Abstract
Background
American Indian and Alaska Natives (AI/AN) suffer pervasive health disparities, including disproportionately high rates of HIV. Sexual network dynamics, including concurrency and sexual mixing patterns, are key determinants of HIV disparities.
Methods
We analyzed data from the first national study of gay, lesbian, bisexual, and transgender (GLBT) AI/AN to examine prevalence of concurrency, sex and race of partners, and level of risk across different partnership patterns. Egocentric network data were analyzed at the level of the respondents, who were grouped according to the sex of their last three partners.
Results
Overall rates of HIV and concurrency were high in this population. HIV prevalence (34%) and cumulative prevalence of concurrency (55%) were highest among men who had sex with only men, while women who had sex with only women reported lower concurrency and HIV. Women who had sex with women and men also had high HIV prevalence (15%) and reported slightly higher concurrency risk and low condom use, making them effective bridge populations.
Conclusions
The uniformly high rates of Native partner selection creates the potential for amplification of disease spread within this small community, while the high rates of selecting partners of other races creates the potential for bridging to other groups in the transmission network. These findings provide some of the first insights into sexual networks and concurrency among Native GLBT populations and suggest that both men and women deserve attention in HIV prevention efforts at individual, dyadic and population levels.
Keywords: Concurrency, network analysis, HIV, sexual risk, American Indians, Native Americans, two-spirit, GLBT
INTRODUCTION
There are an estimated 4.4 million American Indians and Alaska Natives (AI/AN in the United States (U.S.) with 562 federally and over 70 state recognized tribes.[1–3] Despite their wealth of tribal diversity and cultural traditions, AI/AN in the U.S. suffer pervasive health disparities. [3, 4] In particular, epidemiologic evidence points to excessively high rates of sexually transmitted infections (STI) among AI/AN compared to the general population, including the second highest rates of chlamydia and gonorrhea. Through December 2007, a cumulative total of 3,492 AIDS cases among AI/AN were reported to the Center of Disease Control (CDC), primarily among men who have sex with men and injection drug users. In addition, 29% of AI/AN diagnosed with AIDS are women or girls, a percentage second only to African Americans (35%). [4] Along with African Americans, AI/AN were the only racial group to experience an increase in HIV incidence between 1990 and 2004. [5, 6] To avoid an escalation of the epidemic, researchers need to examine more closely STI transmission processes among AI/AN.
HIV and STI epidemiologists have begun to articulate the importance of sexual network dynamics, including concurrent partnerships and selective mixing, as key determinants of HIV racial disparities [7–10] Concurrency – an individual’s having sexual partnerships that overlap in time – can dramatically increase the speed and magnitude of HIV spread. At the individual level, concurrency increases the likelihood that a person will transmit infection by removing the protective effect of partner sequencing. With concurrent partners, a person can relay infection from one partner to the other in either direction, in contrast to serial monogamy, where transmission can only occur from earlier partners to later partners in the sequence. [11] At the population-level, concurrent partnerships link individuals together to create large connected "components" in a network; such connected components allow a pathogen to travel rapidly and efficiently by reducing the time lost in isolated dyads. The longer the duration of overlap for concurrent partnerships, the more opportunity the pathogen has to use this connectivity for rapid spread.
Selective mixing patterns also can influence the spread of HIV. Assortative mixing (i.e., individuals choosing socio-demographically similar sexual partners) leads to segregated networks that can contain infection. A combination of assortative mixing with differential levels of concurrency across groups makes it possible to sustain long-term prevalence differentials across populations. This kind of network pattern may help to explain the persistent racial differentials in HIV and other STI in the US. [12–15] Disassortative mixing (i.e., individuals choosing socio-demographically dissimilar sexual partners) can lead to bridging across groups. This may facilitate the spread of HIV when sexual partnerships occur between high-risk groups and low-risk groups. Among AI/AN men who have sex with men (MSM) and women who have sex with women (WSW), disassortative mixing based on partner racial characteristics or gender characteristics (i.e., men who have sex with men and women (MSMW) and women who have sex with women and men (WSWM)) may be a proxy measure of “bridging” between two sexual networks. [16]
Among AI/AN, MSM and MSMW may be disproportionately affected by HIV. [17] Although there is a presumption of lower HIV transmission risk among Native WSW and WSWM, the disproportionately high rates of STIs and other risk factors for potential HIV exposure suggest that Native WSW, and, in particular WSWM, warrant closer examination as well.[18]
In the present study, we analyzed data from the first national study of gay, lesbian, bisexual, and transgender (GLBT) AI/AN with individual respondents group by sexual behavior of their last three partners. Our goal was to examine prevalence of concurrency, patterns of racial mixing that link these groups to groups outside the AI/AN community, and levels of risk across these different partnership contexts.
METHODS
Respondent Recruitment and Interviewing
Respondents were recruited as part of a multi-site cross-sectional survey of AI/AN GLBT persons from seven metropolitan areas in the U.S.: Seattle-Tacoma, San Francisco-Oakland, Los Angeles, Denver, Tulsa-Oklahoma City, Minneapolis-St. Paul, and New York.[19, 20] To participate, individuals were required to: (1) self-identify as American Indian, Alaska Native, or First Nations and either be enrolled in their tribal nation or report having at least 25% total American Indian blood; (2) self-identify as gay, lesbian, bisexual, transgender, or two-spirit or have engaged in same-sex sexual behavior in the past 12 months; (3) be 18 years of age or older; (4) speak English; and (5) reside, work, or socialize in one of the urban study sites.
Multiple sampling strategies were used to minimize selection bias, including venue-based recruitment, convenience sampling, and respondent-driven sampling (RDS) techniques. Each site coordinator proposed six to eight diverse (by gender and age) first wave “seeds” (n = 36) of which 33 participated. A second wave of RDS generated 58 nominees, of whom 50 participated. Volunteer respondents also were solicited through newsletters, brochures, posters, and word-of-mouth; of 469 volunteers who were solicited, 368 participated. We achieved a total response rate of 80.1%. There were no significant differences between RDS (seeds and nominees) and volunteer respondents for the cohort overall or by site on key socio-demographic variables (i.e., gender, education, employment, income, or housing).
Each respondent received $65.00 for completing a 3–4 hour computer-assisted self-interview. A total of 451 respondents were interviewed between July 2005 and March 2007. Of these, four respondents were later excluded due to ineligibility, leaving a total of 447 participants.
Measures
The interview included, among other items, sections on socio-demographics and health status, sexual risk behavior, and a sexual network module [21] for the last three sexual partners, which provides an egocentric sample of sexual network.
Socio-demographic characteristics
Respondents indicated their current gender identity (coded as male, female, or transgendered); whether they had a penis; sexual orientation; age; and Native blood quantum (combining all tribes’ degree of AI/AN blood (100%, 75%–99%, 50% – 74%, 25%–49%, 24% or less). Socio-economic status variables included education level and income.
Sexual risk
Multiple closed-ended items queried sexual behaviors over the respondent’s lifetime as well as the last 12 months. Respondents indicated whether they had ever traded sex, whether they had a current partner, their HIV status (positive, negative, or unknown) and the number of their lifetime sexual partners. The number of times the respondent had sex without a condom or other barrier (never, once, 2 – 10, 11 – 100, or >100) was collected for each of the respondent’s last 3 partnerships.
Sexual partner characteristics
For each of up to three most recent sexual partners (reported having oral, anal, or vaginal sex), respondents provided partner’s sex; race/ethnicity (coded as AI/AN, non-Hispanic white, non-Hispanic black, other); type of partner (main or steady partner, not a main or steady partner but one with whom respondent had sex more than once, one-time sexual partner, or transactional partner (with whom respondent traded sex for money or other favors). Respondents also were asked whether they had sex with anyone else while they were sexually involved with each of their partners, and whether they believed their partners had additional partners during the course of their partnership.
Sex of partner groupings
Based on respondents’ physical anatomy (i.e., men were defined as those answering yes to the item about whether they had a penis) and reported sex of the last three partners, we divided respondents into four mutually exclusive groups: men who had sex with only men/one man (MSOM), men who had sex with at least one man and at least one woman (MSMW), women who had sex with only women/one woman (WSOW), and women who had sex with at least one woman and at least one man (WSWM). We choose to group respondents based on sexual behavior rather than self-identified sexual orientation because the data shown reported behavior different from reported sexual orientation.
Data Analyses
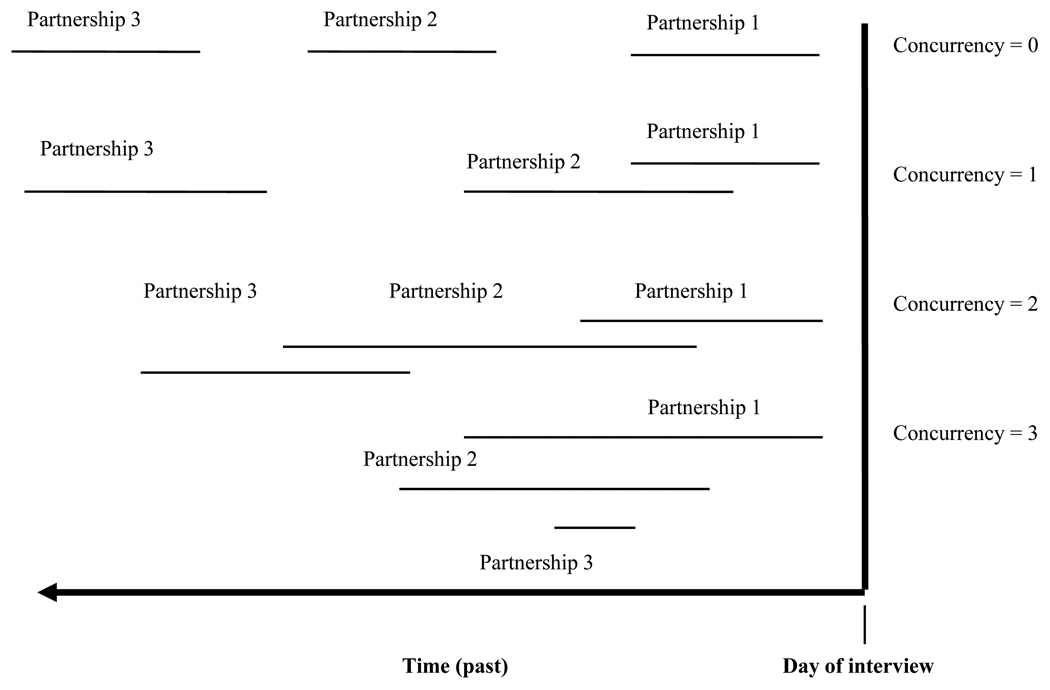
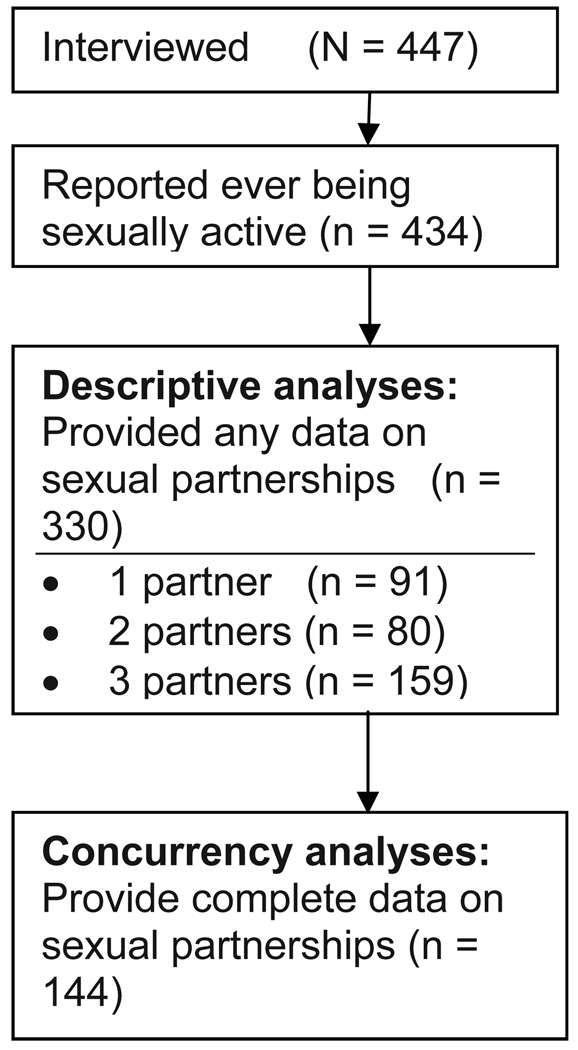
Concurrent partnerships have a number of attributes based on their timing and duration. We calculated three aspects of concurrency for the present analyses: point prevalence, cumulative prevalence, and overlap duration. Point prevalence is defined as having more than one ongoing sexual partnership on the day of the interview. The variable is based on questions that asked whether the respondents expected to have sex again with each of the partners they reported. Cumulative prevalence is defined as reporting any overlapping partnerships among the last three partners. We report two aspects of cumulative prevalence: the first restricted to overlaps that occurred during the past 12 months and the second unrestricted by time. These are based on the reported dates (month/year) of first and last sex with each partner. If one partnership ended and another began in the same month, we conservatively assumed no concurrency. Our findings therefore represent a lower bound estimate of cumulative prevalence. As seen in Figure 1, cumulative concurrency = 0 in two cases: (1) when the respondent practices serial monogamy, which requires each partnership to end before the next one begins, and (2) when respondents have fewer than two partners in their lifetime. Concurrency = 1 when two partnerships overlap only with each other, concurrency = 2 when there are two pairs of overlapping partnerships, and concurrency = 3 if all three partnerships overlap at the same time. Time spent in concurrent partnerships is defined as the number of months that the respondent has more than one partner. Figure 2 illustrates the sample and the differences between the analytic sample among those who reported sexual activity and partnership data.
Figure 1.
Possible concurrency configurations (last three partners)
Figure 2.
Sample size for descriptive analyses and concurrency analyses
Egocentric network data can be analyzed at multiple levels: respondent, partnership, and partnership configuration.[21] This paper reports results of analyses that use the respondent as the unit of analysis. Respondents were grouped according to sex of their last three partners (i.e., MSOM, MSMW, WSOW, WSWM) and descriptive statistics were used to compare sexual network patterns and risk across these four groups. Statistical significance was assessed using chi-square tests (for categorical variables) or ANOVAs (for continuous variables). When these suggested significance, we followed-up with chi-squares and post hoc Bonferroni t tests to pinpoint group differences.
RESULTS
Respondent Characteristics
Table 1 summarizes the socio-demographic characteristics and sexual risk behaviors of the 330 respondents who reported at least one partner in the network module, by grouping based on the sex of their last three sexual partners with no time limit. As seen in the table, there were some important differences beyond the traditional male-female variations in gender, anatomy, sexual orientation, and income.
Table 1.
Sociodemographic and sexual risk related characteristics for 330 sexually active two-spirit Natives, by sexual partnering with last three partners
| Total Networks |
MSOM | MSMW | WSOW | WSWM | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 330) | (n = 134) | (n = 32) | (n = 65) | (n = 99) | |||||||
| Respondent Characteristics | N | % | N | % | N | % | N | % | N | % | |
| Gender *** | |||||||||||
| Male | 155 | 47.0% | 122a | 91.0% | 23b | 71.9% | 4c | 6.2% | 6c | 6.1% | |
| Female | 149 | 45.2% | 0a | 0.0% | 1a | 3.1% | 57b | 87.7% | 91b | 91.9% | |
| Transgendered M-F | 17 | 5.2% | 10a | 7.5% | 7b | 21.9% | 0a, c | 0.0% | 0c | 0.0% | |
| Transgendered F-M | 9 | 2.7% | 2a | 1.5% | 1a | 3.1% | 4a | 6.2% | 2a | 2.0% | |
| Have a penis? *** | 166.0 | 0.5 | 134a | 1.0 | 32a | 1.0 | 0b | 0.0 | 0b | 0.0 | |
| Reported sexual orientation *** | |||||||||||
| Gay/lesbian | 152 | 46.1% | 89a | 61.4 | 5b | 15.6% | 36a | 55.4% | 22b | 22.2% | |
| Bisexual | 90 | 27.3% | 12a | 14.0 | 18b | 56.3% | 10a | 15.4% | 50b | 50.5% | |
| Two-spirit | 51 | 15.5% | 23a, b | 17.4 | 3a, b | 9.4% | 17a | 26.2% | 8b | 8.1% | |
| Heterosexual | 23 | 7.0% | 4a | 2.9 | 3a, b | 9.4% | 0a | 0.0% | 16b | 16.2% | |
| Other | 14 | 4.2% | 6 | 4.3 | 3 | 9.4% | 2 | 3.1% | 3 | 3.0% | |
| Education <=12 years* | 143.0 | 0.4 | 48a | 0.4 | 14a,b | 0.4 | 25a,b | 0.4 | 56b | 0.6 | |
| Monthly income <=$1000* | 195.0 | 0.6 | 67a | 0.5 | 18a, b | 56.3 | 40a, b | 0.6 | 70b | 0.6 | |
| Age (in years) | |||||||||||
| 18 – 24 | 26 | 7.9% | 10 | 7.5% | 4 | 12.5% | 5 | 7.7% | 7 | 7.1% | |
| 25 – 34 | 96 | 29.1% | 39 | 29.1% | 8 | 25.0% | 17 | 26.2% | 32 | 32.3% | |
| 35 – 44 | 96 | 29.1% | 39 | 29.1% | 12 | 37.5% | 18 | 27.7% | 27 | 27.3% | |
| 45 –67 | 112 | 33.9% | 46 | 34.3% | 8 | 25.0% | 25 | 38.5% | 33 | 33.3% | |
| % Native blood | |||||||||||
| <25% | 23 | 7.0% | 8 | 6.0% | 1 | 3.1% | 8 | 12.3% | 6 | 6.1% | |
| 25 – 49% | 102 | 30.9% | 35 | 26.1% | 8 | 25.0% | 26 | 40.0% | 33 | 33.3% | |
| 50 – 74% | 70 | 21.2% | 33 | 24.6% | 10 | 31.3% | 9 | 13.8% | 18 | 18.2% | |
| >=75% | 134 | 40.6% | 58 | 43.3% | 12 | 37.5% | 22 | 33.8% | 42 | 42.4% | |
| HIV status *** | |||||||||||
| HIV-negative | 223 | 67.6% | 79a | 59.0% | 20a, b | 62.5% | 53b | 81.5% | 71a, b | 71.7% | |
| HIV-positive | 70 | 21.2% | 48a | 35.8% | 6a, b | 18.8% | 1b | 1.5% | 15b | 15.2% | |
| HIV-unknown | 36 | 10.9% | 7 | 5.2% | 5 | 15.6% | 11 | 16.9% | 13 | 13.1% | |
| Ever traded sex? *** | 124.0 | 0.4 | 68a | 0.5 | 3a | 0.1 | 15b,c | 0.2 | 38a,c | 0.4 | |
| Have current partner? * | 155 | 51.0% | 50 | 41.0% | 16 | 57.1% | 36 | 58.1% | 53 | 57.6% | |
| >10 lifetime partners? * | 242 | 73.3% | 110a | 82.1% | 22a, b | 68.8% | 43a, b | 66.2% | 67a | 67.7% | |
| Types of partners (% who report any) | |||||||||||
| Main or steady *** | 231 | 70.0% | 73a | 54.5% | 20a, b | 62.5% | 55b | 84.6% | 83b | 83.8% | |
| More than once | 168 | 50.9% | 74 | 55.2% | 18 | 56.2% | 29 | 44.6% | 47 | 47.5% | |
| One time only** | 94 | 28.5% | 50a | 37.3% | 11a, b | 34.4% | 8b | 12.3% | 25a, b | 25.3% | |
| Transactional | 11 | 3.3% | 7 | 5.2% | 1 | 3.1% | 1 | 1.5% | 2 | 2.0% | |
| Partnership duration in months (n = 298)τ | |||||||||||
| <= 1 mo (% who report any)** | 97 | 32.7% | 50a, c | 41.3% | 8a, b, c | 28.6% | 8b | 13.8% | 31c | 34.4% | |
| N | median | N | median | N | median | N | median | N | median | ||
| Median (Avg w/in person) | 298 | 26 | 121 | 16 | 28 | 31 | 58 | 37.8 | 90 | 28 | |
| IQR (Avg w/in person) | 7 – 70 | 4 – 51 | 8 – 91 | 16 – 70 | 3 – 75 | ||||||
| Condom use (always with all partners) | |||||||||||
| Vaginal ** (n = 196) | 12 | 6.1% | - | -- | 0a | 0.0% | 9b | 13.8% | 3a | 3.0% | |
| Anal (n = 265) | 43 | 16.2% | 17 | 12.7% | 4 | 12.5% | - | -- | 22 | 22.2% | |
| All sex ** (n = 330) | 29 | 8.8% | 17 | 12.7% | 0 | 0.0% | 9 | 13.9% | 3 | 3.0% | |
• Notes. MSOM = ‘men who have sex only with men’ - anatomic males with male partners only
MSMW = ‘men who have sex with men and women’ - anatomic males with at least 1 female partner
WSOW = ‘women who have sex with only women’ - anatomic females with female partners only
WSWM = ‘women who have sex with women and men’ - anatomic females with at least 1 male partner
Analyses are conducted using chi-square test
Partnership duration is reported on 298 as 32 respondents did not report data on the duration of partnerships for any of their partners.
p<0.05,
p<0.01,
p<0.001
values which do not share a common superscript are significantly different (p < 0.05)
Over 20% of the sample self-reported being HIV-positive, with an additional 11% unsure of their status. Percentage was highest for MSOM (36%), followed by MSMW (19%), WSWM (15%), and WSW (2%). Over one third of the sample (38%) reported trading sex, which was highest among MSOM (51%) and WSWM (38%). Just over half of each group (57–58%) reported a current, steady partner except for MSOM (41%). About two thirds of each group (66–69%) reported 10 or more lifetime partners except for MSOM (82%). Per each age category 46.9% of the 18–25 years, 78.9% of the 26 – 34 year olds, 78.6 of the 35–44 years old and 74.7 of the 45 years and over reported more than 10 partners.
Partnership Characteristics
Table 1 presents findings on type of partner, partnership duration, and condom use among respondents’ last three partners (no time limit) by grouping based on the sex of their last three sexual partners with no time limit. A total of 728 partnerships were reported. With respect to partner type, men were less likely than women to report at least one of their last three partners was a steady partner (55–63% versus 84–85%), and more likely to report a non-steady partner with whom they had had sex more than once (55–56% versus 45–48%), a one-time partner; 34–37% versus 12–25%), and a transactional partner (i.e., one with whom they traded sex; 3–5% versus 1–2%).
Median partnership duration was 26 months, and 33% of respondents reported at least one partnership lasting less than one month. MSOM (41%) and WSWM (34%) were most likely to report a partnership of less than one month.
Consistent condom use with all partners was very low: only 9% of the respondents reporting having always used condoms with all partners. Consistent condom use for anal sex ranged from 13–22%, which was generally higher than for vaginal sex (0–14%).
Concurrency
Concurrency findings, based on the sub-sample of 144 respondents with “complete” partnership data, are reported in Table 2. Of those that reported complete partnership data three of the 144 respondents had one partner only: one was MSOM, one was WSOM, and one was MSMW. The point prevalence of concurrency was 24%, with 17% reporting 2 ongoing partners, and 7% reported 3 ongoing partners on the day of the interview. Concurrency varied by sex of partners, and was generally higher among MSOM and WSWM.
Table 2.
Concurrency characteristics for 144 two-spirit Natives, by sexual partnering with last three partners
| Total | MSOM | MSMW | WSOW | WSWM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 144) | (n = 60) | (n = 10) | (n = 35) | (n = 39) | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| Point prevalence | ||||||||||
| Any concurrency * | 34 | 23.6% | 20 | 33.3%a | 1 | 10.0%a,b | 3 | 8.6%b | 10 | 25.6% |
| 2 ongoing partners | 24 | 16.7% | 14 | 23.3% | 0 | 0.0% | 1 | 2.9% | 9 | 23.1% |
| 3 ongoing partners | 10 | 6.9% | 6 | 10.0% | 1 | 10.0% | 2 | 5.7% | 1 | 2.6% |
| Cumulative prevalence: last 3 partners in last 12 months | ||||||||||
| Any concurrency ** | 58 | 40.3% | 33 | 55.0%a | 2 | 20.0%a,b | 9 | 25.0%b | 14 | 35.0% |
| 1 concurrency | 22 | 15.3% | 12 | 20.0% | 1 | 10.0% | 3 | 8.6% | 6 | 15.4% |
| 2 concurrencies | 19 | 13.2% | 10 | 16.7% | 0 | 0.0% | 3 | 8.6% | 6 | 15.4% |
| 3 concurrencies | 17 | 11.8% | 11 | 18.3% | 1 | 10.0% | 3 | 8.6% | 2 | 5.1% |
| Mean number of concurrencies | 144 | 0.76 | 60 | 1.08 | 10 | 0.40 | 35 | 0.50 | 39 | 0.60 |
| Cumulative prevalence: last 3 partners no time limit | ||||||||||
| Any concurrency ** | 85 | 59.0% | 42 | 70.0%a | 3 | 30.0%a | 21 | 60.0%a,b | 19 | 48.7% |
| 1 concurrency | 39 | 27.1% | 16 | 26.7% | 2 | 20.0% | 11 | 31.4% | 10 | 25.6% |
| 2 concurrencies | 23 | 16.0% | 13 | 21.7% | 0 | 0.0% | 4 | 11.4% | 6 | 15.4% |
| 3 concurrencies | 23 | 16.0% | 13 | 21.7% | 1 | 10.0% | 6 | 17.1% | 3 | 7.7% |
| Mean number of concurrencies | 144 | 0.88 | 60 | 1.35 | 10 | 0.5 | 35 | 1.09 | 39 | 0.79 |
| Duration of Overlap (n = 85) | ||||||||||
| <=1 mo (% who report any) | 24 | 28.2% | 17 | 40.5% | 1 | 33.3% | 3 | 14.3% | 3 | 15.8% |
| Median (Avg w/in person) | 85 | 4 | 42 | 2.25 | 3 | 1 | 21 | 15.3 | 19 | 6 |
| IQR (Avg w/in person) | 1 – 22 | 1 – 20 | 0 – 3 | 3 – 35 | 2 – 17 | |||||
| Condom use with concurrent partners (no time limit) (n = 85) | ||||||||||
| Ever unsafe with <= one partner only | 24 | 28.2% | 10 | 23.8% | 0 | 0.0% | 9 | 42.9% | 5 | 26.3% |
| Ever unsafe with more than one partner | 61 | 71.8% | 32 | 76.2% | 3 | 100% | 12 | 57.1% | 14 | 73.7% |
| Think partner had concurrent partners | 67 | 78.8% | 32 | 76.2% | 3 | 100% | 16 | 76.2% | 16 | 84.2% |
Notes. MSOM = ‘men who have sex only with men’ - anatomic males with male partners only
MSMW = ‘men who have sex with men and women’ - anatomic males with at least 1 female partner
WSOW = ‘women who have sex with only women’ - anatomic females with female partners only
WSWM = ‘women who have sex with women and men’ - anatomic females with at least 1 male partner
Analyses are conducted using chi-square test
p<0.05,
p<0.01,
p<0.001
values which do not share a common superscript are significantly different (p < 0.05)
Cumulative prevalence of concurrency in the last 12 months was 40%, with 15% reporting 1 concurrency, 13% reporting 2, and 12% reporting 3. The upper limit, assuming concurrency if one partnership ended and another began in the same month, was 45%. Again, cumulative prevalence was highest for MSOM and WSWM. Removing the time restriction, cumulative prevalence of concurrency rose to 59% (upper limit was 70%); 27% reported 1 concurrency; 16% reported 2; and 16% reported 3. Prevalence was significantly higher for MSOM.
The overall median duration of overlap in concurrent partnerships was 4 months, with 28% of respondents reporting an overlap of one month or less. Among MSOM, 41% reported at least one overlap in partners with duration of less than one month, compared to 14–33% for the other groups. Median duration of overlap was 2.25 months for MSOM, compared to 1–15 months for the other groups.
Among respondents who reported at least one concurrency (no time limit), 72% reported unprotected sex with more than one of their partners. Most respondents (79%) believed their concurrent partners also were having sex with other partners.
Concurrency (no time limit) was related to gender, HIV, number of partners, and reported sex of the last three partners, but not self-reported sexual orientation, education, age, income, percent Native blood, and whether the respondent had ever traded sex or has a current partner (data not shown in table). Specifically, mean concurrency was significantly higher among respondents who were male (n = 60) than female or transgendered (n = 84; M[SD] = 1.33 [1.13] versus = 0.88 [1.04], p < 0.01). In fact, 70% (42 of 60) of the males reported concurrency of at least one. Concurrency also was significantly higher among HIV-positive respondents (n = 21) compared to HIV-negative or HIV-unknown respondents (n = 123; M[SD]= 1.62[1.25] versus 0.98[1.05], p < 0.01). Respondents with greater than 10 lifetime partners compared to those with less than 10 (M[SD] = 1.27[1.15] versus 0.74[0.93], p < 0.01) reported more concurrent partners compared to those with less than 10 (M[SD] = 1.27[1.15] versus 0.74[0.93], p < 0.01).
Partnership Mixing Across Race
Table 3 shows the breakdown of the racial composition of respondent's networks by sex of partner groupings. More than half (68%) of the sample reported all of their last 1–3 partners were of the same race/ethnicity: all Native (18%), all non-Hispanic Black (3%), all non-Hispanic White (28%), or all of “other” race/ethnicity (19%). MSOM were least likely to report only Native partners (11% versus 22–24%) and most likely to report only White partners (40% versus 18–22%). About a third of the sample reported that they had had partners of various races (by definition, these respondents reported more than one partner). Of the 239 respondents who reported the race of two or more partners, 57% reported partners of the same race, with 25/239 (10%) reporting all Native partners. Forty three percent of the respondents with more than one partner reported partners of various races (data not shown in table).
Table 3.
Partnership mixing by race of partner for 330 two-spirit Natives, by sexual partnering with last three partners
| Total | MSOM | MSMW | WSOW | WSWM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Respondent with partners | ||||||||||
| all of the same race | 220 | 66.7% | 94 | 70.1% | 20 | 62.5% | 38 | 58.5% | 69 | 69.7% |
| Only Native partners * | 60 | 18.2% | 14 | 10.5%a | 7 | 21.9%a,b | 15 | 23.1%a,b | 24 | 24.2% |
| Only Black partners | 9 | 2.7% | 6 | 4.5% | 0 | 0.0% | 2 | 3.1% | 1 | 1.0% |
| Only White partners*** | 90 | 27.5% | 54 | 40.3%a | 7 | 21.9%a,b | 12 | 18.5%b | 18 | 18.2% |
| Only Other partners | 61 | 18.5% | 20 | 14.9% | 6 | 18.8% | 9 | 13.9% | 26 | 26.3% |
| Respondents with partners | ||||||||||
| of different races | 110 | 33.3% | 40 | 29.9% | 12 | 37.5% | 27 | 41.5% | 30 | 30.3% |
| Native + other races | 57 | 17.2% | 15 | 11.9% | 8 | 25.0% | 15 | 23.1% | 18 | 18.2% |
| Only non-Native races | 53 | 16.1% | 25 | 18.7% | 4 | 12.5% | 12 | 18.5% | 12 | 12.1% |
Notes. MSOM = ‘men who have sex only with men’ - anatomic males with male partners only
MSMW = ‘men who have sex with men and women’ - anatomic males with at least 1 female partner
WSOW = ‘women who have sex with only women’ - anatomic females with female partners only
WSWM = ‘women who have sex with women and men’ - anatomic females with at least 1 male partner
Analyses are conducted using chi-square test
p<0.05,
p<0.01,
p<0.001
values which do not share a common superscript are significantly different (p < 0.05)
DISCUSSION
Results from this study, the first national survey of GLBT AI/AN, revealed an alarmingly high prevalence of HIV (22%), indicating that this population merits attention in terms of STI prevention efforts. Rivaling HIV prevalence reported for African-American MSM and sub-Saharan Africans, MSOM in our study had the highest HIV prevalence (36%), followed by MSMW (19%) and WSWM (15%).
Further analyses suggested the high levels of HIV may be due to high numbers of lifetime partners, multiple short partnerships, and limited condom use. Men were more likely to have had a non-steady sexual partner more than once, a one-time partner, and a transactional partner than women. Nevertheless, nearly half of the women reported having multiple partnerships. Additionally, consistent condom use with all partners was very low.
Concurrent sexual partnerships may be another critical factor in spreading HIV in GLBT AI/AN populations. Prevalence of concurrency in this population was quite high (24% point prevalence, 40% cumulative over last 12 months), with MSOM and WSWM reporting the highest levels (55% and 35%) of cumulative prevalence of concurrency in the past year. These levels are higher than the prevalence of concurrency in the past year among African-American populations (11%–21%)[22] and are consistent with levels found in sub-Saharan Africa (e.g., Lesotho-55% and Cote d’Ivoire-36%)[8] and among MSM in China (33%).[23] Of the 85 respondents who reported at least one concurrency, 72% reported unprotected sex with more than one of their partners. Moreover, more than 75% of MSOM and 84% of the WSWM believed that at least one of their sexual partners also had concurrent partners. This combination of concurrency and inconsistent condom use may enhance the spread of HIV at the network level.
Additionally, an examination of partnership mixing by race indicated potential network patterns that could contain the epidemic within the small GLBT community (i.e., concurrency and assortative mixing) and bridge to other populations (disassortative mixing). Purely assortative mixing by race (i.e., Native partners only) occurred most frequently among women (WSOW and WSWM) and MSMW (22 – 24%). Additionally, almost half of the respondents in these groups reported at least one Native partner. The uniformly high rate of AI/AN partner selection creates the potential for amplification of disease spread within this small community. Disassortive mixing by race occurred mostly among MSOM. Thus, MSOM who have high levels of unsafe sex, short partnership durations, and high prevalence of concurrency are also the ones partnering the most with non-AI/AN. Combined with high rates of selecting partners of other races, MSOM create the potential for effective bridging to other groups in the transmission network.
Bridging also can occur between the sexes. Although the MSMW sub-sample is fairly small and the findings should be interpreted with caution, there are a few trends in the data that are consistent with non-AI/AN MSMW populations.[24–27] Specifically, our findings demonstrate that MSMW had a high number of sexual partners, engaged in high rates of unprotected anal sex with male and female partners, and engaged in unprotected vaginal sex with female partners. Thus, MSMW may be an effective bridge population across sex. The combination of high rates of concurrency and low condom use also makes WSWM an effective bridge population across race and sex. Specifically, WSWM in our study had very high rates of concurrency, second only to MSOM, and they reported 22% consistent condom use for anal sex and only 3% for vaginal sex.
There were several limitations to our study. As with other self-reports of sensitive information, our data are subject to the possible influences of social desirability and recall bias. Although we used computer-assisted self-interviewing to reduce inhibitions about disclosing, the accuracy of participants’ responses cannot be determined. It is likely that any inaccuracies were related to under-reporting of risk behaviors, however, meaning our findings are likely conservative estimates. Although the overall sample was rather large, some of the groups and specific pairings included small numbers of respondents. Additionally, a programming error, possibly exacerbated by the lengthy interview, led to some missing data on partnerships. This was less of an issue in the partnering analysis (n = 330, in which the analytic sub-sample was very similar to the overall sample) than the concurrency analysis (n = 144, in which the analytic sub-sample included fewer males, was more educated, was younger, and had higher incomes than those in the total sample). Also, the concurrency analyses were based only on respondents with “complete” data on in the network module, which could have biased it in either direction. Those with incomplete data who were excluded might include both individuals in long-term monogamous partnerships who could not recall details about early partners (which would inflate our estimate of concurrency) or highly sexually active individuals with multiple and concurrent partnerships who did not know the details about their partners (which would lower our estimate of concurrency). We did not include as concurrent partnerships that started and ended in the same month, which would also lower our estimate of concurrency. Finally, the network module only inquired about the last three partners. For many respondents, this was a small subset of their total sexual partners. For others currently in long-term partnerships, these may represent partners from many years ago for which their memory may be less reliable and their behaviors less representative of their current situation.
Despite these limitations, the findings provide some of the first insights into sexual networks and concurrency among GLBT AI/AN populations, a group that has been virtually ignored in HIV prevention efforts. The data demonstrate that GLBT AI/AN men and women deserve attention in HIV prevention efforts at individual, dyadic and population levels.
Acknowledgments
Disclosure of Funding
Funding for this research has been provided in part by NIH K99 HD057553, UW-SPRC-CFAR, NIAID P30 A127747 to Cassels, NIMH 6587, the National Institute of Mental Health (MH R01 065821), the Office of Research on Women’s Health, the Office of AIDS Research, and the National Center on Minority Health and Health Disparities to Pearson and Walters, NIMH 58986, NIMH 074364 to Simoni, and NIAID P30 A127747 to Morris. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the University of Washington or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Census Bureau. Census 2000 American Indian and Alaska Native Summary File (AIANSF) 2007
- 2.Freeman C, Fox M. Status and trends in the education of American Indians and Alaska natives. In: U.S. Department of Education NCfES, editor. Washington, DC: U.S. Government Printing Office; 2005. [Google Scholar]
- 3.World Health Organization. The world health report 2002 - Reducing Risks, Promoting Healthy Life. 2002 doi: 10.1080/1357628031000116808. [DOI] [PubMed]
- 4.Centers for Disease Control and Prevention. Vol. 19. Atlanta: US Department of Health and Human Services, CDC; 2009. HIV/AIDS Surveillance Report, 2007. [Google Scholar]
- 5.Bertolli J, McNaghten AD, Campsmith M, et al. Surveillance systems monitoring HIV/AIDS and HIV risk behaviors among American Indians and Alaska Natives. AIDS Educ Prev. 2004;16(3):218–237. doi: 10.1521/aeap.16.3.218.35442. [DOI] [PubMed] [Google Scholar]
- 6.The Henry Kaiser Family Foundation. AIDS at 25: An overview of major trends in the U.S. Epidemic. 2006 http://www.kff.org/hivaids/upload/7525.pdf.
- 7.Hudson CP. Concurrent partnerships could cause AIDS epidemics. Int J STD AIDS. 1993;4(5):249–253. doi: 10.1177/095646249300400501. [DOI] [PubMed] [Google Scholar]
- 8.Mah TL, Halperin DT. Concurrent Sexual Partnerships and the HIV Epidemics in Africa: Evidence to Move Forward. AIDS Behav. 2008 doi: 10.1007/s10461-008-9433-x. [DOI] [PubMed] [Google Scholar]
- 9.Morris M. Sexual networks and HIV. AIDS. 1997;11(Supplement A):S209–S216. [PubMed] [Google Scholar]
- 10.Morris M, Kurth AE, Hamilton DT, Moody J, Wakefield S The Network Modeling Group. Concurrent partnerships and HIV prevalence disparities by race: Linking science and public health practice. Am J Public Health. 2009;99(6):1023–1031. doi: 10.2105/AJPH.2008.147835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris M, Kretzschmar M. Concurrent partnerships and the spread of HIV. AIDS. 1997;11(5):641–683. doi: 10.1097/00002030-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Adimora AA, Schoenbach VJ. Social context, sexual networks, and racial disparities in rates of sexually transmitted infections. J Infectious Dis. 2005;191:S115–S122. doi: 10.1086/425280. [DOI] [PubMed] [Google Scholar]
- 13.Adimora AA, Schoenbach VJ, Bonas DM, Martinson FEA, Donaldson KH, Stancil TR. Concurrent sexual partnerships among women in the United States. Epidemiology. 2002;13(3):320–327. doi: 10.1097/00001648-200205000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Gupta S, Anderson RM, May RM. Networks of sexual contacts - Implications for pattern of spread of HIV. AIDS. 1989;3(12):807–817. [PubMed] [Google Scholar]
- 15.Morris M, Handcock MS, Miller WC, et al. Prevalence of HIV Infection Among Young Adults in the United States: Results From the Add Health Study. Am J Public Health. 2006;96(6):1091–1097. doi: 10.2105/AJPH.2004.054759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorbach PM, Drumright LN, Holmes KK. Discord, discordance, and concurrency: comparing individual and partnership-level analyses of new partnerships of young adults at risk of sexually transmitted infections. Sex Transm Dis. 2005;32(1):7–12. doi: 10.1097/01.olq.0000148302.81575.fc. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control. Department of Health and Human Services; 2007. HIV/AIDS Surveillance Report. [Google Scholar]
- 18.Mitchell CM, Kaufman CE, Beals J. Identifying diverse HIV risk groups among American Indian young adults: the utility of cluster analysis. AIDS Behav. 2004;8(3):263–275. doi: 10.1023/B:AIBE.0000044074.46636.c2. [DOI] [PubMed] [Google Scholar]
- 19.Chae DH, Walters KL. Racial discrimination and racial identity attitudes in relation to self-rated health and physical pain and impairment among two-spirit American Indians/Alaska Natives. Am J Public Health. 2009;99(Suppl 1):S144–S151. doi: 10.2105/AJPH.2007.126003. Epub 2009 Feb 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehavot K, Walters KL, Simoni JM. Abuse, mastery, and health among lesbian, bisexual, and two-spirit American Indian and Alaska Native women. Cultur Divers Ethnic Minor Psychol. 2009;15(3):275–284. doi: 10.1037/a0013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris M. Network Epidemiology: A handbook for survey design and data collection. Oxford: Oxford University Press; 2004. International Studies in Demography series) [Google Scholar]
- 22.Adimora AA, Schoenbach VJ, Doherty IA. HIV and African Americans in the Southern United States: Sexual Networks and Social Context. Sex Transm Dis. 2006;33(7):S39–S49. doi: 10.1097/01.olq.0000228298.07826.68. [DOI] [PubMed] [Google Scholar]
- 23.Choi K-H, Hudes ES, Steward WT. Social Discrimination, Concurrent Sexual Partnerships, and HIV Risk Among Men Who have Sex with Men in Shanghai. China AIDS Behav. 2008;12(Supplement 1):71–77. doi: 10.1007/s10461-008-9394-0. [DOI] [PubMed] [Google Scholar]
- 24.Boulton M, Schramm Evans Z, Fitzpatrick R, Hart G. Bisexual Men: Women, Safer Sex and HIV Transmission. In: Aggleton P, Davies P, Hart G, editors. AIDS: Responses, Interventions and Care. Goldsmiths' College, University of London: The Falmer Press; 1991. [Google Scholar]
- 25.McKirnan DJ, Stokes JP, Doll L, Burzette RG. Bisexually active men: Social characteristics and sexual behavior. J Sex Research. 1995;32:64–75. [Google Scholar]
- 26.Siegel K, Schrimshaw EW, Lekas HM, Parsons JT. Sexual behaviors of non-gay identified non-disclosing men who have sex with men and women. Arch Sex Behav. 2008;37(5):720–735. doi: 10.1007/s10508-008-9357-6. [DOI] [PubMed] [Google Scholar]
- 27.Stokes JP, Vanable P, McKirnan DJ. Comparing gay and bisexual men on sexual behavior, condom use, and psychosocial variables related to HIV/AIDS. Arch Sex Behav. 1997;26(4):383–397. doi: 10.1023/a:1024539301997. [DOI] [PubMed] [Google Scholar]