Abstract
Autism spectrum disorder (ASD) often involves sensory and motor problems, yet the proprioceptive sense of limb position has not been directly assessed. We used three tasks to assess proprioception in adolescents with ASD who had motor and sensory perceptual abnormalities, and compared them to age- and IQ-matched controls. Results showed no group differences in proprioceptive accuracy or precision during active or passive tasks. Both groups showed (a) biases in elbow angle accuracy that varied with joint position, (b) improved elbow angle precision for active versus passive tasks, and (c) improved precision for a fingertip versus elbow angle estimation task. Thus, a primary proprioceptive deficit may not contribute to sensorimotor deficits in ASD. Abnormalities may arise at later sensory processing stages.
Keywords: Proprioception, Motor control, Sensory processing
Introduction
The varied symptoms experienced by individuals with autism spectrum disorder (ASD)—poor social interaction and communication, repetitive behaviors and interests, excessive focus on details (American Psychiatric Association 2000)—may in part result from atypical use of sensory input to generate movements and to interact with others and the environment. For example, actively interacting with others and adopting ‘second-person perspective,’ abilities that heavily rely on proper sensory processing, are proposed to be essential for social cognition (Schilbach 2010). Despite the importance of proper sensory processing for more complex behaviors, sensory abnormalities have not been extensively explored in ASD.
Responses from questionnaires have revealed general differences in sensory experiences between children with and without ASD, including differences in auditory, visual, tactile, and movement processing (Baker et al. 2007; Baranek et al. 2006; Kern et al. 2007; Kientz and Dunn 1997; Tomchek and Dunn 2007; Watling et al. 2001; Wiggins et al. 2009). Quantitative studies of somatosensation suggest that there may be differences between control and ASD groups within this modality. Autistic individuals, for example, show improved tactile discrimination of high frequency vibrations (Blakemore et al. 2006) but appear to have no advantage in discriminating low frequency vibrations (Blakemore et al. 2006; Cascio et al. 2008) or in detecting touch (Cascio et al. 2008; O’Riordan and Passetti 2006). This suggests inconsistent patterns of performance in mechanoreception between individuals with ASD and controls. Similarly, ASD and control adults are comparable at detecting innocuous temperatures, but individuals with ASD show reduced thresholds for painful temperatures (Cascio et al. 2008), suggesting that there may also be differences between these populations in thermoception (the perception of temperature) and nociception (the perception of pain).
While few studies have tried to systematically examine somatosensation in autism, results of other studies also suggest impairments in the use of somatosensory information. Molloy et al. found that children with ASD show greater impairments in balance performance compared to controls when visual input is removed (i.e., subjects close their eyes) as opposed to when somatosensory input is distorted (i.e., subjects stand on foam pads) (Molloy et al. 2003). The authors interpreted these findings to indicate that compared to control children, children with ASD rely more on visual information than somatosensory and vestibular information to maintain balance. Looking across studies, one cannot form a unifying hypothesis explaining what is occurring within this modality, even whether differences are driven by changes at the receptor, afferent, or cortical level.
Looking within somatosensation at proprioception, even less is understood. Proprioception—the sense of position and movement of the parts of the body (Sherrington 1906)—plays a crucial role in daily life, contributing to motor skills and the general ability to successfully interact with the environment. Many individuals with ASD have relayed anecdotes suggesting impairments in proprioception:
“I could not point at objects for many reasons. The most important reason is that I had very little sensation of my body” (Tito Rajarshi Mukhopadhyay in (Biklen 2005)).
“…in childhood I had real problems in knowing exactly where my connectional limbs and truck were, where they would move to next, and, even more frighteningly, where they had last been positioned” (Lucy Blackman in (Biklen 2005)).
Although proprioceptive impairments appear evident from anecdotes, no studies have systematically examined proprioception in autism. A few studies, however, suggest deficits within this modality. For example, Weimer et al. used a battery of motor tests and found that children and adolescents with ASD showed comparable performance to controls on tests that involved visual input (e.g., finger tapping, inserting pegs into a board) but were worse on tests in which a lack of visual input required subjects to depend on proprioception (e.g., repetitive finger-thumb apposition, one-leg balance with eyes closed) (Weimer et al. 2001).
Given the importance of proprioception for even the most basic motor behaviors, directly examining proprioceptive processing in individuals with ASD is crucial for understanding the underlying causes of the social, communicative, and motor impairments. Using a paradigm previously used to explore proprioception in control adults (Fuentes and Bastian 2010), in this study we tested whether the accuracy and precision of proprioceptive estimates are comparable between adolescents with and without ASD. We also compared the accuracy and precision of proprioceptive estimates across tasks, testing whether proprioceptive ability changes in adolescents with or without autism depending on what is being localized (e.g., fingertip position versus joint angle) or whether static positions are actively versus passively reached. Overall, we aimed to establish whether there are proprioceptive differences in ASD on a peripheral level or in how the information is neurally represented and integrated with efferent information.
Methods
Participants
Twenty-four right-handed adolescents between 12 and 16 years of age participated in the study: 12 with ASD (1 female) and 12 typically-developing controls (1 female). Based on performance on either the Wechsler Intelligence Scale for Children IV (WISC-IV) (Wechsler 2003) or the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler 1999), all subjects had full-scale IQs (FSIQ) greater than 80 with the exception of one subject with ASD who had marked discrepancies between factor scores on the WISC-IV (Perceptual Reasoning Index 94, Working Memory Index 62, FSIQ 70). There was no significant difference between groups in age or full-scale IQ (see Table 1).
Table 1.
Demographics
| Characteristic | ASDs (n = 12) | Controls (n = 12) | p-value |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age (years) | 14.4 (1.4) | 13.8 (1.2) | 0.316 |
| Full scale IQ | 111.7 (16.4) | 116.7 (12.1) | 0.422 |
| PANESS total | 34.8 (9.5) | 15.6 (4.0) | <0.001* |
| -Overflow movements | 13.0 (4.8) | 5.6 (3.1) | <0.001* |
| -Gait/stations | 13.6 (5.3) | 5.8 (2.5) | <0.001* |
| -Timed movements | 21.3 (7.4) | 9.8 (2.7) | <0.001* |
| SP low registration: vision | 2.5 (1.0) | 1.7 (0.5) | 0.016* |
| SP sensory avoiding: taste/smell | 3.3 (0.9) | 2.2 (1.2) | 0.016* |
| SP sensory seeking: movement | 3.0 (1.2) | 4.1 (0.9) | 0.014* |
| SP high threshold: movement | 2.4 (0.6) | 3.1 (0.9) | 0.034* |
PANESS, physical and neurological examination for subtle (motor) signs SP, sensory profile
p values reflect significance of two-tailed Student t-tests
Adolescents with ASD were recruited from outpatient clinics at the Kennedy Krieger Institute in Baltimore, from the Interactive Autism Network operated by the Kennedy Krieger Institute, and from local Autism Society of America chapters, schools, social groups, and pediatricians’ offices. All ASD participants met Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV) criteria for ASD: nine met criteria according to the Autism Diagnostic Observation Schedule-Generic (ADOS-G) (Lord et al. 2000) and the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al. 1994) administered at the Kennedy Krieger Institute and three received a diagnosis elsewhere (diagnoses for these participants were confirmed by the Social Responsiveness Scale (Constantino 2005) and clinical observation by Stewart Mostofsky, M.D., a medical specialist with extensive experience diagnosing ASD). Adolescents with identifiable causes of autism (e.g., Fragile X syndrome) and known neurological disorders were excluded.
All participants were tested for motor and sensory abnormalities. We assessed motor function using the Revised Physical and Neurological Examination for Subtle (Motor) Signs (PANESS) (Denckla 1985). The PANESS has several categories such as stressed gaits, balance, and timed movements and has previously been used to show motor impairments in children with ASD (Fuentes et al. 2009; Jansiewicz et al. 2006; Mandelbaum et al. 2006; Mostofsky et al. 2007).
Sensory abnormalities were assessed with the Adolescent/Adult Sensory Profile, which is a self-questionnaire that addresses behavioral responses to everyday sensory experiences (Brown and Dunn 2002). The profile consists of 60 statements (e.g., “I become dizzy easily (for example, after bending over or getting up too fast)”). For each statement subjects reply with how frequently they have the experience or behavior, scoring the statement on a scale of 1 (Never) to 5 (Always). Statements are designed to assess subjects’ neurological thresholds (i.e., the amount of stimuli required for a response) and their amount of behavioral response/self-regulation (i.e., the way in which response strategies are constructed in relation to thresholds). In previous studies, multiple versions of the Sensory Profile have revealed sensory differences between ASD and control groups (Baker et al. 2007; Baranek et al. 2006; Kern et al. 2007; Kientz and Dunn 1997; Tomchek and Dunn 2007; Watling et al. 2001).
Written assent to participate was obtained from participants, and written consent was obtained from a parent or guardian. Protocols were approved by the Johns Hopkins Institutional Review Board.
Proprioceptive Tasks
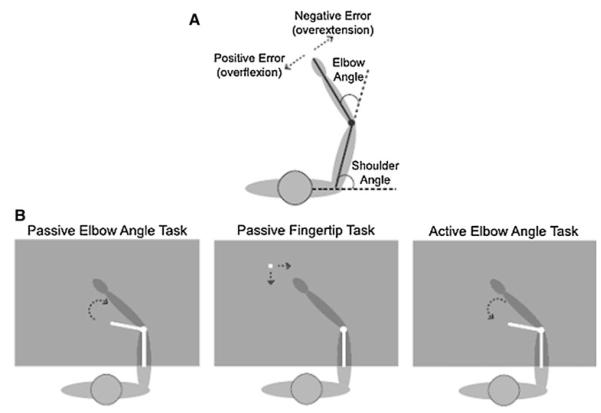
Experiments were performed using the KINARM (BKIN Technologies), an exoskeleton robotic arm that allows for individual application of torques to the elbow and shoulder joints. For all tasks, subjects sat with their right arm in the KINARM, and the robot was calibrated so that the right arm moved in a shoulder-level horizontal plane. The left arm remained out of the robot. Using a reflected rear projection system, subjects viewed images that appeared to be in the same plane as their right arm, and during test blocks vision of their right arm was always blocked by a screen. Throughout each experiment subjects saw a dot projected over their elbow joint and a line over their upper arm. At the start of each trial the elbow dot and upper arm line were red, indicating that the KINARM was moving the subject’s right arm into a new configuration (movements were made along a bell-shaped trajectory profile with a maximum fingertip velocity of 0.5 m/s). Once in position for that trial, the KINARM held the elbow and shoulder in place (only the shoulder in the active elbow angle matching task). The elbow dot and upper arm line then turned green, and an additional element appeared on the screen depending on the task (Fig. 1b).
Fig. 1.
Methods. a Angle definitions. b In all tasks subjects’ arms were blocked from view with a screen and only the white elements were visible on the display. In the passive elbow task subjects used a joystick to rotate a line until it matched the orientation of their forearm. In the passive fingertip task subjects moved a dot until it matched the position of their fingertip. In the active elbow task subjects rotated their forearm to match the orientation of a line
Passive Elbow Angle Matching Task
A line was projected out of the elbow dot at a random angle at least ± 15° from the forearm but no farther than ± 30°. Subjects used a joystick in their left hand to rotate the second line about the elbow dot until they perceived that it was aligned over their forearm, with the angle of the upper arm line and the rotated line matching their elbow angle. Trials were all timed at 12 s so that if subjects finished early they were instructed to wait. At the completion of the 12 s, the rotating line disappeared and the elbow dot and upper arm line again turned red as the arm was passively moved into the next trial’s configuration. There were a total of eight tested arm configurations: shoulder angles of 75 and 90° each paired with elbow angles of 30, 45, 60, and 75° (see Fig. 1a for angle definitions). Each configuration was presented once in a pseudorandom order within a block. Subjects completed five to six blocks of eight test trials.
Passive Fingertip Matching Task
A dot appeared at a random position between 8.5 and 17 cm from the subject’s right index finger. Subjects used a joystick in their left hand (in a similar fashion to the passive elbow angle matching task) to move the dot until they perceived that it was positioned over their index fingertip. Trials were timed at 18 s. At the completion of the trial the fingertip dot disappeared and the elbow dot and upper arm line again turned red as the arm was passively moved into the next trial’s configuration. The same eight arm configurations were tested and presented in the same order within each block as in the passive elbow angle matching task. Subjects completed five to six blocks of eight test trials.
Active Elbow Angle Matching Task
A line was projected out of the elbow dot as in the passive elbow angle matching task, but instead of rotating the line to match the location of their fixed forearm subjects rotated their forearm about the elbow to match the location of the fixed line. Trials were set to 10 s each. The same eight configurations were presented as were used in the passive tasks, and the presentation order, as well as experimental protocol, were the same as the previous tasks (i.e., five to six blocks of eight trials without vision of the right arm).
The order of the passive elbow angle matching task and the passive fingertip matching task was randomized across subjects, but all subjects performed the active elbow angle matching task last. This design ensured that subjects’ responses in the passive tasks were not influenced by remembered images from the active task.
Control Trials
Each experiment ended with two blocks of eight trials in which subjects could see their arm (i.e., two control trials per configuration). Since subjects could see their arms, errors on these trials were not due to abnormalities in proprioception.
EMG Recordings
During the passive elbow angle matching task and the passive fingertip matching task, EMG recordings were collected from three muscles in the right arm: brachialis, biceps brachii, and triceps brachii. Amplifier gains were set to 10,000, and signals were sampled at a rate of 1,000 Hz. EMG signals were monitored online to ensure that no muscle activity could contribute to proprioceptive estimates. To ensure that subjects were passive in both passive tasks, trials in which activity was noted were discarded from later analyses.
Analysis
For all three tasks, the average error for each configuration from the two trials with vision (i.e., control trials) was taken as a measure of error not due to proprioception. This error was subtracted from each test trial’s error, and analyses were conducted on these purely proprioceptive errors.
For the two elbow angle matching tasks, the angular difference between the displayed forearm line and the actual elbow angle provided a measure of accuracy for each trial, while the standard deviation of the perceived angle across trials provided a measure of precision. For each subject an average elbow angle error was calculated for each of the eight arm configurations.
For the fingertip matching task, the final x and y positions of the fingertip dot on each trial were taken as measurement of a subject’s perceived position of his fingertip. This perceived position was compared to the actual fingertip position for each trial. The corresponding elbow angle error and absolute end point error were then calculated based on the known length of the forearm.
Repeated measures ANOVA (2 groups × 3 tasks × 4 elbow angles × 2 shoulder angles) was performed on group data for average elbow angle errors (i.e., accuracy) and standard deviation of elbow angle estimates (i.e., precision). Group accuracy and precision for each arm configuration for each task were compared with two-tailed Student t-tests. Repeated measures ANOVA (2 groups × 4 elbow angles × 2 shoulder angles) was also performed for accuracy and precision of absolute endpoint errors on the fingertip matching task.
Results
Table 1 shows the results from all assessments used in this study. As expected, there were significant differences between groups on the motor and sensory scales.
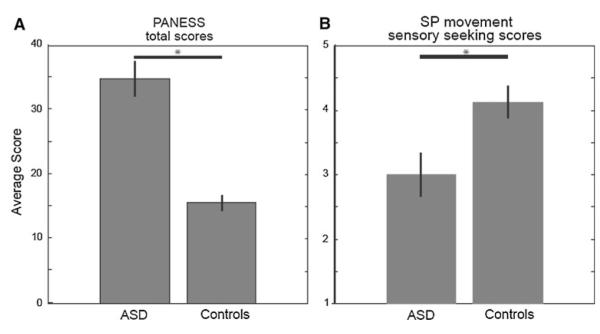
Participants with ASD demonstrated motor impairments relative to controls. Table 1 shows that the ASD group was more impaired than controls on all categories of the PANESS. Participants with ASD performed worse overall (p < 0.01, Fig. 2a) and also performed worse in the over-flow movements, gait/stances, and timed movements subcategories (all p < 0.01, Table 1).
Fig. 2.
Group performance on motor and sensory assessments. a The ASD group averaged higher total PANESS scores than did the control group, denoting worse performance (p < 0.001). b The ASD group averaged lower movement “sensory seeking” scores on the Sensory Profile, suggesting hypersensitivity and more passive behaviors (p = 0.014). Error bars represent standard error
The Adolescent/Adult Sensory Profile showed that participants with ASD were different from controls across a number of sensory categories (Table 1). The ASD group had higher “low registration” scores within visual processing (p = 0.02) and activity level (p = 0.05), suggesting higher neurological thresholds (i.e., hyposensitivity) and more passive behavior. Within taste/smell processing, the ASD group had higher “sensory avoiding” scores (p = 0.02), suggesting lower neurological thresholds (i.e., hypersensitivity) and more active behavior. In a category termed “movement processing,” the ASD group was hypersensitive to proprioceptive and vestibular stimuli and demonstrate more passive behaviors based on lower “sensory seeking” scores (p = 0.01, Fig. 2b) and lower high neurological threshold scores (p = 0.03).
Elbow Angle Accuracy
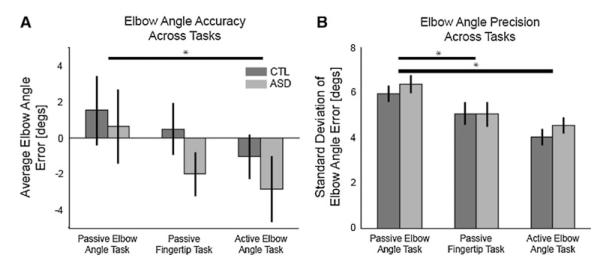
A repeated measures ANOVA (2 groups × 3 tasks × 4 elbow angles × 2 shoulder angles) was performed for elbow angle estimate error. Collapsing across tasks and arm configurations, there was no effect of group (p = 0.35). Because our collapsed data revealed no differences between groups, we checked for more subtle and specific differences. To confirm that there were no differences between groups in any individual task or configuration, we took the additional step of breaking out elbow angle estimate errors for each of the 24 possible cases (3 tasks, 8 elbow-shoulder configurations). These configuration and task specific errors were compared between control and ASD groups with two-tailed Student t-tests. No group differences were found for 23 of the 24 comparisons (accuracy only differed for the 75° shoulder, 30° elbow configuration in the active elbow angle task, p = 0.04). Thus, we think that the lack of a difference between control and ASD groups is robust.
Repeated measures ANOVA did, however, show a strong trend for task (p = 0.06). This task effect was driven by differences between the passive and active elbow angle tasks, such that estimates in the active task were more biased toward extension than in the passive task (planned comparison, p = 0.01) (Fig. 3a).
Fig. 3.
Group performance across proprioceptive tasks. a There was no group difference for accuracy (p = 0.35). Estimates were more extended in the active versus passive elbow angle task (p = 0.009, planned comparison). b There was also no group difference for precision (p = 0.48). Compared to the passive elbow task, both groups were more precise at identifying the angle of their elbow in the fingertip task (p = 0.002, planned comparison) and the active elbow task (p < 0.001). Error bars represent standard errors
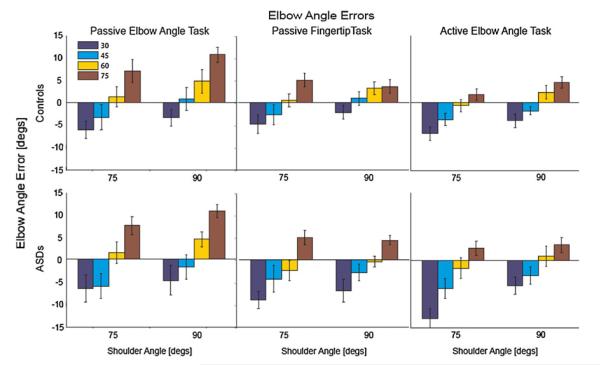
There were also significant effects for shoulder angle (p < 0.01) and elbow angle (p < 0.01). This suggests that arm configuration influences elbow angle estimate accuracy. Indeed, within each task a pattern in accuracy was observed across space: subjects were most accurate when the joints were at intermediate angles and the hand was in the middle of the workspace, and estimates became biased toward or away from the body (i.e., overly flexed or extended) as the hand moved closer or father from the middle of the workspace (i.e., as joint angles became more flexed or extended, respectively). Figure 4 shows average group accuracy for each configuration: consistent across groups and tasks, biases depend on true elbow and shoulder angle.
Fig. 4.
Accuracy across joint space. The bar graphs show group errors (top row, controls; bottom row, ASDs) for each arm configuration tested, for each task (error bars represent standard errors). Both groups demonstrated the same accuracy pattern across joint space: for all tasks, elbow angle estimates were overextended as the elbow became more extended and overflexed as the elbow became more flexed
For none of the three tasks did absolute accuracy of elbow angle estimates correlate with PANESS scores or with Sensory Profile neurological threshold scores for touch or movement (all p > 0.2).
Elbow Angle Precision
As with accuracy, a repeated measures ANOVA for standard deviation (i.e., precision) of elbow angle estimates (2 groups × 3 tasks × 4 elbow angles × 2 shoulder angles) found no effect of group (p = 0.48). Here again we took the additional step of breaking out errors for the 24 possible cases (3 tasks, 8 elbow-shoulder configurations) to confirm that there were no specific differences between groups. Two-tailed Student t-tests of group precision for each arm configuration for each task revealed no difference for 23 of the 24 comparisons (precision only differed for the 75° shoulder, 75° elbow configuration in the passive elbow angle task, p = 0.02). As with accuracy, these data strongly support the finding of comparable precision across groups.
Repeated measures ANOVA showed a strong effect of task on precision (p < 0.01). Figure 3b shows that subjects in both groups were the least precise at identifying their elbow angle when their arms were moved passively and they reported their angle with the visual display (passive elbow angle task). Compared to the passive elbow angle task, subjects were more precise at estimating their elbow angles in the passive fingertip task (planned comparison, p < 0.01) and the active elbow angle task (planned comparison, p < 0.01).
There was also an effect of elbow angle (p < 0.01) and shoulder angle (p < 0.01). Elbow angle estimates were more precise when the elbow was at 75° compared to all other angles (planned comparisons: 30 versus 75°, p < 0.01; 45 versus 75°, p < 0.01; 60 versus 75°, p < 0.01; no other significant comparisons) and when the shoulder was at 90° compared to 75°. Subjects were most precise at estimating elbow angle when their arm was positioned such that the elbow and shoulder were most flexed (i.e., the arm was closest to the body).
Precision on the active elbow angle task correlated with high neurological threshold movement scores on the Sensory Profile, such that subjects with lower scores (i.e., hypersensitivity) were less precise (controls: r = −0.646, p = 0.02; ASD: r = −0.524, p = 0.08; all: r = −0.610, p = 0.002). For no task did precision correlate with PANESS scores (all p > 0.3).
Fingertip Accuracy and Precision
In the fingertip matching task, actual finger endpoint errors could be assessed in addition to the calculated elbow angle errors. A repeated measures ANOVA (2 groups × 4 elbow angles × 2 shoulder angles) for endpoint accuracy (i.e., the absolute distance between subjects’ perceived fingertip endpoints and the actual positions of their index fingertip) revealed no effect of group (p = 0.21). There were, however, effects of elbow angle (p < 0.01), shoulder angle (p < 0.01), and elbow × shoulder angle interaction (p = 0.01) such that fingertip estimates were more accurate at flexed joint configurations (i.e., when the hand was closer to the body).
Similarly, a repeated measures ANOVA for endpoint precision (i.e., the average standard deviation of perceived endpoint positions) revealed no effect of group (p = 0.12) but effects of elbow angle (p = 0.02) and shoulder angle (p = 0.05). In addition to being more accurate at estimating fingertip position at flexed joint configurations, subjects were on average also more precise.
Within the control group only, absolute fingertip accuracy correlated with total PANESS scores (r = 0.64, p = 0.03). Fingertip accuracy did not correlate with Sensory Profile neurological threshold scores for touch or movement (all p > 0.2).
Discussion
Here we have shown that the accuracy and precision of proprioceptive estimates are comparable between adolescents with and without ASD. Additionally, we found that compared to the passive elbow angle task, participants with and without ASD demonstrate similar improvements in elbow angle precision for the fingertip task and the active elbow angle task.
Our ASD group demonstrated motor and sensory impairments consistent with other studies. The ASD group performed significantly worse than the control group on the PANESS, a motor assessment on which children with ASD have previously demonstrated impairments relative to typically-developing controls (Dowell et al. 2009; Fuentes et al. 2009; Jansiewicz et al. 2006; Mandelbaum et al. 2006; Mostofsky et al. 2007). Many of the tasks in the PANESS involve balance and rapid execution of finely controlled movements, skills that require good processing of proprioceptive and vestibular sensory information. Successful performance on the PANESS therefore requires good sensory processing, so the impairments demonstrated by participants with ASD may reflect impaired sensory processing in addition to impaired motor control.
Responses from the Adolescent/Adult Sensory Profile further support the finding that our ASD group demonstrated abnormalities in sensory processing. This is consistent with previous studies that have uncovered sensory differences between children with and without ASD using other versions of the Sensory Profile (Baker et al. 2007; Baranek et al. 2006; Kern et al. 2007; Kientz and Dunn 1997; Tomchek and Dunn 2007; Watling et al. 2001; Wiggins et al. 2009). Of particular interest were differences within the movement processing category of the questionnaire, which suggest that in regards proprioceptive and vestibular processing our ASD group had lower neurological thresholds (i.e., was hypersensitive) relative to controls. Based on performance on the PANESS and responses to the Adolescent/Adult Sensory Profile, then, our participants with ASD demonstrated movement-related sensory and motor execution impairments.
Despite motor and sensory impairments, participants with ASD showed comparable proprioceptive accuracy and precision to controls when identifying the angle of their elbow and the position of their fingertip. For elbow angle accuracy, both groups demonstrated biases across joint space that are consistent with patterns observed in adults (Fuentes and Bastian 2010): subjects were most accurate at intermediate angles and became biased toward sensing over-extension or over-flexion as the elbow became more extended or flexed, respectively. We have previously suggested that these biases may reflect the engagement of peripheral receptors, such as joint and cutaneous receptors, that protectively signal when joints are moving towards extremes (Fuentes and Bastian 2010). The lack of group differences in proprioceptive accuracy and precision on these three tasks and the comparable pattern in accuracy across joint space suggest that movement processing impairments in ASD do not arise from peripheral proprioceptive deficits.
In addition to showing comparable overall proprioceptive accuracy and precision, both participants with and without ASD showed improved precision of elbow angle estimates when these estimates were integrated into estimates of fingertip position versus directly reported after passive movements. Improved precision of elbow angle estimates for fingertip position sense has been demonstrated in adults and is thought to reflect the central nervous system’s direct calculation of limb endpoint position from peripheral sensory signals, whereas isolated joint angle estimates may need to be extracted from these representations (Fuentes and Bastian 2010). This hypothesis is supported by neurophysiological recordings in cats and primates showing that proprioceptive information along afferent pathways and within primary cortical areas is represented in terms of limb endpoint position rather than joint angles and segment lengths (Bosco et al. 2000; Prud’homme and Kalaska 1994; Tillery et al. 1996). The improved elbow angle precision demonstrated by the ASD group for the fingertip versus passive elbow angle task suggests that there are no differences in the low-level neural representation of limb position; like typically developing controls, adolescents with ASD appear to represent limb position in terms of endpoint position.
Also like typically developing controls, adolescents with ASD showed improved proprioceptive precision when arm position was reported after active versus passive movements. Improved precision after active versus passive movements has previously been demonstrated in controls (Adamovich et al. 1998; Craske and Crawshaw 1975; Fuentes and Bastian 2010; Gritsenko et al. 2007; Gurfinkel’ et al. 1985; Laufer et al. 2001; Paillard and Brouchon 1968; Zia et al. 2002). This difference is thought to result from the additional position information available in active movements from efference copies of motor commands and alpha-gamma motor neuron coactivation. Similar to controls, individuals with ASD appear to combine efferent information with afferent information to form more precise sensory estimates. This provides additional evidence that individuals with ASD can form internal models (Gidley Larson et al. 2008; Haswell et al. 2009).
The correlation between Sensory Profile high neurological threshold scores for movement processing and precision on the active elbow angle task reveals that across groups subjects with lower thresholds (i.e., hypersensitivity) are less precise. The ASD group had significantly lower movement processing thresholds, suggesting that imprecise position estimates after movements may be related to proprioceptive hypersensitivity. However, despite demonstrating motor and sensory impairments, the ASD group showed no impairments on all tested proprioceptive tasks. It therefore appears that ASD impairments in motor control and position sense cannot be accounted for by impaired peripheral proprioceptive signals, differing low-level neural representations of limb position, or an inability to utilize efference information to improve sensory estimates. It is still possible, however, that there exist autism-associated differences in the cortical organization and integration of proprioceptive information. Studies in ASD have uncovered smaller, less compact cortical minicolumns (Casanova et al. 2002), increased short-range fiber connections (Herbert et al. 2004), reduced long-range fiber connections (Courchesne and Pierce 2005), and abnormal spacing of representations in somatosensory cortical maps (Coskun et al. 2009). These differences in cortical structure may result in behavioral differences in how sensory modalities are utilized to control movements and learn about the environment (Haswell et al. 2009). Important next steps will be to see if proprioceptive abilities are still intact in ASD when individuals have to integrate position information across multiple joints and if efferent information contributes to sensory estimates after more complicated movements. In terms of the results of this study, the knowledge that lower-level proprioceptive information appears to be intact in ASD will allow researchers and therapists to better focus sensorimotor training, utilizing intact proprioception to improve the teaching of such skills as tool use and imitation.
Acknowledgments
The authors wish to thank all participants in this research study, as well as Jessica O’Brien, Lindsay Koenig, and particularly Lauren Dowell for assistance with subject recruitment and data collection. We would also like to thank the Interactive Autism Network. This research was supported by an Autism Speaks Pre-Doctoral Fellowship (AJB and CTF), NIH Grant R01 NS048527 (SHM), and the Johns Hopkins University School of Medicine Institute for Clinical and Translational Research, an NIH/NCRR CTSA Program, UL1 RR025005 (SHM).
Contributor Information
Christina T. Fuentes, Kennedy Krieger Institute, 707 N Broadway—G05, Baltimore, MD 21205, USA Department of Neuroscience, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Stewart H. Mostofsky, Department of Neurology, Johns Hopkins School of Medicine, Baltimore, MD, USA
Amy J. Bastian, Department of Neurology, Johns Hopkins School of Medicine, Baltimore, MD, USA Kennedy Krieger Institute, 707 N Broadway—G05, Baltimore, MD 21205, USA.
References
- Adamovich SV, Berkinblit MB, Fookson O, Poizner H. Pointing in 3D space to remembered targets. I. Kinesthetic versus visual target presentation. Journal of Neurophysiology. 1998;79:2833–2846. doi: 10.1152/jn.1998.79.6.2833. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders IV—Text Revision. American Psychiatric Publishing, Inc.; Washington, DC: 2000. Pervasive developmental disorders; pp. 69–70. [Google Scholar]
- Baker AE, Lane A, Angley MT, Young RL. The relationship between sensory processing patterns and behavioural responsiveness in autistic disorder: A pilot study. Journal of Autism and Developmental Disorders. 2007;38(5):867–875. doi: 10.1007/s10803-007-0459-0. [DOI] [PubMed] [Google Scholar]
- Baranek GT, David FJ, Poe MD, Stone WL, Watson LR. Sensory experiences questionnaire: discriminating sensory features in young children with autism, developmental delays, and typical development. Journal of Child Psychology and Psychiatry. 2006;47:591–601. doi: 10.1111/j.1469-7610.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Biklen D. Autism and the Myth of the person alone. NYU Press; 2005. [Google Scholar]
- Blakemore SJ, Tavassoli T, Calo S, et al. Tactile sensitivity in Asperger syndrome. Brain and Cognition. 2006;61:5–13. doi: 10.1016/j.bandc.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Bosco G, Poppele RE, Eian J. Reference frames for spinal proprioception: limb endpoint based or joint-level based? Journal of Neurophysiology. 2000;83:2931–2945. doi: 10.1152/jn.2000.83.5.2931. [DOI] [PubMed] [Google Scholar]
- Brown CE, Dunn W. Adolescent/adult sensory profile. Pearson; San Antonio, TX: 2002. [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Cascio C, McGlone F, Folger S, et al. Tactile perception in adults with autism: A multidimensional psychophysical study. Journal of Autism and Developmental Disorders. 2008;38(1):127–137. doi: 10.1007/s10803-007-0370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN. Social responsiveness scale (SRS) Western Psychological Services; Los Angeles, CA: 2005. [Google Scholar]
- Coskun MA, Varghese L, Reddoch S, et al. How somatic cortical maps differ in autistic and typical brains. Neuroreport. 2009;20:175–179. doi: 10.1097/WNR.0b013e32831f47d1. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: Local over-connectivity but long-distance disconnection. Current Opinion in Neurobiology. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Craske B, Crawshaw M. Shifts in kinesthesis through time and after active and passive movement. Perceptual and Motor Skills. 1975;40:755–761. doi: 10.2466/pms.1975.40.3.755. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Revised neurological examination for subtle signs. Psychopharmacology Bulletin. 1985;21:773–800. [PubMed] [Google Scholar]
- Dowell LR, Mahone EM, Mostofsky SH. Associations of postural knowledge and basic motor skill with dyspraxia in autism: Implication for abnormalities in distributed connectivity and motor learning. Neuropsychology. 2009;23(5):563–570. doi: 10.1037/a0015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes CT, Bastian AJ. Where is your arm? Variations in proprioception across space and tasks. Journal of Neurophysiology. 2010;103:164–171. doi: 10.1152/jn.00494.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes CT, Mostofsky SH, Bastian AJ. Children with autism show specific handwriting impairments. Neurology. 2009;73:1532–1537. doi: 10.1212/WNL.0b013e3181c0d48c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidley Larson JC, Bastian AJ, Donchin O, Shadmehr R, Mostofsky SH. Acquisition of internal models of motor tasks in children with autism. Brain. 2008;131:2894–2903. doi: 10.1093/brain/awn226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritsenko V, Krouchev NI, Kalaska JF. Afferent input, efference copy, signal noise, and biases in perception of joint angle during active versus passive elbow movements. Journal of Neurophysiology. 2007;98:1140–1154. doi: 10.1152/jn.00162.2007. [DOI] [PubMed] [Google Scholar]
- Gurfinkel’ VS, Debreva EE, Levik Y. Relationship between perception of the position of parts of the body and movement. Human Physiology. 1985;11:4–7. [PubMed] [Google Scholar]
- Haswell CC, Izawa J, Dowell LR, Mostofsky SH, Shadmehr R. Representation of internal models of action in the autistic brain. Nature Neuroscience. 2009;12:970–972. doi: 10.1038/nn.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, et al. Localization of white matter volume increase in autism and developmental language disorder. Annals of Neurology. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Jansiewicz EM, Goldberg MC, Newschaffer CJ, Denckla MB, Landa R, Mostofsky SH. Motor signs distinguish children with high functioning autism and Asperger’s syndrome from controls. Journal of Autism and Developmental Disorders. 2006;36:613–621. doi: 10.1007/s10803-006-0109-y. [DOI] [PubMed] [Google Scholar]
- Kern JK, Trivedi MH, Grannemann BD, et al. Sensory correlations in autism. Autism. 2007;11:123–134. doi: 10.1177/1362361307075702. [DOI] [PubMed] [Google Scholar]
- Kientz MA, Dunn W. A comparison of the performance of children with and without autism on the sensory profile. American Journal of Occupational Therapy. 1997;51:530–537. doi: 10.5014/ajot.51.7.530. [DOI] [PubMed] [Google Scholar]
- Laufer Y, Hocherman S, Dickstein R. Accuracy of reproducing hand position when using active compared with passive movement. Physiotherapy Research International. 2001;6:65–75. doi: 10.1002/pri.215. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le CA. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mandelbaum DE, Stevens M, Rosenberg E, et al. Sensorimotor performance in school-age children with autism, developmental language disorder, or low IQ. Developmental Medicine and Child Neurology. 2006;48:33–39. doi: 10.1017/S0012162206000089. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Dietrich KN, Bhattacharya A. Postural stability in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2003;33:643–652. doi: 10.1023/b:jadd.0000006001.00667.4c. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Burgess MP, Gidley Larson JC. Increased motor cortex white matter volume predicts motor impairment in autism. Brain. 2007;130:2117–2122. doi: 10.1093/brain/awm129. [DOI] [PubMed] [Google Scholar]
- O’Riordan M, Passetti F. Discrimination in autism within different sensory modalities. Journal of Autism and Developmental Disorders. 2006;36:665–675. doi: 10.1007/s10803-006-0106-1. [DOI] [PubMed] [Google Scholar]
- Paillard J, Brouchon M. Active and passive movements in the calibration of position sense. In: Freedman SJ, editor. The neuropsychology of spatially oriented behavior. Dorsey Press; 1968. pp. 37–55. [Google Scholar]
- Prud’homme MJ, Kalaska JF. Proprioceptive activity in primate primary somatosensory cortex during active armr eaching movements. Journal of Neurophysiology. 1994;72:2280–2301. doi: 10.1152/jn.1994.72.5.2280. [DOI] [PubMed] [Google Scholar]
- Schilbach L. A second-person approach to other minds. Nature Reviews Neuroscience. 2010;11:449. doi: 10.1038/nrn2805-c1. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. The integrative action of the nervous system. Yale University Press; New Haven, CT: 1906. [Google Scholar]
- Tillery SI, Soechting JF, Ebner TJ. Somatosensory cortical activity in relation to arm posture: nonuniform spatial tuning. Journal of Neurophysiology. 1996;76:2423–2438. doi: 10.1152/jn.1996.76.4.2423. [DOI] [PubMed] [Google Scholar]
- Tomchek SD, Dunn W. Sensory processing in children with and without autism: a comparative study using the short sensory profile. American Journal of Occupational Therapy. 2007;61:190–200. doi: 10.5014/ajot.61.2.190. [DOI] [PubMed] [Google Scholar]
- Watling RL, Deitz J, White O. Comparison of sensory profile scores of young children with and without autism spectrum disorders. American Journal of Occupational Therapy. 2001;55:416–423. doi: 10.5014/ajot.55.4.416. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children. 4th ed The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- Weimer AK, Schatz AM, Lincoln A, Ballantyne AO, Trauner DA. “Motor” impairment in Asperger syndrome: evidence for a deficit in proprioception. Journal of Developmental and Behavioral Pediatrics. 2001;22:92–101. doi: 10.1097/00004703-200104000-00002. [DOI] [PubMed] [Google Scholar]
- Wiggins LD, Robins DL, Bakeman R, Adamson LB. Brief report: Sensory abnormalities as distinguishing symptoms of autism spectrum disorders in young children. Journal of Autism and Developmental Disorders. 2009;39:1087–1091. doi: 10.1007/s10803-009-0711-x. [DOI] [PubMed] [Google Scholar]
- Zia S, Cody FW, O’Boyle DJ. Identification of unilateral elbow-joint position is impaired by Parkinson’s disease. Clinical Anatomy. 2002;15:23–31. doi: 10.1002/ca.1087. [DOI] [PubMed] [Google Scholar]