Abstract
Objective
We investigated effects of three weekly courses of fetal betamethasone (βM) exposure on motivation and cognition in juvenile baboon offspring utilizing the Cambridge Neuropsychological Test Automated Battery.
Study design
Pregnant baboons (Papio sp.) received two injections of saline control (C) or 175 μg/kg βM 24h apart at 0.6, 0.65 and 0.7 gestation. Offspring [Female (FC), n = 7 and Male (MC), n = 6; Female (FβM), n = 7 and Male (MβM), n = 5] were studied at 2.6–3.2 years with a progressive ratio test for motivation, simple discriminations (SD) and reversals (SR) for associative learning and rule change plasticity, and an intra-dimensional/extra-dimensional (IDED) set-shifting test for attention allocation.
Results
βM exposure decreased motivation in both sexes. In IDED testing, FβM made more errors in the SR [mean difference of errors (FβM minus MβM) = 20.2 ± 9.9; P≤0.05], compound discrimination [mean difference of errors = 36.3 ± 17.4; P≤0.05] and compound reversal [mean difference of errors = 58 ± 23.6; P<0.05] stages as compared to the MβM offspring.
Conclusion
This central nervous system developmental programming adds growing concerns of long-term effects of repeated fetal synthetic glucocorticoid exposure. In summary, behavioral effects observed show sex specific differences in resilience to multiple fetal βM exposures.
Keywords: developmental programming, neurodevelopment, neuropsychological testing, operant conditioning, nonhuman primates, synthetic glucocorticoids
INTRODUCTION
Fetal life is a period of intensive developmental plasticity during which an orchestrated and interactive pattern of organ development occurs 1. Postnatal survival depends on an adequate level of maturation of key systems such as the lung. In sheep, the prenatal rise in fetal cortisol that occurs in the final twenty days of gestation 2 has been shown to be essential for terminal differentiation of the fetal lung 3. Administration of synthetic glucocorticoids (sGC) to pregnant women threatening premature labor has clearly proven to be beneficial in enhancing survival of offspring by accelerating lung maturation and decreasing the incidence of neonatal morbidity and mortality 4;5. However several studies by others and ourselves in rodents, sheep and baboons have shown that fetal exposure to sGC in the dosing regimen administered clinically have unwanted effects on the development of key fetal systems such as the peripheral vasculature 6–8, endocrine glands 9, and brain 10;11. Alterations in the trajectory of development are inevitable since the very purpose of the treatment is to produce circulating fetal glucocorticoid (GC) levels that are higher than those appropriate for the current stage of maturation of key fetal organs. There are limited findings on the long-term clinical effects of betamethasone (βM), a common sGC, on neurological development and cognition 12–19. Although no rigorous studies have been conducted on GC effects in humans, a recent paper negatively correlates maternal stress and amniotic cortisol with behavior/cognitive outcomes in infants 20. To date there have been no studies to evaluate postnatal effects of sGC administration to pregnant non-human primates when given by the intramuscular clinical route and in the doses used clinically.
In this study, we sought to investigate the effects of βM administration on cognitive function and motivation in juvenile baboons exposed prenatally to levels of GC that were inappropriate for the stage of gestation utilizing the Cambridge Neuropsychological Test Automated Battery (CANTAB) system. The behaviors assessed were motivation (progressive ratio task), associative learning (simple discrimination task), rule change flexibility (simple discrimination reversal task), selective attention (intra-dimensional set task) and attentional allocation (extra-dimensional set shift task). These behaviors rely on higher cortical function and normally developed limbic components 21;22. Our experimental design based βM administration on National Institute of Child Health and Human Development recommendation for prevention of complications associated with newborn prematurity. The timing of maternal treatment corresponded to the human clinical protocol at weeks 26, 27 and 28 (term 40 weeks). As in the case of human pregnancy GC do not change the length of nonhuman primate pregnancy in contrast to other experimental species such as sheep 1–4 and thus represent a translatable model to human fetuses exposed to sGC that deliver at term. Although records are difficult to obtain, some authorities indicate that in excess of 50% of human babies who are exposed to inappropriate amounts of GC as a result of this efficacious therapy deliver at term thus enhancing the value of this study 12;23.
METHODS
Subjects
All animal procedures were performed in accordance with accepted standards of humane animal care, approved by the Southwest Foundation for Biomedical Research and University of Texas Health Science Center at San Antonio (UTHSCSA) Institutional Animal Care and Use Committee, and conducted in AAALAC, Inc.-approved facilities. Pregnant baboons (Papio sp, n = 25) 10–15 years of age from the colony maintained at the Southwest National Primate Research Center (SNPRC, San Antonio, TX, USA) were housed in outdoor metal and concrete gang cages, each containing 10–16 females and 1 breeding male. Animals were observed three times per week for evaluation of perineal skin swelling. Gestational age was calculated, using estimated day of conception (the time of last maximal perineal skin swelling minus 2 days).
Normal baboon gestation lasts 184 days. At 90 days of gestation (dG) (0.5 gestation, 0.5 G), pregnant baboons were weighed, underwent a detailed ultrasound examination, and were placed in individual indoor cages. Baboons were randomized to receive saline (n= 13) or betamethasone phosphate (n=12, Celestan Solubile, Essex Pharma, Munich, Germany) in doses of 175 μg·kg−1·day−1 – a weight-adjusted dose equivalent to 12 mg administered to a 70 kg woman. Treatments were administered intramuscularly once daily at 0800 hours at 111, 112, 118, 119, 125, and 126 dG [approximately equivalent to 24 (0.6G), 26 (0.65G) and 28 (0.7G) weeks of human pregnancy]. Mothers delivered spontaneously at full term and offspring were reared with their mother in group housing until young adolescence 24. To ensure that testing was conducted at the same stage of post-natal development, offspring (saline Control (C): Female (FC), n = 7 and Male (MC), n = 6 or 175 μg/kg betamethasone (βM): Female (FβM), n = 7 and Male (MβM), n = 5) were transferred to the UTHSCSA in four different cohorts of 5–7 subjects across two years and housed individually in sight of at least one other subject in the UTHSCSA Laboratory Animal Resources facility.
Subjects were behaviorally tested at 2.9 ± 0.03 years of age when males weighed between 7–12 kg and females weighed between 6–9 kg, all being within the normal weight for this age 25. Training and testing were conducted between 9 a.m. and 4 p.m., Monday through Friday. If an animal was tested in the morning (9–11:30 a.m.), feeding occurred at 12 and 5 p.m. or if tested in the afternoon (1–4 p.m.), feeding occurred at 9:00 a.m. and 5 p.m. Nonhuman primate chow (2050 Teklad Global 20% protein, 2.7 kcal/g metabolizable energy, Harlan Laboratories, USA) was given no less than 4 hours preceding behavioral sessions and at the end of the day (5 p.m.) with the exception of the progressive ratio task. For the progressive ratio task, each subject was fed 2 hours before the task was administered regardless of when the animal was tested and a second time at 5 p.m. Daily chow rations were calculated prior to training by administering food ad libitum over a course of two weeks and measuring consumption. Each subject would then be fed half this amount twice per day over the course of the study (3 months). In this manner feed was adjusted to each individual subject and no refusal to eat was observed. Water was always available and fruit and vitamins supplemented the diet on Monday, Wednesday, and Friday. The light cycle was set with lights on at 7 a.m. and off at 9 p.m.
Equipment/CANTAB Training and Testing
Our use of the CANTAB system for baboon behavioral testing has been described in detail 26, also see appendix 1.
Data Analysis
Data are summarized with the mean ± standard error. Mean contrasts with regard to treatment (Control versus Betamethasone - βM) and sex (Female, F versus Male, M) were performed using either a linear model or a repeated measures linear model with an autoregressive correlation structure to model the association between successive trials. The interaction of treatment and sex was included in all models. All subjects provided data for all tasks except one subject (a male control), which was excluded from participation in the IDED test for failing to reach this stage contemporaneously with the others. SAS Version 9.2 (SAS institute, Cary, NC) was used to conduct analyses; statistical significance was set at p ≤ 0.05.
RESULTS
Touch-screen training
Analysis of the initial touch-screen training (shrinking program) comparing control and β M groups on percent errors revealed no effect of treatment or sex (data not shown). In contrast, touch-screen training during the moving stimulus task did reveal differences. Analysis of percent errors made (incorrect trials/total trials × 100) during the moving stimulus sessions revealed a significant treatment effect [mean difference (FβM minus FC) = 18 ± 8.3; P<0.05, Figure 1] between the FC and the FβM offspring with the exposed group showing increased percent errors made, but no significant treatment effect among the male subjects. No significant treatment differences were determined for the two-position stimulus touch-screen training program that followed the progressive ratio sessions (data not shown).
Figure 1.

Analysis of touch screen training (moving stimulus task) shows a treatment effect in the Female βM offspring (closed bar, n=7) increased percent error versus Female Control (open bar, n=7), but no effect between the male groups or between sex within treatment. Male Control (small squares, n=6) and Male βM (big squares, n=5). Mean ± SEM; p<0.05, *.
Progressive ratio (motivation)
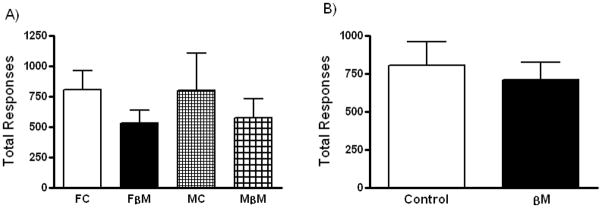
Repeated measures analysis for means of total rewards, total responses or breakpoint per ten sessions did not vary significantly between groups (Figure 2A, data not shown for rewards or breakpoint). However, when treatment groups were pooled, comparison of total responses between control and the βM group revealed a borderline difference (p=0.09), with fewer responses on average in the βM exposed group (Figure 2B).
Figure 2.
A) Analysis of progressive ratio responses during 10 sessions revealed no differences between treatment or sex. B) Pooled groups showed a borderline difference (p=0.09), βM exposed offspring responded less. Female Control (open bar, n=7), Female βM exposed (closed bar, n=7), Male Control (small squares, n=6) and Male βM exposed (big squares, n=5). Mean ± SEM.
Simple discrimination and reversal tasks (associative learning and rule change flexibility)
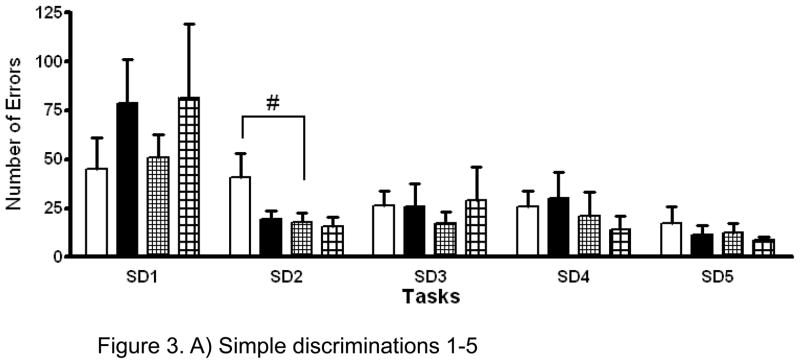
Among controls, the mean number of errors was greater among females than among males in the SD2 task [mean difference (FC minus MC = 22.9 ± 11.2; P≤0.05] (Figure 3A). In addition, the mean number of errors was significantly increased in the FβM group relative to the MβM group in the SR1, SR2 and SR3 tasks [SR1: mean difference (FβM minus MβM) = 17.6 ± 7.6; P<0.05, SR2: mean difference = 52.3 ± 22; P<0.05, SR3: mean difference = 31.9 ± 16.6; P=0.07] (Figure 3B). The only significant treatment effect was observed in the SR2 task with FβM group showing an increase in mean number of errors made compared with FC group [mean difference (FβM minus FC)= 45 ± 20.1; P<0.05] (Figure 3B). There were no significant differences between the MC and MβM groups in the mean number of SD and SR errors.
Figure 3.
A) Analysis of errors made during simple discrimination (SD) 1–5 tasks revealed a sex effect with the Female Control (open bars, n=7) making more errors than the Male Control (small squares, n=6) offspring during SD2 (p≤0.05, #). Female βM exposed (closed bars, n=7) and Male βM exposed (big squares, n=5). Mean ± SEM.
B) Analysis of errors made during simple discriminations (SD) followed by reversals (SR) revealed a treatment effect with the Female βM exposed offspring (closed bars, n=7) making more errors than the Female Control (open bars, n=7) during SR2 (p<0.05, *) and a sex effect as compared to the Male βM exposed offspring (big squares, n=5) during SR1 and SR2 (p<0.05, #), and SR3 (p=0.07, †). Male Control (small squares, n=6). Mean ± SEM.
IDED Test (selective attention and attention allocation)
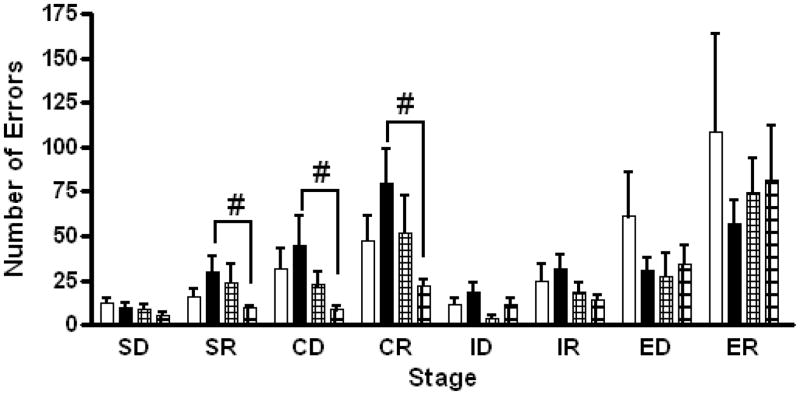
Analysis of the eight stages of the IDED test revealed no overall treatment difference between the control and βM group (Figure 4). The mean number of errors made by FβM offspring was, however, significantly increased relative to MβM offspring for the SR, CD and CR tests stages [SR: mean difference (FβM minus MβM) = 20.2 ± 9.9; P≤0.05, CD: mean difference = 36.3 ± 17.4; P≤0.05, CR: mean difference = 58 ± 23.6; P<0.05]. There were no significant differences between FC and MC, FC and FβM or MC and MβM groups in the mean number of errors made in the IDED test stages.
Figure 4.
Analysis of errors made in the stages of the Intra-/Extra-dimensional Attention Set Shifting test revealed a sex effect between the Female βM exposed (closed bars, n=7) and the Male βM exposed (big squares, n=5) offspring in the SR, CD, and CR stages (p≤0.05, #). Female βM offspring made more errors. Female Control (open bars, n=7) and Male Control (small squares, n=5). Mean ± SEM.
COMMENT
We have developed a nonhuman primate operant test battery to assess cognitive ability in different physiological states 26. There is growing concern from animal models of adverse outcomes following fetal exposure at critical periods of development to levels of GC that are higher than those normally experienced at that stage of development 5;27–30. In view of the paucity of information in primates on any developing system, our initial aim was to use the CANTAB system to evaluate specific developmental outcomes among juvenile baboons exposed to sGC in dosing regimens that mimic those to which the human fetus is exposed. Young baboons are one of the best models available for translation to human outcomes because of their genotypic and phenotypic similarity to young human subjects in terms of fetal growth, postnatal neurodevelopment and cognitive ability 31–33. Also, the singly housed subjects were tested in a non-stressful environment in their home cage in view of age matched peers thereby eliminating the confounds accompanying paired housing 34. The CANTAB tests are a powerful and well characterized tool in biomedical research for their cognitive diagnostic capacity in human psychological assessment and their adaptability for use with nonhuman primates and other animal species 35–40.
Our results revealed an effect of fetal baboon βM exposure on several CANTAB operant tasks. A treatment effect was found between the FC and FβM offspring in the moving stimulus task in which the FβM offspring performed less accurately. This effect suggests impairment at some level (i.e. in anxiety, attention, motor coordination) in the FβM treatment group. Subsequently, no effect was found in the two-position touch-screen training, which suggests intact motor coordination by all groups. Assessing motivation during progressive ratio sessions revealed no overall treatment effect for rewards or breakpoint. For progressive ratio total responses the p-value equaled 0.09 revealing a borderline difference in the βM exposed group responding less than control animals. Results from associative learning, cognitive flexibility (during reversals) and attention based tasks revealed a sex effect in FβM offspring making more errors in the SR, CD and CR tasks during training and testing stages compared with the MβM offspring. A treatment effect was also found in the SR2 task in females. This indicates that the FβM group exhibited impaired reversal learning, increased perseverative behavior and reduced selective attention.
There are several explanations for the potential difference in motivation between the control and βM group in the progressive ratio task. One possibility is that the βM exposed subjects were distractible or hyperactive, i.e. increased displacement behavior, which prevented them from exhibiting the necessary increase in response. Substantiating this argument is evidence indicating that children exposed to sGC from maternal treatment or maternal stress have a higher risk of emotional and behavioral abnormalities including aggressive/destructive behavior, increased distractibility, attention deficit and hyperactivity 17;41–44 and neuromotor development impairment 18. Female exposed children are more likely to develop behavioral disturbances which are in agreement with our observation of greater effects in females than in males 18. Furthermore, in utero effects to the hypothalamic-pituitary adrenal (HPA) axis by sGC exposure may have long lasting ramifications and potentially contribute to behavioral abnormalities 19;45;46.
The known effects of prenatal sGC exposure on the developing brain may provide some explanation for the cognitive performance differences. A clinical study found that exposure to βM in fetal life significantly reduces whole brain cortex convolution (an index of surface area) in infants born at or near term 47. At the subcortical level there is evidence of decreased hippocampal size during adolescence in humans and primates 48;49 and hippocampal atrophy in psychiatric patients administered multiple courses of sGC 50. In rhesus monkeys, repeated prenatal βM exposure reduces brain weights at 165 days of gestation 51. Also, prenatal dexamethasone (another commonly administered sGC) administration after a single or multiple injections on days 132 and 133 of gestation in pregnant rhesus monkeys causes a dose-dependent decrease in the number of pyramidal neurons in all hippocampal regions and of granular neurons in the dentate gyrus at 135 and 162 days of gestation 49 and a 30% reduction in hippocampal size and segmental volume in offspring 52. Lastly, prenatal stress (maternal acoustic startle for 25% of gestation) has been shown to diminish neurogenesis in the dentate gyrus of juvenile rhesus monkey offspring 53.
These effects of GC exposure on neuronal viability could potentially manifest in the behavioral and cognitive deficits observed here. Although the orbital frontal and dorsal lateral prefrontal cortex are important cortical areas in learning simple/reversal discrimination and attention/set-shifting 54, respectively, the subcortical area of importance is the hippocampus 55–58. Hippocampal involvement in different aspects of learning and memory including simple discrimination tasks has been well documented 59–63 as well as its involvement in the regulation of anxiety through various neurotransmitter receptor types including GC receptors 64–69. Consequently, the participation and convergence of different neurotransmitter systems in this brain area adds to the complexity of the mechanistic processes in this critical brain region 15. In view of evidence that the hippocampal formation is sensitive to sGC treatment, the results of our study indicate the need for further investigation to elucidate the cellular mechanisms of the hippocampus and other brain areas relevant to performance of attention, learning and memory based tasks. There is also need for studies investigating potential differences between sexes of sGC exposure during neurodevelopment. Such studies were not possible in this cohort which we propose to maintain for longitudinal life time studies and thus cannot provide access to brain tissue for histological and biochemical analysis. Finally, the impairments observed in the FβM versus the MβM offspring mainly involved reversal task performance. The prefrontal cortex is important for reversal discrimination suggesting a sex-specific neurodevelopmental effect of βM exposure on this area of the brain. Effects on the prefrontal cortex by βM exposure and more specifically the mechanisms by which this exposure affects female subjects remain unknown.
Reports in various experimental nonprimate animal species support our findings. In guinea pigs, repeated prenatal sGC treatment produces sex-specific effects with females exhibiting hyperactivity in an open-field test and a concomitant decrease in expression of the hippocampal N-Methyl-D-Aspartate receptor subunit 70 as well as altered HPA regulation 71. Likewise, young and adult rats born to dams treated with dexamethasone display sex-specific changes in locomotor and habituation activity in an anxiety related behavioral test 72 and prenatally stressed rats display significantly more depressive and anxious behaviors 73–75. Emgard et al. 76 has shown prenatal dexamethasone exposure inhibits Morris water maze learning, a hippocampal dependent task, possibly by damaging cholinergic neurons. Furthermore, rat and sheep studies show a decrease in neural cell number and size in the forebrain 77, as well as decreased brain weight and myelination in the optic nerve and corpus callosum following repeated courses of antenatal sGC, respectively 78;79. We hypothesize that high βM exposure induced change in the hippocampus and forebrain structures during development causing long-term behavioral and neurological consequences in female offspring.
The possibility exist the sex differences we observed in the treatment group are due to the influence of βM exposure occurring at different critical windows of development in the two sexes rather than a quantitative difference on systems with a similar trajectory of development. These sex specific effects may depend on timing of drug exposure and concurrent neurodevelopmental stage. Accordingly, differential neurodevelopment between sex has been reported in nonhuman primates with cerebral (prefrontal) cortical maturation occurring earlier in males than females 80. Indeed pre-adolescent human imaging studies confirm whole brain volume is larger in males than females 81. Thus if neuronal maturation is advancing more rapidly in male than in females then βM could potentially impact brain maturation at that stage in the more developmentally advanced males. It is well known that GC halts cellular differentiation and accelerates tissue and organ maturation. Conversely βM exposure in females, which would be lagging developmentally, results in impaired cognition. Brain development consists of many phases including cellular proliferation, migration, differentiation, synaptogenesis and myelination 82. In our model βM treatment could potentially affect differentiation, synaptogenesis and/or myelination according to the time scale of brain development 82. This difference in the time line of brain maturation and the timing of βM exposure could potentially explain the sex specific effects we observed. An alternate explanation of female susceptibility relates to a clinical study which shows increased 11β hydroxysteroid dehydrogenase 2 activity and cortisol in umbilical artery at term induced by maternal stress in female infants 83. However, studies such as fetal or newborn brain imaging are required to characterize the precise stages of central nervous system development that are affected differentially between sexes.
The effects of repeated prenatal βM exposure were sex-specific with female exposed subjects exhibiting impaired performance in the CANTAB operant tasks which are in agreement with clinical studies that show female behavior and cognitive impairments following antenatal sGC treatment 17;18. To our knowledge this is the first report of such findings in a nonhuman primate model of learning and attention, although others have administered similar CANTAB tests to rhesus and marmoset monkeys 58;84;85 with only one of those groups measuring motivation and cognitive ability following prenatal sGC administration 84. That study reported impaired skilled motor reaching at juvenile age, no effect on motivation but enhanced reversal learning in adolescence with no sex differences after dexamethasone exposure during late gestation. The specific βM treatment used here reveals detrimental neurodevelopment effects in female offspring.
However, the exact reasons for these sex-specific effects are not known. Although speculative, one potential explanation is that male βM exposed offspring show resilience to the in utero challenge while female exposed offspring are more vulnerable. Resilience in general refers to a pattern of functioning indicative of positive adaptation in the context of significant risk or adversity 86. This lack of resilience in females may stem from an imbalance in the mineralocorticoid and glucocorticoid receptor levels in the brain and concomitant HPA axis dysregulation, which have been shown to result in abnormality of cognitive ability among other health concerns 87. Further work on potential mechanisms is required including evaluation of these receptors in the central nervous system and HPA axis. Additionally, timing of prenatal GC exposure appears to have variable effects on offspring mental development with early (15 week gestation) exposure having positive outcomes and late (37 weeks gestation) exposure having negative outcomes in humans 88 and varying effects in marmosets 84. In our model βM administration occurred during late 2nd through early 3rd trimester human pregnancy equivalence, which based on human prenatal stress studies are less likely to cause effects on offspring neurodevelopment 88. In conclusion, we have shown that repeated administration of prenatal βM causes long-lasting behavioral effects (prenatal programming) that manifest in adolescent age raising many questions on clinical dosing regimens, which as we point out have never been rigorously tested for dose response effects.
Supplementary Material
Acknowledgments
Financial Support: NICHD-021350 and a supplement to JSR.
Authors thank Ralf Wimmer, Sue Jenkins and Dr. Thomas McDonald for their advice and assistance in this study. Additional thanks go to Dr. Joel Michalek and Yumin Chen from the Department of Biostatistics and Epidemiology for statistical work. This work was supported by NICHD 21350.
Footnotes
Presentation Information: Presidential Plenary Session, Society for Gynecological Investigation 57th Annual Meeting, Orlando, FL. March 25, 2010.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Nijland MJ, Ford SP, Nathanielsz PW. Prenatal origins of adult disease. Curr Opin Obstet Gynecol. 2008;20:132–38. doi: 10.1097/GCO.0b013e3282f76753. [DOI] [PubMed] [Google Scholar]
- 2.Magyar DM, Fridshal D, Elsner CW, Glatz T, Eliot J, Klein AH, et al. Time-trend analysis of plasma cortisol concentrations in the fetal sheep in relation to parturition. Endocrinology. 1980;107:155–59. doi: 10.1210/endo-107-1-155. [DOI] [PubMed] [Google Scholar]
- 3.Liggins GC, Kitterman JA. Development of the fetal lung. Ciba Found Symp. 1981;86:308–30. doi: 10.1002/9780470720684.ch15. [DOI] [PubMed] [Google Scholar]
- 4.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–25. [PubMed] [Google Scholar]
- 5.Newnham JP, Moss TJ. Antenatal glucocorticoids and growth: single versus multiple doses in animal and human studies. Semin Neonatol. 2001;6:285–92. doi: 10.1053/siny.2001.0064. [DOI] [PubMed] [Google Scholar]
- 6.Docherty CC, Kalmar-Nagy J, Engelen M, Koenen SV, Nijland MJ, Kuc RE, et al. Effect of in vivo fetal infusion of dexamethasone at 0.75 GA on fetal ovine resistance artery responses to ET-1. Am J Physiol Regul Integr Comp Physiol. 2001;281(1):R261–R268. doi: 10.1152/ajpregu.2001.281.1.R261. [DOI] [PubMed] [Google Scholar]
- 7.Docherty CC, Kalmar-Nagy J, Engelen M, Nathanielsz PW. Development of the fetal vascular responses to endothelin-1 and acetylcholine in the sheep. Am J Physiol Regul Integr Comp Physiol. 2001;280:R554–R562. doi: 10.1152/ajpregu.2001.280.2.R554. [DOI] [PubMed] [Google Scholar]
- 8.Molnar J, Nijland MJM, Howe DC, Nathanielsz PW. Evidence for microvascular dysfunction after prenatal dexamethasone at 0.7, 0.75, and 0.8 gestation in sheep. Am J Physiol Regul Integr Comp Physiol. 2002;283:R561. doi: 10.1152/ajpregu.00031.2002. [DOI] [PubMed] [Google Scholar]
- 9.Thomas AL, Krane EJ, Nathanielsz PW. Changes in the fetal thyroid axis after induction of premature parturition by low dose continuous intravascular cortisol infusion to the fetal sheep at 130 days of gestation. Endo. 1978;103:17–23. doi: 10.1210/endo-103-1-17. [DOI] [PubMed] [Google Scholar]
- 10.Antonow-Schlorke I, Schwab M, Li C, Nathanielsz PW. Glucocorticoid exposure at the dose used clinically alters cytoskeletal proteins and presynaptic terminals in the fetal baboon brain. J Physiol (Lond) 2003;547:117. doi: 10.1113/jphysiol.2002.025700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonow-Schlorke I, Kuhn B, Muller T, Schubert H, Sliwka U, Nathanielsz PW, et al. Antenatal betamethasone treatment reduces synaptophysin immunoreactivity in presynaptic terminals in the fetal sheep brain. Neurosci Lett. 2001;297:147–50. doi: 10.1016/s0304-3940(00)01605-0. [DOI] [PubMed] [Google Scholar]
- 12.Wapner RJ, Sorokin Y, Thom EA, Johnson F, Dudley DJ, Spong CY, et al. Single versus weekly courses of antenatal corticosteroids: evaluation of safety and efficacy. Am J Obstet Gynecol. 2006;195:633–42. doi: 10.1016/j.ajog.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 13.Wapner RJ, Sorokin Y, Mele L, Johnson F, Dudley DJ, Spong CY, et al. Long-term outcomes after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1190–98. doi: 10.1056/NEJMoa071453. [DOI] [PubMed] [Google Scholar]
- 14.Church MW, Wapner RJ, Mele LM, Johnson F, Dudley DJ, Spong CY, et al. Repeated courses of antenatal corticosteroids: are there effects on the infant’s auditory brainstem responses? Neurotoxicol. Teratol. 2010;32:605–10. doi: 10.1016/j.ntt.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connors SL, Levitt P, Matthews SG, Slotkin TA, Johnston MV, Kinney HC, et al. Fetal mechanisms in neurodevelopmental disorders. Pediatr Neurol. 2008;38:163–76. doi: 10.1016/j.pediatrneurol.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Dessens AB, Haas HS, Koppe JG. Twenty-year follow-up of antenatal corticosteroid treatment. Pediatrics. 2000;105:E77. doi: 10.1542/peds.105.6.e77. [DOI] [PubMed] [Google Scholar]
- 17.French NP, Hagan R, Evans SF, Mullan A, Newnham JP. Repeated antenatal corticosteroids: effects on cerebral palsy and childhood behavior. Am J Obstet Gynecol. 2004;190:588–95. doi: 10.1016/j.ajog.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Karemaker R, Heijnen CJ, Veen S, Baerts W, Samsom J, Visser GH, et al. Differences in behavioral outcome and motor development at school age after neonatal treatment for chronic lung disease with dexamethasone versus hydrocortisone. Pediatr Res. 2006;60:745–50. doi: 10.1203/01.pdr.0000246200.76860.de. [DOI] [PubMed] [Google Scholar]
- 19.Karemaker R, Kavelaars A, ter WM, Tersteeg-Kamperman M, Baerts W, Veen S, et al. Neonatal dexamethasone treatment for chronic lung disease of prematurity alters the hypothalamus-pituitary-adrenal axis and immune system activity at school age. Pediatrics. 2008;121:e870–e878. doi: 10.1542/peds.2007-2454. [DOI] [PubMed] [Google Scholar]
- 20.Bergman K, Sarkar P, Glover V, O’Connor TG. Maternal prenatal cortisol and infant cognitive development: moderation by infant-mother attachment. Biol Psychiatry. 2010;67:1026–32. doi: 10.1016/j.biopsych.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–52. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 22.Parkinson JA, Cardinal RN, Everitt BJ. Limbic cortical-ventral striatal systems underlying appetitive conditioning. Prog Brain Res. 2000;126:263–85. doi: 10.1016/S0079-6123(00)26019-6. [DOI] [PubMed] [Google Scholar]
- 23.Polyakov A, Cohen S, Baum M, Trickey D, Jolley D, Wallace EM. Patterns of antenatal corticosteroid prescribing 1998–2004. Aust N Z J Obstet Gynaecol. 2007;47:42–45. doi: 10.1111/j.1479-828X.2006.00677.x. [DOI] [PubMed] [Google Scholar]
- 24.Schlabritz-Loutsevitch NE, Lopez-Alvarenga JC, Comuzzie AG, Miller MM, Ford SP, Li C, et al. The prolonged effect of repeated maternal glucocorticoid exposure on the maternal and fetal leptin/insulin-like growth factor axis in Papio species. Reprod Sci. 2009;16:308–19. doi: 10.1177/1933719108325755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glassman DM, Coelho AM, Jr, Carey KD, Bramblett CA. Weight growth in savannah baboons: a longitudinal study from birth to adulthood. Growth. 1984;48:425–33. [PubMed] [Google Scholar]
- 26.Zurcher NR, Rodriguez JS, Jenkins SL, Keenan K, Bartlett TQ, McDonald TJ, et al. Performance of juvenile baboons on neuropsychological tests assessing associative learning, motivation and attention. J Neurosci Methods. 2010;188:219–25. doi: 10.1016/j.jneumeth.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2010 doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Matthews SG. Antenatal glucocorticoids and the developing brain: mechanisms of action. Semin Neonatol. 2001;6:309–17. doi: 10.1053/siny.2001.0066. [DOI] [PubMed] [Google Scholar]
- 29.Nathanielsz PW. Animal models that elucidate basic principles of the developmental origins of adult diseases. ILAR J. 2006;47:73–82. doi: 10.1093/ilar.47.1.73. [DOI] [PubMed] [Google Scholar]
- 30.Weaver IC. Epigenetic effects of glucocorticoids. Semin Fetal Neonatal Med. 2009;14:143–50. doi: 10.1016/j.siny.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Roberts AC. Comparison of cognitive function in human and non-human primates. Brain Res Cogn Brain Res. 1996;3:319–27. doi: 10.1016/0926-6410(96)00017-1. [DOI] [PubMed] [Google Scholar]
- 32.Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- 33.Goldman-Rakic PS. Development of cortical circuitry and cognitive function. Child Dev. 1987;58:601–22. [PubMed] [Google Scholar]
- 34.Hotchkiss CE, Paule MG. Effect of pair-housing on operant behavior task performance by rhesus monkeys. Contemp Top Lab Anim Sci. 2003;42:38–41. [PubMed] [Google Scholar]
- 35.Rodefer JS, Nguyen TN. Naltrexone reverses age-induced cognitive deficits in rats. Neurobiol Aging. 2008;29:309–13. doi: 10.1016/j.neurobiolaging.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 36.McCoy JG, Tartar JL, Bebis AC, Ward CP, McKenna JT, Baxter MG, et al. Experimental sleep fragmentation impairs attentional set-shifting in rats. Sleep. 2007;30:52–60. doi: 10.1093/sleep/30.1.52. [DOI] [PubMed] [Google Scholar]
- 37.Garner JP, Thogerson CM, Wurbel H, Murray JD, Mench JA. Animal neuropsychology: validation of the Intra-Dimensional Extra-Dimensional set shifting task for mice. Behav Brain Res. 2006;173:53–61. doi: 10.1016/j.bbr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Brigman JL, Bussey TJ, Saksida LM, Rothblat LA. Discrimination of multidimensional visual stimuli by mice: intra- and extradimensional shifts. Behav Neurosci. 2005;119:839–42. doi: 10.1037/0735-7044.119.3.839. [DOI] [PubMed] [Google Scholar]
- 39.Spinelli S, Pennanen L, Dettling AC, Feldon J, Higgins GA, Pryce CR. Performance of the marmoset monkey on computerized tasks of attention and working memory. Brain Res Cogn Brain Res. 2004;19:123–37. doi: 10.1016/j.cogbrainres.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Weed MR, Taffe MA, Polis I, Roberts AC, Robbins TW, Koob GF, et al. Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Brain Res Cogn Brain Res. 1999;8:185–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- 41.Talge NM, Neal C, Glover V. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J Child Psychol Psychiatry. 2007;48:245–61. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez A, Bohlin G. Are maternal smoking and stress during pregnancy related to ADHD symptoms in children? J Child Psychol Psychiatry. 2005;46:246–54. doi: 10.1111/j.1469-7610.2004.00359.x. [DOI] [PubMed] [Google Scholar]
- 43.O’Connor TG, Heron J, Golding J, Glover V. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J Child Psychol Psychiatry. 2003;44:1025–36. doi: 10.1111/1469-7610.00187. [DOI] [PubMed] [Google Scholar]
- 44.O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. 2002;180:502–08. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- 45.Kapoor A, Petropoulos S, Matthews SG. Fetal programming of hypothalamic-pituitary-adrenal (HPA) axis function and behavior by synthetic glucocorticoids. Brain Res Rev. 2008;57:586–95. doi: 10.1016/j.brainresrev.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal ’programming’ of adult pathophysiology. Nat Clin Pract Endocrinol Metab. 2007;3:479–88. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- 47.Modi N, Lewis H, Al-Naqeeb N, jayi-Obe M, Dore CJ, Rutherford M. The effects of repeated antenatal glucocorticoid therapy on the developing brain. Pediatr Res. 2001;50:581–85. doi: 10.1203/00006450-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uno H, Lohmiller L, Thieme C, Kemnitz JW, Engle MJ, Roecker EB, et al. Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques. I. Hippocampus. Brain Res Dev Brain Res. 1990;53:157–67. doi: 10.1016/0165-3806(90)90002-g. [DOI] [PubMed] [Google Scholar]
- 50.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–35. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 51.Johnson JW, Mitzner W, Beck JC, London WT, Sly DL, Lee PA, et al. Long-term effects of betamethasone on fetal development. Am J Obstet Gynecol. 1981;141:1053–64. doi: 10.1016/s0002-9378(16)32697-7. [DOI] [PubMed] [Google Scholar]
- 52.Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, et al. Neurotoxicity of glucocorticoids in the primate brain. Horm Behav. 1994;28:336–48. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- 53.Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, et al. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry. 2003;54:1025–34. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- 54.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 55.McDonald RJ, King AL, Hong NS. Neurotoxic damage to the dorsomedial striatum exaggerates the behavioral influence of a context-specific inhibitory association mediated by the ventral hippocampus. Behav Neurosci. 2008;122:27–35. doi: 10.1037/0735-7044.122.1.27. [DOI] [PubMed] [Google Scholar]
- 56.Hornak J, O’Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, et al. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci. 2004;16:463–78. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- 57.Meunier M, Bachevalier J, Mishkin M. Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia. 1997;35:999–1015. doi: 10.1016/s0028-3932(97)00027-4. [DOI] [PubMed] [Google Scholar]
- 58.Nagahara AH, Bernot T, Tuszynski MH. Age-related cognitive deficits in rhesus monkeys mirror human deficits on an automated test battery. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greene AJ. Human hippocampal-dependent tasks: is awareness necessary or sufficient? Hippocampus. 2007;17:429–33. doi: 10.1002/hipo.20296. [DOI] [PubMed] [Google Scholar]
- 60.McDonald RJ, King AL, Wasiak TD, Zelinski EL, Hong NS. A complex associative structure formed in the mammalian brain during acquisition of a simple visual discrimination task: dorsolateral striatum, amygdala, and hippocampus. Hippocampus. 2007;17:759–74. doi: 10.1002/hipo.20333. [DOI] [PubMed] [Google Scholar]
- 61.Thompson RF. In search of memory traces. Annu Rev Psychol. 2005;56:1–23. doi: 10.1146/annurev.psych.56.091103.070239. [DOI] [PubMed] [Google Scholar]
- 62.Leuner B, Shors TJ. New spines, new memories. Mol Neurobiol. 2004;29:117–30. doi: 10.1385/MN:29:2:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wiltgen BJ, Brown RA, Talton LE, Silva AJ. New circuits for old memories: the role of the neocortex in consolidation. Neuron. 2004;44:101–08. doi: 10.1016/j.neuron.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 64.Lanfumey L, Mongeau R, Cohen-Salmon C, Hamon M. Corticosteroid-serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci Biobehav Rev. 2008;32:1174–84. doi: 10.1016/j.neubiorev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 65.Viveros MP, Marco EM, Llorente R, Lamota L. The role of the hippocampus in mediating emotional responses to nicotine and cannabinoids: a possible neural substrate for functional interactions. Behav Pharmacol. 2007;18:375–89. doi: 10.1097/FBP.0b013e3282d28fb4. [DOI] [PubMed] [Google Scholar]
- 66.Engin E, Treit D. The role of hippocampus in anxiety: intracerebral infusion studies. Behav Pharmacol. 2007;18:365–74. doi: 10.1097/FBP.0b013e3282de7929. [DOI] [PubMed] [Google Scholar]
- 67.Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kellendonk C, Gass P, Kretz O, Schutz G, Tronche F. Corticosteroid receptors in the brain: gene targeting studies. Brain Res Bull. 2002;57:73–83. doi: 10.1016/s0361-9230(01)00638-4. [DOI] [PubMed] [Google Scholar]
- 69.Lupien SJ, Lepage M. Stress, memory, and the hippocampus: can’t live with it, can’t live without it. Behav Brain Res. 2001;127:137–58. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- 70.Owen D, Matthews SG. Repeated maternal glucocorticoid treatment affects activity and hippocampal NMDA receptor expression in juvenile guinea pigs. J Physiol. 2007;578:249–57. doi: 10.1113/jphysiol.2006.122887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dunn E, Kapoor A, Leen J, Matthews SG. Prenatal synthetic glucocorticoid exposure alters hypothalamic-pituitary-adrenal regulation and pregnancy outcomes in mature female guinea pigs. J Physiol. 2010;588:887–99. doi: 10.1113/jphysiol.2009.182139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kreider ML, Levin ED, Seidler FJ, Slotkin TA. Gestational dexamethasone treatment elicits sex-dependent alterations in locomotor activity, reward-based memory and hippocampal cholinergic function in adolescent and adult rats. Neuropsychopharmacology. 2005;30:1617–23. doi: 10.1038/sj.npp.1300716. [DOI] [PubMed] [Google Scholar]
- 73.Nagano M, Ozawa H, Suzuki H. Prenatal dexamethasone exposure affects anxiety-like behaviour and neuroendocrine systems in an age-dependent manner. Neurosci Res. 2008;60:364–71. doi: 10.1016/j.neures.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 74.Patin V, Lordi B, Vincent A, Caston J. Effects of prenatal stress on anxiety and social interactions in adult rats. Brain Res Dev Brain Res. 2005;160:265–74. doi: 10.1016/j.devbrainres.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 75.van den Hove DL, Blanco CE, Aendekerk B, Desbonnet L, Bruschettini M, Steinbusch HP, et al. Prenatal restraint stress and long-term affective consequences. Dev Neurosci. 2005;27:313–20. doi: 10.1159/000086711. [DOI] [PubMed] [Google Scholar]
- 76.Emgard M, Paradisi M, Pirondi S, Fernandez M, Giardino L, Calza L. Prenatal glucocorticoid exposure affects learning and vulnerability of cholinergic neurons. Neurobiol Aging. 2007;28:112–21. doi: 10.1016/j.neurobiolaging.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 77.Kreider ML, Tate CA, Cousins MM, Oliver CA, Seidler FJ, Slotkin TA. Lasting effects of developmental dexamethasone treatment on neural cell number and size, synaptic activity, and cell signaling: critical periods of vulnerability, dose-effect relationships, regional targets, and sex selectivity. Neuropsychopharmacology. 2006;31:12–35. doi: 10.1038/sj.npp.1300783. [DOI] [PubMed] [Google Scholar]
- 78.Dunlop SA, Archer MA, Quinlivan JA, Beazley LD, Newnham JP. Repeated prenatal corticosteroids delay myelination in the ovine central nervous system. J Matern Fetal Med. 1997;6:309–13. doi: 10.1002/(SICI)1520-6661(199711/12)6:6<309::AID-MFM1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 79.Huang WL, Harper CG, Evans SF, Newnham JP, Dunlop SA. Repeated prenatal corticosteroid administration delays myelination of the corpus callosum in fetal sheep. Int J Dev Neurosci. 2001;19:415–25. doi: 10.1016/s0736-5748(01)00026-0. [DOI] [PubMed] [Google Scholar]
- 80.Goldman PS, Crawford HT, Stokes LP, Galkin TW, Rosvold HE. Sex-dependent behavioral effects of cerebral cortical lesions in the developing rhesus monkey. Science. 1974;186:540–42. doi: 10.1126/science.186.4163.540. [DOI] [PubMed] [Google Scholar]
- 81.Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van EH. Anatomical MRI of the developing human brain: what have we learned? J Am Acad Child Adolesc Psychiatry. 2001;40:1012–20. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 82.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108 (Suppl 3):511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stark MJ, Wright IM, Clifton VL. Sex-specific alterations in placental 11beta-hydroxysteroid dehydrogenase 2 activity and early postnatal clinical course following antenatal betamethasone. Am J Physiol Regul Integr Comp Physiol. 2009;297:R510–R514. doi: 10.1152/ajpregu.00175.2009. [DOI] [PubMed] [Google Scholar]
- 84.Hauser J, Knapman A, Zurcher NR, Pilloud S, Maier C, az-Heijtz R, et al. Effects of prenatal dexamethasone treatment on physical growth, pituitary-adrenal hormones, and performance of motor, motivational, and cognitive tasks in juvenile and adolescent common marmoset monkeys. Endocrinology. 2008;149:6343–55. doi: 10.1210/en.2008-0615. [DOI] [PubMed] [Google Scholar]
- 85.Weed MR, Bryant R, Perry S. Cognitive development in macaques: attentional set-shifting in juvenile and adult rhesus monkeys. Neuroscience. 2008;157:22–28. doi: 10.1016/j.neuroscience.2008.08.047. [DOI] [PubMed] [Google Scholar]
- 86.Ong AD, Bergeman CS, Boker SM. Resilience comes of age: defining features in later adulthood. J Pers. 2009;77:1777–804. doi: 10.1111/j.1467-6494.2009.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oitzl MS, Champagne DL, van d V, de Kloet ER. Brain development under stress: hypotheses of glucocorticoid actions revisited. Neurosci Biobehav Rev. 2010;34:853–66. doi: 10.1016/j.neubiorev.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 88.Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81:131–48. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.