cystically dilated glands. Only 14% of exposed samples lacked this feature. In contrast, while only about half of exposed samples showed abnormal vessel morphology, its presence in this study was quite specific for drug exposure. It was not seen in any unexposed samples. Given that other conditions, particularly those associated with hormonal disruption or prolonged progesterone exposure can cause changes in vessel morphology, vessel dysmorphology alone may show lower specificity for detection of drug effects in clinical practice than we observed in this selected sample. These features could be used to compare effects from different doses, different schedules of administration (e.g. weekly vs. daily administration), and conduct endometrial studies following drug cessation. Different doses or agents might have variable effects on vessels or glands. Future studies could also examine the time course to resolution of these characteristic features.
These findings are limited by a relatively modest sample size, endometrial biopsies that were not synchronized with women's ovulatory cycles, by absence of objective measure and use of Pipelle biopsies (which typically do not sample from the basalis tissue). Studies with larger populations and longer follow-up are needed to more fully characterize the effects of long-term low dose exposure to mifepristone. Studies are also needed to confirm that these changes resolve following cessation of the drug.
Nonetheless, these findings demonstrate that use of mifepristone, even at very low doses is associated with characteristic changes in the endometrium, and that these changes can be reliably assessed. These findings offer a way for investigators to monitor the effect of very low doses of mifepristone on the endometrium and correlate changes seen in the endometrium with clinical findings.
ACKNOWLEDGEMENTS
Study supported by funding from the National Institute for Child Health and Human Development (Grant # R01-HD042578-01A1) and Athenium.
We appreciate the assistance of Adjuah van Keken in preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
None of the authors reports any conflict of interest, financial or otherwise.
REFERENCES
- 1.Eisinger SH, Meldrum S, Fiscella K, le Roux HD, Guzick DS. Low-dose mifepristone for uterine leiomyomas. Obstet Gynecol. 2003;101:243–250. doi: 10.1016/s0029-7844(02)02511-5. [DOI] [PubMed] [Google Scholar]
- 2.Fiscella K, Eisinger SH, Meldrum S, et al. Effect of mifepristone for symptomatic leiomyomas on quality of life and uterine size: a randomized controlled trial. Obstet Gynecol. 2006;108:1381–1387. doi: 10.1097/01.AOG.0000243776.23391.7b. [DOI] [PubMed] [Google Scholar]
- 3.Bagaria M, Suneja A, Vaid NB, Guleria K, Mishra K. Low-dose mifepristone in treatment of uterine leiomyoma: a randomised double-blind placebo-controlled clinical trial. Aust NZ J Obstet Gynecol. 2009;49:77–83. doi: 10.1111/j.1479-828X.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- 4.Eisinger SH, Fiscella J, Bonfiglio T, Meldrum S, Fiscella K. Open-label study of ultra low-dose mifepristone for the treatment of uterine leiomyomas. Eur J Obstet Gynecol Reprod Biol. 2009 doi: 10.1016/j.ejogrb.2009.06.004. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 5.Carbonell Esteve JL, Acosta R, Heredia B, et al. Mifepristone for the treatment of uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2008;112:1029–1036. doi: 10.1097/AOG.0b013e31818aa930. [DOI] [PubMed] [Google Scholar]
- 6.Levens E, Potlog-Nahari C, Armstrong A, et al. CDB-2914 for Uterine Leiomyomas Treatment: A Randomized Controlled Trial. Obstet Gynecol. 2008;111:1129–1136. doi: 10.1097/AOG.0b013e3181705d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engman M, Granberg S, Williams AR, et al. Mifepristone for treatment of uterine leiomyoma. A prospective randomized placebo controlled trial. Hum Reprod. 2009;24:1870–1879. doi: 10.1093/humrep/dep100. [DOI] [PubMed] [Google Scholar]
- 8.Lakha F, Ho PC, Van der Spuy ZM, et al. A novel estrogen-free oral contraceptive pill for women: multicentre, double-blind, randomized controlled trial of mifepristone and progestogen-only pill (levonorgestrel). Hum Reprod. 2007;22:2428–2436. doi: 10.1093/humrep/dem177. [DOI] [PubMed] [Google Scholar]
- 9.Simpson GM, El SA, Loza N, et al. An 8-week open-label trial of a 6-day course of mifepristone for the treatment of psychotic depression. J Clin Psychiatr. 2005;66:598–602. doi: 10.4088/jcp.v66n0509. [DOI] [PubMed] [Google Scholar]
- 10.Spitz IM. Clinical utility of progesterone receptor modulators and their effect on the endometrium. Curr Opin Obstet Gynecol. 2009;21:318–324. doi: 10.1097/GCO.0b013e32832e07e8. [DOI] [PubMed] [Google Scholar]
- 11.Slayden OD, Brenner RM. Hormonal regulation and localization of estrogen, progestin and androgen receptors in the endometrium of nonhuman primates: effects of progesterone receptor antagonists. Arch Histol Cytol. 2004;67:393–409. doi: 10.1679/aohc.67.393. [DOI] [PubMed] [Google Scholar]
- 12.Narvekar N, Cameron S, Critchley HO, et al. Low-dose mifepristone inhibits endometrial proliferation and up-regulates androgen receptor. J Clin Endocrinol Metab. 2004;89:2491–2497. doi: 10.1210/jc.2003-031945. [DOI] [PubMed] [Google Scholar]
- 13.Mutter GL, Bergeron C, Deligdisch L, et al. The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod Pathol. 2008;21:591–598. doi: 10.1038/modpathol.2008.19. [DOI] [PubMed] [Google Scholar]
- 14.Holland BS, Copenhaver MD. Improved Bonferroni-type multiple testing procedures. Psych Bull. 1988;104:145–149. [Google Scholar]
- 15.Harrell FE, Jr., Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3:143–52. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- 16.Croxatto HB, Kovacs L, Massai R, et al. Effects of long-term low-dose mifepristone on reproductive function in women. Hum Reprod. 1998;13:793–798. doi: 10.1093/humrep/13.4.793. [DOI] [PubMed] [Google Scholar]
- 17.Brown A, Cheng L, Lin S, Baird DT. Daily low-dose mifepristone has contraceptive potential by suppressing ovulation and menstruation: a double-blind randomized control trial of 2 and 5 mg per day for 120 days. J Clin Endocrinol Metab. 2002;87:63–70. doi: 10.1210/jcem.87.1.8140. [DOI] [PubMed] [Google Scholar]
- 18.Hildenbrand A, Stavreus-Evers A, Lalitkumar PG, et al. Aquaporin 1 is expressed in the human endometrium during normal cycle and increases after mifepristone treatment. Int J Molecul Med. 2008;22:49–53. [PubMed] [Google Scholar]
- 19.Papp C, Schatz F, Krikun G, Hausknecht V, Lockwood CJ. Biological mechanisms underlying the clinical effects of mifepristone (RU 486) on the endometrium. Early Preg. 2000;4:230–239. [PubMed] [Google Scholar]
- 20.Girling JE, Lederman FL, Walter LM, Rogers PA. Progesterone, but not estrogen, stimulates vessel maturation in the mouse endometrium. Endocrinol. 2007;148:5433–5441. doi: 10.1210/en.2007-0856. [DOI] [PubMed] [Google Scholar]
- 21.Ioffe OB, Zaino RJ, Mutter GL. Endometrial changes from short-term therapy with CDB-4124, a selective progesterone receptor modulator. Mod Pathol. 2009;22:450–459. doi: 10.1038/modpathol.2008.204. [DOI] [PubMed] [Google Scholar]
- 22.Williams AR, Critchley HO, Osei J, et al. The effects of the selective progesterone receptor modulator asoprisnil on the morphology of uterine tissues after 3 months treatment in patients with symptomatic uterine leiomyomas. Hum Reprod. 2007;22:1696–1704. doi: 10.1093/humrep/dem026. [DOI] [PubMed] [Google Scholar]
- 23.Wilkens J, Chwalisz K, Han C, et al. Effects of the selective progesterone receptor modulator asoprisnil on uterine artery blood flow, ovarian activity, and clinical symptoms in patients with uterine leiomyomas scheduled for hysterectomy. J Clin Endocrinol Metab. 2008;93:4664–4671. doi: 10.1210/jc.2008-1104. [DOI] [PubMed] [Google Scholar]