Abstract
In humans, aromatase (CYP19) gene expression is regulated via alternative promoters. Activation of each promoter gives rise to a CYP19 mRNA species with a unique 5′-untranslated region. Inhibition of aromatase has been reported to downregulate lung tumor growth. The genetic basis for CYP19 gene expression and aromatase activity in lung cancer remains poorly understood. We analyzed tissues from 15 patients with non-small cell lung cancer (NSCLC) to evaluate CYP19 promoter usage and promoter-specific aromatase mRNA levels in NSCLC tumor tissues and adjacent non-malignant tissues. CYP19 promoter usage was determined by multiplex RT-PCR and aromatase mRNA levels were measured with real-time RT-PCR. In non-malignant tissues, aromatase mRNA was primarily derived from activation of CYP19 promoter I.4. Although promoter I.4 usage was also dominant in tumor tissues, I.4 activation was significantly lower compared with adjacent non-malignant tissues. Activity of promoters I.3, I.1 and I.7 was significantly higher in tumor tissues compared with non-malignant tissues. In 4 of 15 cases of non-small cell lung cancer, switching from CYP19 promoter I.4 to the alternative promoters II, I.1 or I.7 was observed. In 9 cases, there were significantly higher levels of aromatase mRNA in lung tumor tissues compared with adjacent non-malignant tissues. These findings suggest aberrant activation of alternative CYP19 promoters that may lead to upregulation of local aromatase expression in some cases of NSCLC. Further studies are needed to examine the impact of alternative CYP19 promoter usage on local estrogen levels and lung tumor growth.
Keywords: non-small cell lung cancer, estrogen, aromatase, CYP19, promoter, promoter usage
Introduction
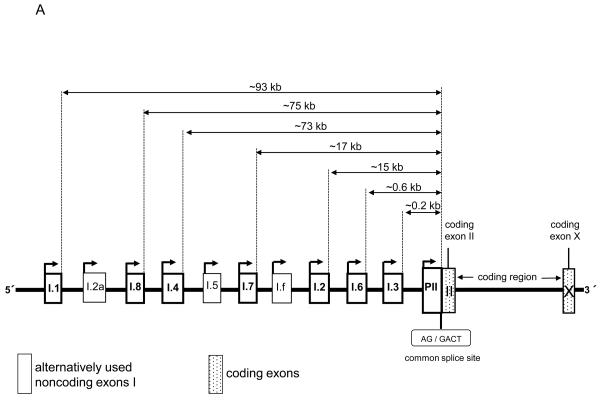
Aromatase catalyzes the rate-limiting step in estrogen biosynthesis, the aromatization of androgens. A single gene (CYP19) encodes aromatase, and activation determines the presence or absence of aromatase enzyme activity in cells or tissues [1]. The entire aromatase gene spans approximately 123 kb and is transcribed from the telomere to the centromere [1-3]. The 30-kb 3′-end of the gene contains 9 exons (II-X) that encode the aromatase protein. The ATG translation initiation site is located 38 bp downstream of a common splice acceptor site in coding exon II. The 93-kb 5′-flanking region of the gene contains a number of tissue-specific promoters with unique 5′-untranslated first exons that are spliced onto a common splice junction such that each promoter-specific mRNA species encodes an identical aromatase protein [1].
The farthest upstream promoter is I.1, and its activity causes splicing of exon I.1 onto the common splice acceptor site, 93 kb downstream. The most proximal promoters, the gonad-specific promoter II and two other proximal promoters, I.3 (expressed in adipose tissue and breast cancer) and I.6 (expressed in bone) are located within the 1-kb region upstream of the common splice junction. The promoters specific for the brain (I.f) and skin (I.4) are localized at approximately 33 kb and 73 kb, respectively, upstream of the common splice junction [1, 4]. In adipose tissue or cultured adipose fibroblasts from breast, abdomen, buttocks and thighs, promoters I.4 (major) and I.3/II (minor) are used [4].
Estrogens contribute to differentiation and maturation in normal lung [5] and also stimulate growth and progression of non-small cell lung cancer (NSCLC) [6, 7]. As in breast, aromatase mediates the synthesis of local estrogen in lung tissues, which may affect lung tumor progression in estrogen receptor-expressing malignancies [8-10]. Aromatase inhibitors have recently been reported to repress lung tumor growth in vitro and in xenografts in nude mice [11].
The molecular basis for the CYP19 gene regulation in the lung remains poorly understood. In the present study, we demonstrate that CYP19 promoter I.4 is dominant in both lung tumor tissue and non-malignant tissues, though at a lower level than in non-malignant tissues, and that there is aberrant activation of alternative CYP19 promoters and increased aromatase mRNA levels in some NSCLC tumor tissues.
Materials and Methods
Patients
We assessed tumor tissue and adjacent non-malignant tissue from 15 patients with NSCLC. The patient characteristics are listed in Table 1. The subjects without a smoking history were regarded as non-smokers, those who had a smoking history of one year or longer but did not smoke as ex-smokers, and those who smoked as current smokers. The Brinkman Index was calculated for the ex- and current smokers based on the number of cigarettes smoked each day and the number of smoking years. The purpose of the study was explained, and written informed consent was obtained from all study patients. The study protocol was approved by the ethics committees of University of Fukui Hospital and Northwestern University Feinberg School of Medicine. Tissue samples were collected at the time of surgery or autopsy at the University of Fukui Hospital. All samples were stabilized at 4°C overnight by RNA-later liquid (Applied Biosystems, Foster City, CA) immediately after surgery or autopsy, and then moved to −20°C until analyses. Tumor tissue and adjacent non-malignant tissue were in the same lobe and approximately 10 cm apart from each other.
Table 1.
Patient characteristics
| Sex | Age | Histology | Smoking history |
Brinkman index |
Exposure to asbestos |
Stage | Tissue collection |
|
|---|---|---|---|---|---|---|---|---|
| 1 | M | 63 | Well differentiated adenocarcinoma |
Current smoker |
400 | No | 1A | Surgical resection |
| 2 | M | 64 | Squamous cell carcinoma |
Ex- smoker |
2400 | No | 1A | Surgical resection |
| 3 | M | 72 | Squamous cell carcinoma |
Ex- smoker |
1500 | No | 3A | Surgical resection |
| 4 | F | 76 | Well differentiated adenocarcinoma |
Never | 0 | No | 3A | Surgical resection |
| 5 | M | 75 | Poorly differentiated adenocarcinoma |
Current smoker |
1100 | No | 1A | Surgical resection |
| 6 | M | 59 | Squamous cell carcinoma |
Current smoker |
600 | Yes | 1A | Surgical resection |
| 7 | F | 58 | Poorly differentiated adenocarcinoma |
Never | 0 | No | 3A | Surgical resection |
| 8 | F | 38 | Poorly differentiated adenocarcinoma |
Never | 0 | No | 3A | Surgical resection |
| 9 | M | 70 | Poorly differentiated adenocarcinoma |
Ex- smoker |
2000 | No | 1A | Surgical resection |
| 10 | F | 66 | Poorly differentiated adenocarcinoma |
Never | 0 | No | 1A | Surgical resection |
| 11 | M | 62 | Poorly differentiated adenocarcinoma |
Current smoker |
1680 | No | 4 | Autopsy |
| 12 | F | 77 | Well differentiated adenocarcinoma |
Never | 0 | No | 2B | Surgical resection |
| 13 | M | 82 | Squamous cell carcinoma |
Ex- smoker |
1200 | No | 1A | Surgical resection |
| 14 | M | 75 | Well differentiated adenocarcinoma |
Ex- smoker |
680 | No | 1A | Surgical resection |
| 15 | F | 80 | Squamous cell carcinoma |
Current smoker |
800 | No | 1B | Surgical resection |
M, male; F, female
Multiplex RT-PCR
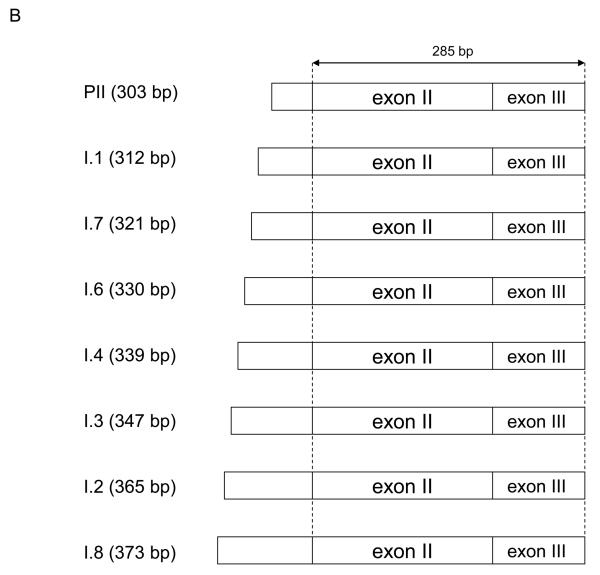
To study promoter usage in the CYP19 gene by multiplex RT-PCR, we designed 9 different amplicons from 303 to 373 bp (Fig. 1), as described previously [12]. This method has been validated in 2 different reports [12, 13]. The primers used in this study were: I.1 forward, 5′-ctg tgc tcg gga tct tcc-3′, I.2 forward, 5′-ggc ttc ctg act ttc aac ag −3′, I.3 forward, 5′-cct tgt ttt gac ttg taa cca-3′, I.4 forward, 5′-gac caa ctg gag cct gac ag-3′, I.6 forward, 5′-taa att gat tgt ctt gca cag g-3′, I.7 forward, 5′-gaa gta aga ccg gag aaa ggg-3′, I.8 forward, 5′- tca tat tgg gag gag ctt gg -3′, PII forward, 5′-tcc ctt tga ttt cca cag gac tc-3′, common reverse, 5′-ctc cat aca ccc ggt tgt ag-3′. The reverse primer was located at common exon III and labeled with 6-carboxyfluorescein [FAM] to quantitate promoter-specific mRNA species. The PCR reaction was set as follows: denaturation at 96°C for 5 min, then 45 cycles at 96°C for 30 sec, 58°C for 30 sec and 72°C for 30 sec. After PCR amplification, RT-PCR products were separated on an ABI3100 capillary sequencer and quantified by GeneScan software (Applied Biosystems).
Figure 1.
Assessment of alternative CYP19 promoter usage. (A) Schematic of alternative tissue-specific exons I and promoters of the CYP19 gene. (B) RT-PCR products for multiplex PCR. 8 different amplicons from 303 to 373 bp were designed to detect tissue-specific first exons.
Real-time RT-PCR
mRNA was reverse-transcribed from total RNA using Superscript III (Invitrogen, Carlsbad, CA) and random hexamer primers. Real-time PCR was then performed to amplify the coding region of the CYP19 gene and GAPDH as a control, using TaqMan Gene Expression Assays (Applied Biosystems). PCR amplifications were performed in an ABI PRISM 7900 Sequence Detection System (Applied Biosystems) according to the manufacturer's instructions. cDNA (2 μl) was added to the PCR mixture in a final volume of 20 μl. Thermal conditions were 50 °C for 2 min, 95°C for 10 min and then 40 cycles of 95°C for 15 sec and 60°C for 1 min. PCR amplifications were performed in triplicate.
Statistical analysis
All statistical analyses were conducted using the StatView statistical software. Statistical comparison of CYP19 gene promoter usage and expression levels in lung tumor and adjacent non-malignant tissues was done by Wilcoxon signed-ranks test or Student's t-test. P values of <0.05 were considered statistically significant.
Results
CYP19 promoter usage in lung tumor and adjacent non-malignant tissues
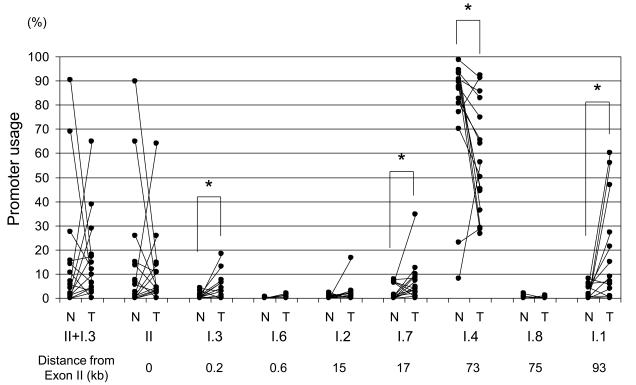
Non-malignant lung tissues from almost all cases employed promoter I.4, though two cases primarily used promoters I.3/II (Fig. 2). Although I.4 was the dominant promoter used in tumor tissues, I.4 usage was significantly lower in lung tumor tissues compared with adjacent non-malignant tissues. Tumor tissues also showed use of promoters I.3, I.1 and I.7 (Fig. 2). In tumor tissues from 4 of the 15 cases, promoter switching from I.4 to II, I.1 or I.7 was observed. These observations suggest aberrant activation of alternative CYP19 promoters in lung cancer.
Figure 2.
Tissue-specific CYP19 gene promoter usage in lung tumor and adjacent non-malignant tissues by multiplex RT-PCR. Significant differences in promoter usage were observed for promoters I.3, I.1, I.4 and I.7, indicating aberrant activation of promoters I.3, I.1, and I.7 in lung cancer. The horizontal line represents the median, the box encompasses the 25th to 75th percentile and error bars encompass the 10th to 90th percentile. *p<0.05, by Wilcoxon signed-ranks test.
Aromatase mRNA levels in lung tumor and adjacent non-malignant tissues
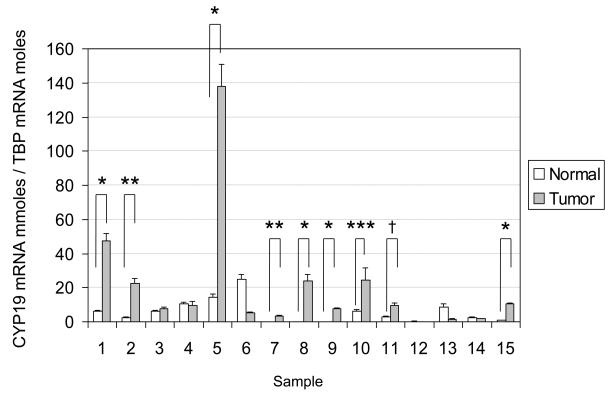
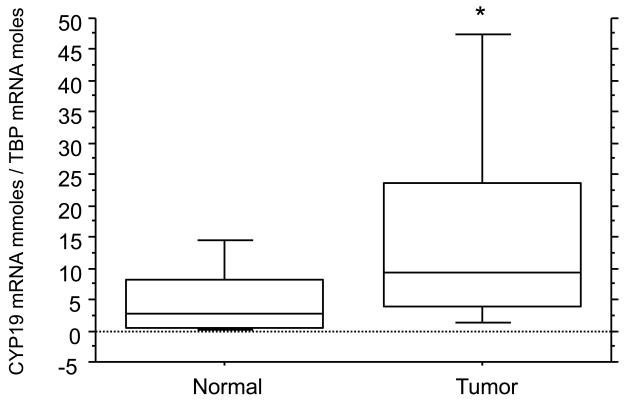
Aromatase mRNA levels in 9 of 15 lung tumor tissues were significantly higher than those in adjacent non-malignant tissues (Fig. 3). A statistically significant difference in aromatase mRNA levels between lung tumor and corresponding non-malignant tissues was observed (Fig. 4).
Figure 3.
Comparison of aromatase mRNA levels in lung tumor and adjacent non-malignant tissues. In 9 out of 15 paired samples, there was significantly higher aromatase expression in lung tumor tissues compared with adjacent normal tissues. *p<0.0001, **p<0.001, ***p<0.05, †p<0.01, by two tailed Student's t-test.
Figure 4.
Boxplot summary of CYP19 mRNA levels in lung tumor and adjacent non-malignant tissues. There were significantly higher aromatase mRNA levels in lung tumor tissues compared with adjacent non-malignant tissues. The horizontal line represents the median, the box encompasses the 25th to 75th percentile and error bars encompass the 10th to 90th percentile. *p<0.05, by Wilcoxon signed-ranks test.
Discussion
The poor long-term survival rate in patients with NSCLC has driven the search for novel therapeutic targets through a better understanding of the disease pathology. The findings of a number of previous studies suggest that treatments targeted to estrogen signaling pathways may have anti-tumor efficacy in lung cancer [7, 11, 14-18]. Aromatase inhibitors have been used in therapeutic regimens for estrogen-dependent disorders such as breast cancer, endometriosis and uterine fibroids, but their application in lung cancer has not been considered. Our study adds to the growing body of evidence that aromatase inhibition may be a new therapeutic option for patients with lung malignancies.
Our results show that non-malignant lung tissue from NSCLC patients maintained low levels of aromatase expression, primarily via promoter I.4, which lies 73 kb upstream of the common coding region. Lung tumor tissue also primarily employed promoter I.4, though at a lower level than adjacent non-malignant tissue. The most distal promoter, I.1, was used only minimally in non-malignant lung tissue, but was used at much higher levels in lung tumor tissue. Promoters I.3 and I.7 were also upregulated in lung tumor tissue. Thus, total aromatase expression in lung tumor tissue was comprised of the sum of mRNA species arising from four promoters (I.4, I.1, I.3 and I.7), while normal lung tissue aromatase expression arose almost exclusively from promoter I.4 activation. Future studies will determine the impact of CYP19 promoter switching in lung cancer on aromatase activity and local production of estrogen.
Epithelial-stromal interactions enhance estrogen formation, desmoplastic reaction, and angiogenesis in a breast tumor [19, 20]. Breast cancer appears to take advantage of four promoters (II, I.3, I.7, and I.4) for aromatase expression [21]. The use of proximal promoters II/I.3 are strikingly up-regulated in adipose tissue adjacent to breast cancer and in breast cancer tissue per se. Aromatase is overexpressed in vascular endothelial cells of breast tumor tissue via promoter I.7. We observed that activity of promoters I.3, I.7 and I.4 was up-regulated in lung tumor tissue. Complex tumor-stromal interactions may favor angiogenesis and increased local estrogen concentrations in lung tumors.
Alternative promoters of the CYP19 gene have been shown to be differentially regulated. Cyclic AMP (cAMP)-dependent transcription factors regulates proximal promoters PII/I.3 via two proximal cAMP response elements [22], whereas the glucocorticoid receptor (GR) induces promoter I.4 transcription via a GR binding element [23]. Interaction between the transcription factor GATA-2 and a specific GATA cis-regulatory element in promoter I.7 plays a critical role in its regulation [24]. These facts suggest that the activation of a particular CYP19 promoter may be specifically blocked, such that promoters found to be aberrantly activated in disease states can be targeted by therapeutics. In this case, a novel therapeutic strategy for estrogen-dependent NSCLC may involve targeting of the signaling pathways responsible for activation of promoters I.1, I.3 and I.7.
Though aromatase inhibitors are well tolerated, they can cause unwanted side effects related to systemic estrogen deprivation, such as bone loss. New therapeutic strategies that more effectively target aromatase expression and activity are needed. Our study provides new information on promoter usage in individual patients with NSCLC. In combination with information about estrogen receptor status, an understanding of CYP19 promoter usage in individual patients with lung cancer may lead to personalized approaches that more specifically suppress aromatase expression only in lung tumor tissues.
In the present study, we revealed that promoter I.4 was dominant in both lung tumor and non-malignant tissues, and that the alternative promoters I.3/II, I.1 and I.7 were aberrantly and heterogeneously activated in the lung tumor tissues from certain patients with NSCLC. Further study is essential to elucidating the trans and/or cis acting factors involved in ectopic activation of these promoters in lung cancer, as well as the impact of CYP19 promoter switching on local aromatase activity and estrogen levels, and lung tumor growth and progression.
Acknowledgement
This work was supported by NIH (C67167), AVON Foundation, Lynn Sage Foundation, and Northwestern Memorial Foundation and Friends of Prentice (S.E.B.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have nothing to disclose.
References
- 1.Sebastian S, Bulun SE. A highly complex organization of the regulatory region of the human CYP19 (aromatase) gene revealed by the Human Genome Project. J Clin Endocrinol Metab. 2001;86:4600–2. doi: 10.1210/jcem.86.10.7947. [DOI] [PubMed] [Google Scholar]
- 2.Bulun SE, Sebastian S, Takayama K, Suzuki T, Sasano H, Shozu M. The human CYP19 (aromatase P450) gene: update on physiologic roles and genomic organization of promoters. J Steroid Biochem Mol Biol. 2003;86:219–24. doi: 10.1016/s0960-0760(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 3.Kamat A, Mendelson CR. Identification of the regulatory regions of the human aromatase P450 (CYP19) gene involved in placenta-specific expression. J Steroid Biochem Mol Biol. 2001;79:173–80. doi: 10.1016/s0960-0760(01)00156-x. [DOI] [PubMed] [Google Scholar]
- 4.Mahendroo MS, Mendelson CR, Simpson ER. Tissue-specific and hormonally controlled alternative promoters regulate aromatase cytochrome P450 gene expression in human adipose tissue. J Biol Chem. 1993;268:19463–70. [PubMed] [Google Scholar]
- 5.Patrone C, Cassel TN, Pettersson K, Piao YS, Cheng G, Ciana P, et al. Regulation of postnatal lung development and homeostasis by estrogen receptor beta. Mol Cell Biol. 2003;23:8542–52. doi: 10.1128/MCB.23.23.8542-8552.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stabile LP, Davis AL, Gubish CT, Hopkins TM, Luketich JD, Christie N, et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002;62:2141–50. [PubMed] [Google Scholar]
- 7.Pietras RJ, Marquez DC, Chen HW, Tsai E, Weinberg O, Fishbein M. Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids. 2005;70:372–81. doi: 10.1016/j.steroids.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Pezzi V, Mathis JM, Rainey WE, Carr BR. Profiling transcript levels for steroidogenic enzymes in fetal tissues. J Steroid Biochem Mol Biol. 2003;87:181–9. doi: 10.1016/j.jsbmb.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Brodie A, Long B, Lu Q. Aromatase expression in the human breast. Breast Cancer Res Treat. 1998;49(Suppl 1):S85–91. doi: 10.1023/a:1006029612990. discussion S109-19. [DOI] [PubMed] [Google Scholar]
- 10.Oyama T, Kagawa N, Sugio K, Uramoto H, Hatano O, Harada N, et al. Expression of aromatase CYP19 and its relationship with parameters in NSCLC. Front Biosci. 2009;14:2285–92. doi: 10.2741/3379. [DOI] [PubMed] [Google Scholar]
- 11.Weinberg OK, Marquez-Garban DC, Fishbein MC, Goodglick L, Garban HJ, Dubinett SM, et al. Aromatase inhibitors in human lung cancer therapy. Cancer Res. 2005;65:11287–91. doi: 10.1158/0008-5472.CAN-05-2737. [DOI] [PubMed] [Google Scholar]
- 12.Demura M, Reierstad S, Innes JE, Bulun SE. Novel promoter I.8 and promoter usage in the CYP19 (Aromatase) gene. Reprod Sci. 2008;15:1044–53. doi: 10.1177/1933719108322441. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa H, Reierstad S, Demura M, Rademaker AW, Kasai T, Inoue M, et al. High aromatase expression in uterine leiomyoma tissues of African-American women. J Clin Endocrinol Metab. 2009;94:1752–6. doi: 10.1210/jc.2008-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taioli E, Wynder EL. Re: Endocrine factors and adenocarcinoma of the lung in women. J Natl Cancer Inst. 1994;86:869–70. doi: 10.1093/jnci/86.11.869. [DOI] [PubMed] [Google Scholar]
- 15.Mollerup S, Jorgensen K, Berge G, Haugen A. Expression of estrogen receptors alpha and beta in human lung tissue and cell lines. Lung Cancer. 2002;37:153–9. doi: 10.1016/s0169-5002(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 16.Kawai H, Ishii A, Washiya K, Konno T, Kon H, Yamaya C, et al. Estrogen receptor alpha and beta are prognostic factors in non-small cell lung cancer. Clin Cancer Res. 2005;11:5084–9. doi: 10.1158/1078-0432.CCR-05-0200. [DOI] [PubMed] [Google Scholar]
- 17.Stabile LP, Lyker JS, Gubish CT, Zhang W, Grandis JR, Siegfried JM. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res. 2005;65:1459–70. doi: 10.1158/0008-5472.CAN-04-1872. [DOI] [PubMed] [Google Scholar]
- 18.Marquez-Garban DC, Chen HW, Goodglick L, Fishbein MC, Pietras RJ. Targeting aromatase and estrogen signaling in human non-small cell lung cancer. Ann N Y Acad Sci. 2009;1155:194–205. doi: 10.1111/j.1749-6632.2009.04116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng L, Zhou J, Sasano H, Suzuki T, Zeitoun KM, Bulun SE. Tumor necrosis factor alpha and interleukin 11 secreted by malignant breast epithelial cells inhibit adipocyte differentiation by selectively down-regulating CCAAT/enhancer binding protein alpha and peroxisome proliferator-activated receptor gamma: mechanism of desmoplastic reaction. Cancer Res. 2001;61:2250–5. [PubMed] [Google Scholar]
- 20.Zhou J, Gurates B, Yang S, Sebastian S, Bulun SE. Malignant breast epithelial cells stimulate aromatase expression via promoter II in human adipose fibroblasts: an epithelial-stromal interaction in breast tumors mediated by CCAAT/enhancer binding protein beta. Cancer Res. 2001;61:2328–34. [PubMed] [Google Scholar]
- 21.Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57:359–83. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Agarwal VR, Mendelson CR, Simpson ER. Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology. 1996;137:5739–42. doi: 10.1210/endo.137.12.8940410. [DOI] [PubMed] [Google Scholar]
- 23.Shozu M, Sumitani H, Segawa T, Yang HJ, Murakami K, Kasai T, et al. Overexpression of aromatase P450 in leiomyoma tissue is driven primarily through promoter I.4 of the aromatase P450 gene (CYP19) J Clin Endocrinol Metab. 2002;87:2540–8. doi: 10.1210/jcem.87.6.8533. [DOI] [PubMed] [Google Scholar]
- 24.Sebastian S, Takayama K, Shozu M, Bulun SE. Cloning and characterization of a novel endothelial promoter of the human CYP19 (aromatase P450) gene that is up-regulated in breast cancer tissue. Mol Endocrinol. 2002;16:2243–54. doi: 10.1210/me.2002-0123. [DOI] [PubMed] [Google Scholar]