Abstract
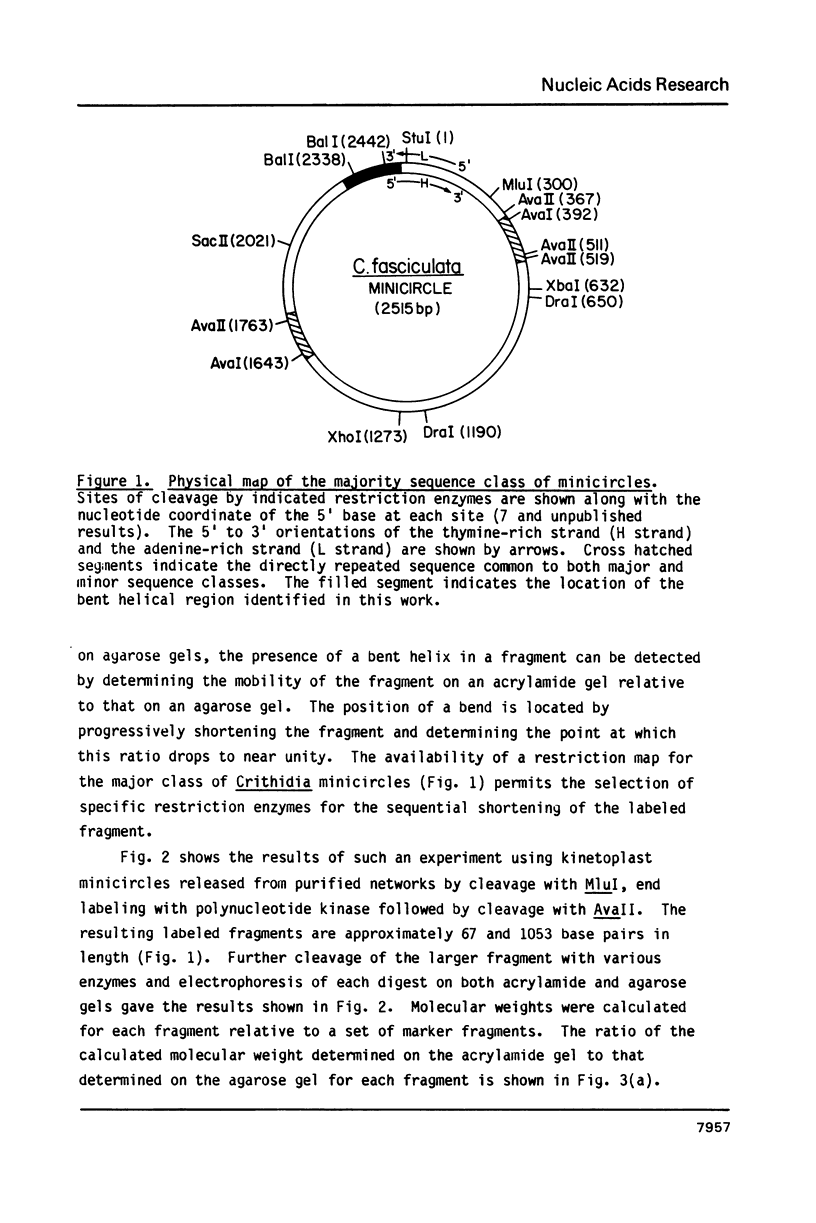
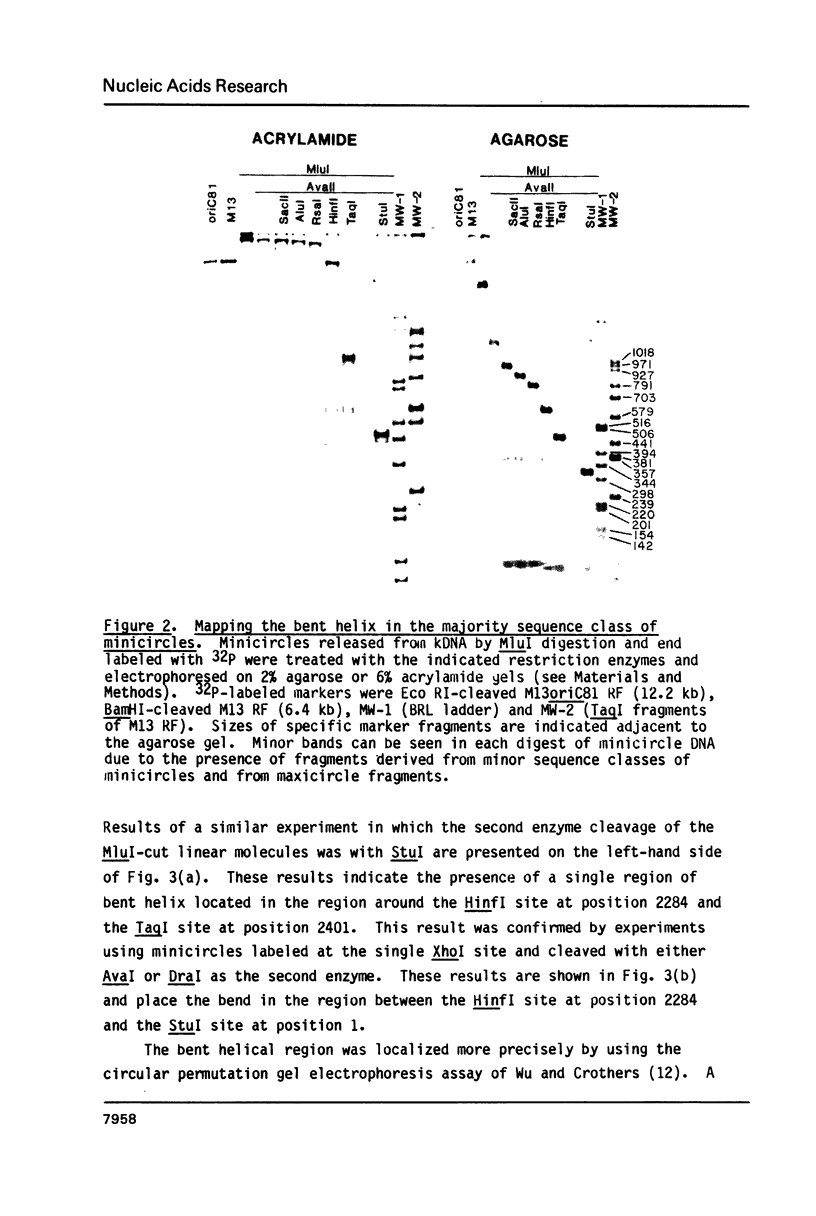
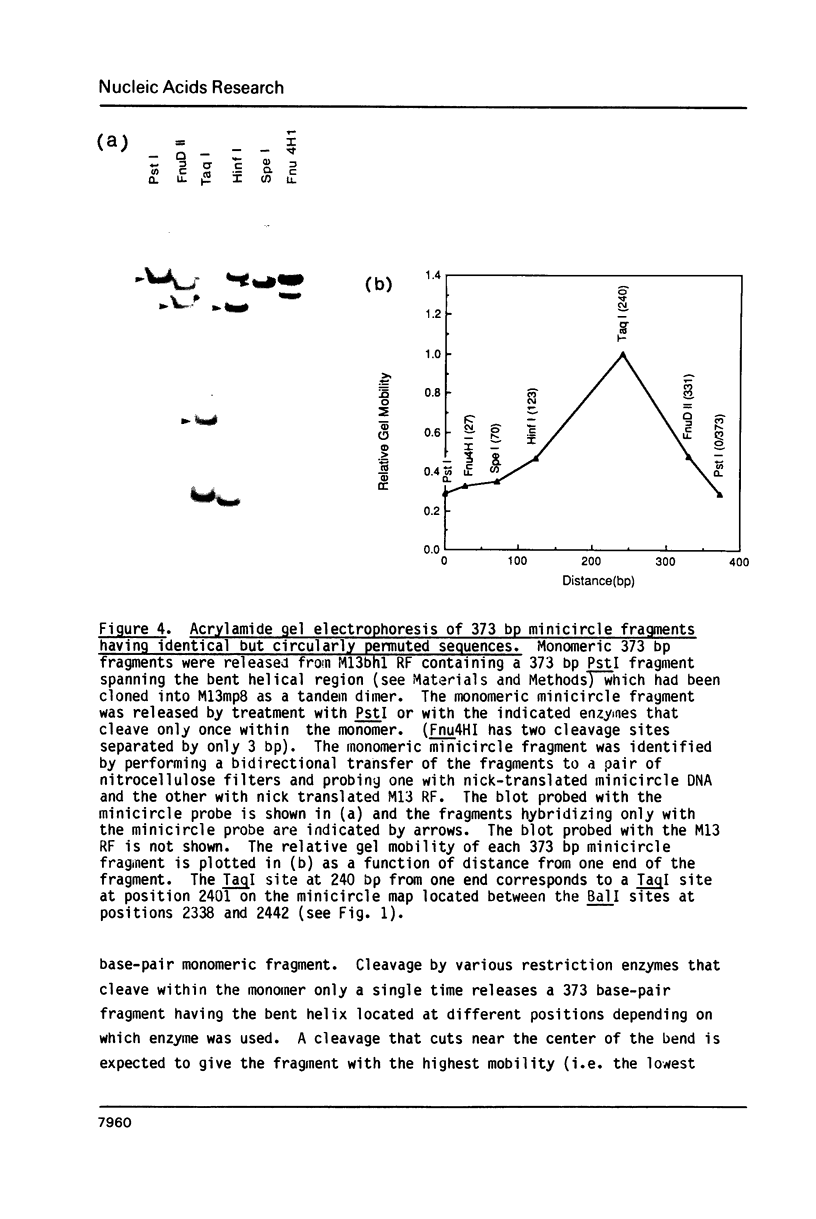
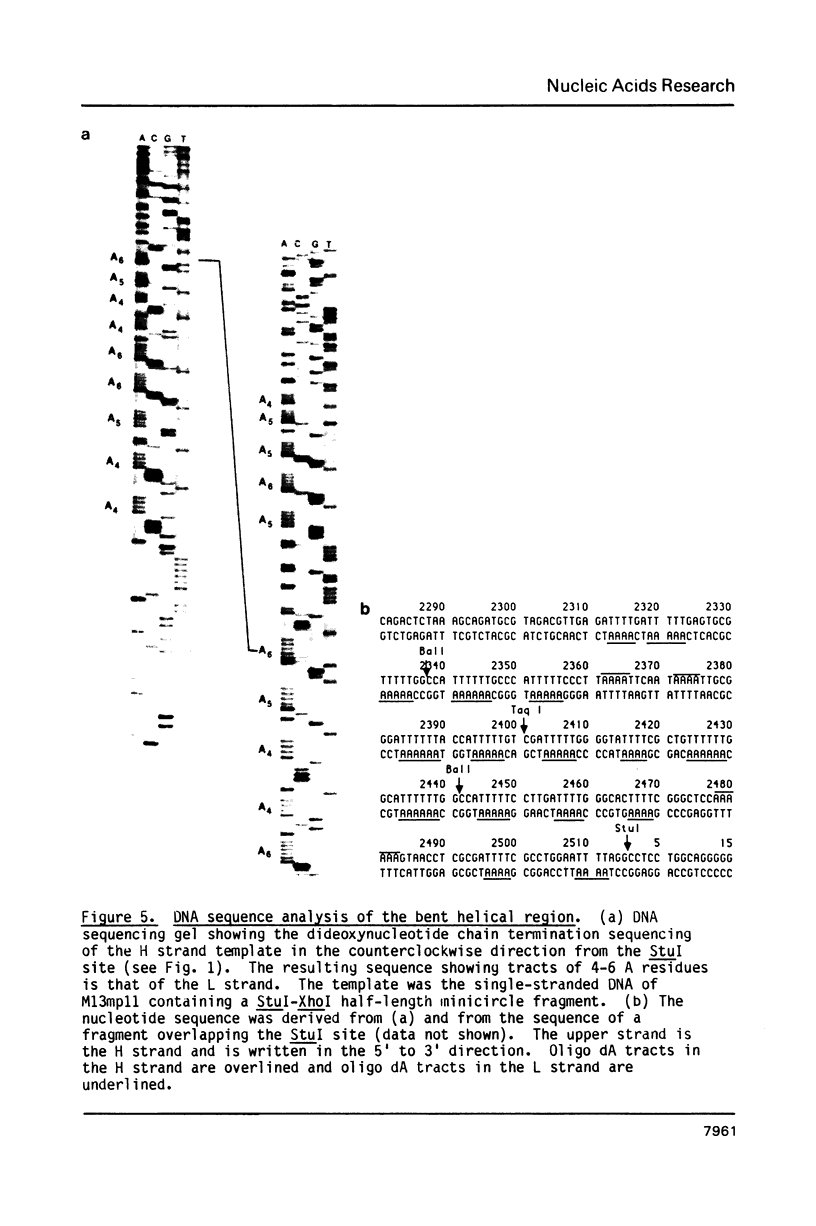
The major sequence class of Crithidia fasciculata minicircles is shown to have a single region of bent helical DNA widely separated from the two replication origins located 180 degrees apart on the minicircle map. The position of the bend in the DNA has been mapped both by gel electrophoretic methods and by direct electron microscopic observation of the DNA. This sequence directed bending is apparently the result of homopolymeric dA X dT tracts 4-6 base pairs long repeated in phase with the helix screw. The region of the bend contains nineteen such homopolymeric tracts in a region of about 200 base pairs with sixteen of the tracts oriented in the same direction.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrois M., Riou G., Galibert F. Complete nucleotide sequence of minicircle kinetoplast DNA from Trypanosoma equiperdum. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3323–3327. doi: 10.1073/pnas.78.6.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeyer L., Ray D. S. Replication of kinetoplast DNA in isolated kinetoplasts from Crithidia fasciculata. Identification of minicircle DNA replication intermediates. J Biol Chem. 1986 Feb 15;261(5):2362–2368. [PubMed] [Google Scholar]
- Birkenmeyer L., Sugisaki H., Ray D. S. The majority of minicircle DNA in Crithidia fasciculata strain CF-C1 is of a single class with nearly homogeneous DNA sequence. Nucleic Acids Res. 1985 Oct 11;13(19):7107–7118. doi: 10.1093/nar/13.19.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Hoeijmakers J. H. Kinetoplast DNA. Plasmid. 1979 Jan;2(1):20–40. doi: 10.1016/0147-619x(79)90003-9. [DOI] [PubMed] [Google Scholar]
- Chen K. K., Donelson J. E. Sequences of two kinetoplast DNA minicircles of Tryptanosoma brucei. Proc Natl Acad Sci U S A. 1980 May;77(5):2445–2449. doi: 10.1073/pnas.77.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. K., Donelson J. E. Sequences of two kinetoplast DNA minicircles of Tryptanosoma brucei. Proc Natl Acad Sci U S A. 1980 May;77(5):2445–2449. doi: 10.1073/pnas.77.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund P. T., Hajduk S. L., Marini J. C. The molecular biology of trypanosomes. Annu Rev Biochem. 1982;51:695–726. doi: 10.1146/annurev.bi.51.070182.003403. [DOI] [PubMed] [Google Scholar]
- Hagerman P. J. Sequence dependence of the curvature of DNA: a test of the phasing hypothesis. Biochemistry. 1985 Dec 3;24(25):7033–7037. doi: 10.1021/bi00346a001. [DOI] [PubMed] [Google Scholar]
- Hines J. C., Ray D. S. Construction and characterization of new coliphage M13 cloning vectors. Gene. 1980 Nov;11(3-4):207–218. doi: 10.1016/0378-1119(80)90061-x. [DOI] [PubMed] [Google Scholar]
- Kidane G. Z., Hughes D., Simpson L. Sequence heterogeneity and anomalous electrophoretic mobility of kinetoplast minicircle DNA from Leishmania tarentolae. Gene. 1984 Mar;27(3):265–277. doi: 10.1016/0378-1119(84)90071-4. [DOI] [PubMed] [Google Scholar]
- Koo H. S., Wu H. M., Crothers D. M. DNA bending at adenine . thymine tracts. Nature. 1986 Apr 10;320(6062):501–506. doi: 10.1038/320501a0. [DOI] [PubMed] [Google Scholar]
- Marini J. C., Effron P. N., Goodman T. C., Singleton C. K., Wells R. D., Wartell R. M., Englund P. T. Physical characterization of a kinetoplast DNA fragment with unusual properties. J Biol Chem. 1984 Jul 25;259(14):8974–8979. [PubMed] [Google Scholar]
- Marini J. C., Levene S. D., Crothers D. M., Englund P. T. A bent helix in kinetoplast DNA. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):279–283. doi: 10.1101/sqb.1983.047.01.033. [DOI] [PubMed] [Google Scholar]
- Marini J. C., Levene S. D., Crothers D. M., Englund P. T. Bent helical structure in kinetoplast DNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7664–7668. doi: 10.1073/pnas.79.24.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntambi J. M., Englund P. T. A gap at a unique location in newly replicated kinetoplast DNA minicircles from Trypanosoma equiperdum. J Biol Chem. 1985 May 10;260(9):5574–5579. [PubMed] [Google Scholar]
- Ntambi J. M., Marini J. C., Bangs J. D., Hajduk S. L., Jimenez H. E., Kitchin P. A., Klein V. A., Ryan K. A., Englund P. T. Presence of a bent helix in fragments of kinetoplast DNA minicircles from several trypanosomatid species. Mol Biochem Parasitol. 1984 Jul;12(3):273–286. doi: 10.1016/0166-6851(84)90084-7. [DOI] [PubMed] [Google Scholar]
- Ponzi M., Birago C., Battaglia P. A. Two identical symmetrical regions in the minicircle structure of Trypanosoma lewisi kinetoplast DNA. Mol Biochem Parasitol. 1984 Sep;13(1):111–119. doi: 10.1016/0166-6851(84)90105-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. Isolation of maxicircle component of kinetoplast DNA from hemoflagellate protozoa. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1585–1588. doi: 10.1073/pnas.76.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Wu H. M., Crothers D. M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]
- de la Cruz V. F., Neckelmann N., Simpson L. Sequences of six genes and several open reading frames in the kinetoplast maxicircle DNA of Leishmania tarentolae. J Biol Chem. 1984 Dec 25;259(24):15136–15147. [PubMed] [Google Scholar]