Abstract
Intercellular adhesion molecule-1 (ICAM-1) in cerebral vascular endothelium induced by ischemic insult triggers leukocyte infiltration and inflammatory reaction. We investigated the mechanism of hypothermic suppression of ICAM-1 in a model of focal cerebral ischemia. Rats underwent 2 hours of middle cerebral artery occlusion and were kept at 37°C or 33°C during occlusion and rewarmed to normal temperature immediately after reperfusion. Under hypothermic condition, robust activation of extracellular signal-regulated kinase-1/2 (ERK1/2) was observed in vascular endothelium of ischemic brain. Hypothermic suppression of ICAM-1 was reversed by ERK1/2 inhibition. Phosphorylation of signal transducer and activator of transcription 3 (STAT3) in ischemic vessel was attenuated by hypothermia. STAT3 inhibitor suppressed ICAM-1 production induced by stroke. ERK1/2 inhibition enhanced phosphorylation and DNA binding activity of STAT3 in hypothermic condition. In this study, we demonstrated that hypothermic suppression of ICAM-1 induction is mediated by enhanced ERK1/2 activation and subsequent attenuation of STAT3 action.
1. Introduction
Intercellular adhesion molecule-1 (ICAM-1) is a member of the immunoglobulin superfamily and the principal ligand for leukocyte function-associated antigen-1 (LFA-1), a member of the integrin superfamily. ICAM-1/LFA-1 adhesion system assists leukocyte movement into the tissue. LFA-1-positive leukocytes are induced to adhere to ICAM-1-positive endothelial surface [1, 2], and then to pass through the basement membrane into the tissue [3, 4]. Many animal and human studies indicate that ICAM-1 is implicated in the pathogenesis of ischemic cardiovascular and cerebrovascular disorders [5–8]. Especially during reperfusion period of stroke, infiltrated leukocytes contribute to the secondary injury by producing toxic substances that damage the brain cells and disrupt the blood-brain barrier [9, 10]. Since ICAM-1 is an important factor of leukocyte infiltration and reperfusion injury in stroke, intervention of ICAM-1 induction has been a promising therapeutic strategy against stroke.
The remarkable benefit of mild hypothermia in brain ischemia has long been recognized and remains one of the most powerful neuroprotective strategies in cerebral ischemia both experimentally and clinically [11]. Many studies indicate that inflammatory response contributes significantly to the secondary injury after ischemia [12, 13], and protection by mild hypothermia is associated with anti-inflammatory processes [14–16]. Even though there is considerable interest in the potential therapeutic role of induced hypothermia, the molecular basis of hypothermic protection remains mostly unknown.
Previously, we and others have demonstrated that hypothermia attenuated ICAM-1 induction [17–21] and neutrophil infiltration after stroke attack [15, 16, 22]. In most studies, hypothermia was applied during ischemic or few hours after ischemia. Therefore, there is a time gap between hypothermia application and ICAM-1 induction when the temperature was already returned to normal body temperature. Since hypothermia is known to interfere with some ischemia related signaling pathways and gene expression [23, 24], we hypothesize that hypothermia applied during ischemia interferes the upstream pathway of ICAM-1 expression and investigated the molecular mechanism in the vascular endothelium of the ischemic brain.
2. Materials and Methods
2.1. Animal Model
Experiments were carried out according to the guidelines for the animal care and use of laboratory animal protocols approved by our university administrative panel on laboratory animal care. Rats were housed with food and water available ad libitum under diurnal lighting conditions and temperature-controlled environment until the day of experiment.
2.2. Focal Cerebral Ischemia by Transient Middle Cerebral Artery Occlusion (MCAO)
Male Sprague-Dawley rats weighing 290 to 320 g were anesthetized with enflurane and maintained during surgical procedures. Physiological parameters were monitored and maintained in the normal range as shown previously [14]. Ischemia was induced using an occluding intraluminal suture. An uncoated 30 mm long segment of 3–0 nylon monofilament suture with the tip rounded by flame was inserted into the stump of the common carotid artery and advanced into the internal carotid artery approximately 19-20 mm from the bifurcation in order to occlude the ostium of middle cerebral artery. After 2 hours of ischemic period, the suture was removed and the animal was allowed to recover. Sham-operated animals were treated in the same manner as the ischemic animals, but no ischemia was applied. During surgery, rectal temperature was maintained between 37-38°C. Mild hypothermia (33°C of rectal temperature) was achieved as previously described using paradigms associated with neuroprotection [14]. Cooling began upon ischemia onset, maintained for 2 hours and terminated immediately after reperfusion. To inhibit ERK1/2 activation, U0126 (0.5 mg/kg, Cell Signaling Technology) was administered via tail vein 30 minutes before MCAO. Cucurbitacin I or JSI-124 (0.1 mg/kg; selective JAK/STAT inhibitor, Calbiochem) was injected intraperitoneally 1 hour before MCAO.
2.3. Brain Endothelial Cell Culture and Oxygen Glucose Deprivation (OGD) Study
bEnd.3 cells, mouse brain endothelial cell line was purchased from American Type Culture Collection (Rockville, MD). The cells were cultured with DMEM containing 10% FBS at 37°C in a humidified 5% CO2 incubator. JSI-124 (10 μM) or U0126 (10 μM) was treated 30 min before OGD. OGD was performed by transferring cells to an anaerobic chamber (Forma) with an atmosphere of 5% CO2, 5% H2, and 90% N2. The culture medium was replaced three times with deoxygenated PBS and serum free media for OGD. Cultures were placed in a humidified 37°C or 33°C incubator within the anaerobic chamber for 4 hr. Oxygen tension was monitored with an oxygen electrode meter and was kept under 0.02%. OGD was ended by adding 5.5 mM glucose to the culture medium, and the cultures were returned to the normoxic (37°C) or hypothermic (33°C) incubator (reperfusion). The cultured cells and media were harvested for further study.
2.4. Tissue Preparation and Infarct Area Measurement
Rats were euthanized by carbon dioxide overdose and perfused with cold normal saline immediately. The brain was quickly removed and sectioned into 2 mm thick slices starting at the frontal pole using a brain matrix slicer. Some slices were immersed in 2% 2,3,5-triphenyl tetrazolium chloride (TTC, Sigma) and incubated at 37°C for 20 minutes. To assess lesion area, TTC-stained slices were photographed and analyzed by Image-J analysis software (public domain software developed at NIH, available at http://rsb.info.nih.gov/ij/). Lesion area was determined as the percentage of the total ipsilateral hemispheric area. Other slices were processed for further studies.
2.5. Immunohistochemistry
From paraffin embedded brain slices, 6 μm thick sections were cut. After deparaffinization, sections were treated with 0.03% H2O2 and blocked in 1% bovine serum albumin and 5% normal serum. Following incubation with primary antibodies against ICAM-1 (1 : 100, Serotec), phosphorylated ERK1/2 (1 : 200, Santa Cruz), total ERK1/2 (1 : 200, Santa Cruz), STAT3 phosphorylated at Ser727 (1 : 200, Cell Signaling), and total STAT3 (1 : 200, Santa Cruz), respectively, biotin-labeled anti-IgG secondary antibody (1 : 200, Vector Labs) was treated. Antibodies were detected using the Vector ABC kit (Elite Vectastain ABC kit, Vector Labs) and colorized with 0.05% diaminobenzidine (DAB, Vector Labs). Negative controls were run in parallel using adjacent sections incubated with IgG instead of the primary antibody. For fluorescence staining, we used FITC or Cy3-labeled anti-IgG secondary antibody (1 : 200, Jackson) instead of biotin-labeled antibody.
2.6. Western Blot Analysis
Brain samples were homogenized in Laemmli's lysis buffer plus protease inhibitors. Aliquots containing 30 μg of protein were subjected to 10% SDS-PAGE. Protein bands were transferred to polyvinylidene difluoride membrane (Millipore), probed by incubating with the primary antibodies, and followed by a horseradish peroxidase conjugated secondary antibody (1 : 2000, Santa Cruz). We used the following primary antibodies raised against ICAM-1 (1 : 500, Serotec), phosphorylated ERK1/2 (1 : 1000, Santa Cruz), total ERK1/2 (1 : 1000, Santa Cruz), phosphorylated STAT3 (1 : 1000, Cell Signaling), and total STAT3 (1 : 1000, Santa Cruz). To determine the specificity of the primary antibodies, we used antibodies preabsorbed with blocking peptides instead of primary antibodies. Blots were visualized using the ECL system (Amersham) according to the manufacturer's directions, and exposed to X-ray film. Equal protein loading was confirmed by measuring β-actin (1 : 5000, anti β-actin, Sigma). Densitometric measurements were made from the film using a GS-700 imaging densitometer (Bio-Rad), then quantified using Multi-Analyst (Bio-Rad). For quantification of relative protein expression, the optical density of the protein band of interest was normalized to the optical density of β-actin on the same gel.
2.7. Microwell Colorimetric STAT3 DNA Binding Assay
The binding ability of STAT3 to its DNA consensus sequences was measured using a commercially available kit (Trans-AMTM STAT3, Active Motif). In this assay, tissue lysates were isolated from the brain tissue and tested for their ability to bind to a double-stranded oligonucleotide probe containing the consensus binding sequence for STAT3. Samples were homogenized in 3 mL ice-cold lysis buffer (20 mmol/L HEPES, pH 7.5; 350 mmol/L NaCl; 20% glycerol; 1% Igepal-CA630; 1 mmol/L MgCl2; 0.5 mmol/L EDTA; 0.1 mmol/L EGTA) per gram tissue. Lysates were centrifuged at 10,000 g for 10 minutes at 4°C. The supernatant was used to measure the protein content by a Bradford-based assay (Bio-Rad). STAT3 activity was determined by the sample's ability to bind to consensus sequences (5′-GGGACTTTCC-3′) in a 96-well plate. A primary antibody that recognizes an epitope on STAT3 and is accessible only when STAT3 is activated and bound to its target DNA was added to the wells, followed by a secondary horseradish peroxidase-conjugated antibody. Developing solution (tetramethylbenzidine) was added and the colorimetric reaction was stopped by adding stop solution (0.5 mol/L H2SO4). After stopping the reaction, absorbance was measured on a spectrophotometer within 5 minutes at 450 nm with a reference wavelength of 625 nm. HeLa whole-cell extract was used as positive control. The STAT3 wild-type and mutated consensus oligonucleotides were used in order to monitor the specificity of the assay.
2.8. Statistical Analysis
Data are given as means ± SEM. Comparisons between groups were performed using standard statistical methods using SigmaStat (SPSS). The data were analyzed by one-way ANOVA, Kruskal-Wallis one-way ANOVA on ranks, or unpaired t-test. Statistical significance was determined at the P < .05 level.
3. Results
3.1. Hypothermia Potentiated ERK1/2 Phosphorylation in the Vascular Endothelium of the Ischemic Brain
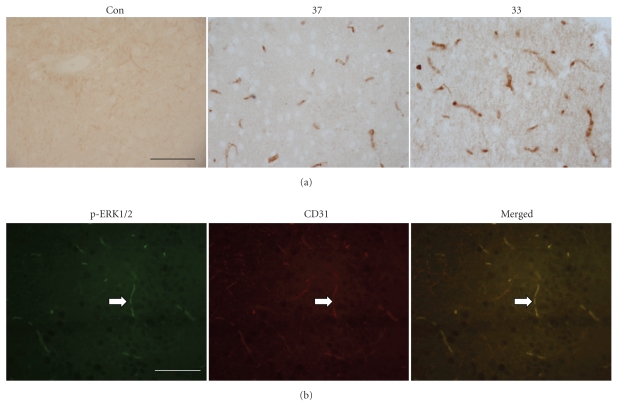
We observed the presence of phosphorylated or activated ERK1/2 in the ischemic brain using immunohistochemistry (Figure 1(a)) and demonstrated its localization in the endothelium using fluorescence double-labeling with antibody against CD31, an endothelial cell marker from the brain tissue at 2 hours after MCAO (Figure 1(b)). To find out the evidence that ERK1/2 pathway in the endothelium is related with ICAM-1 induction, we first investigated the effect of hypothermia on the activation of ERK1/2. From the immunohistochemistry study, the number and intensity of phosphorylated ERK1/2 immunoreactivity were higher in hypothermia group than normothermia (Figure 1(a)). To obtain quantitative data, we performed Western blot analysis and observed that ischemia-induced phosphorylated ERK1/2 level was significantly higher in hypothermia group than normothermia while total ERK1/2 was not affected by the temperature difference (Figure 2(a)).
Figure 1.
Photomicrographs of the cerebral cortex in the ischemic brain with immunohistochemical staining for phosphorylated ERK1/2. (a) Phosphorylated ERK1/2 is detected in the ischemic brain under normothermic (37) or hypothermic (33) condition but not in the nonischemic control brain (sham) at 2 hours after MCAO initiation. The number and intensity of ERK1/2 immunoreactive vessels were higher in hypothermic group. (b) Fluorescence double labeling illustrates the colocalization of CD31 (red), an endothelial marker, and phosphorylated ERK1/2 (green) in the vessels of the hypothermia group at 2 hours after MCAO initiation. Scale bar: 100 μm.
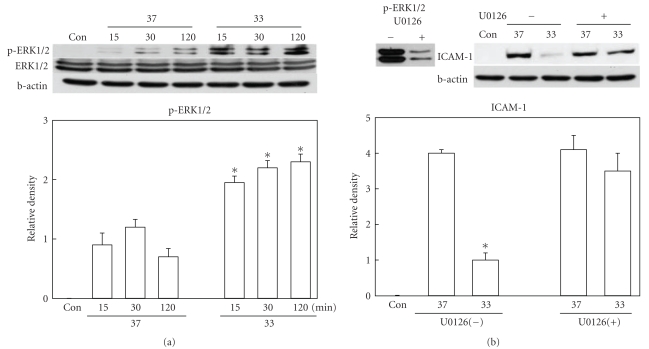
Figure 2.
Western blot analysis of phosphorylated ERK1/2 and ICAM-1. (a) Phosphorylation of ERK1/2 was significantly higher in hypothermic (33) brains than normothermic (37) ones at 15, 30, and 120 minutes after ischemic insult (n = 6 per group). Total ERK1/2 level was not changed. (b) Ischemia-induced increase of ICAM-1 at 24 hours after MCAO was suppressed by hypothermia. But this suppression was not observed when ERK1/2 inhibition was done (U0126, n = 4) at the dose which almost completely inhibited ERK1/2 phosphorylation. *P < .05 versus normothermia.
3.2. ERK1/2 Inhibitor Reversed Hypothermic Suppression of ICAM-1
To demonstrate the role of ERK1/2 in the hypothermic attenuation of ICAM-1 induction, we administered U0126, a MEK inhibitor suppressing phosphorylation of ERK1/2, to the animals and measured the ICAM-1 level in the ischemic brain. The optimal dose of U0126 was evaluated in the pilot study, and activation of ERK1/2 in brain was almost completely suppressed when U0126 (0.5 mg/kg) was administered 30 minutes before MCAO onset (Figure 2(b)). In U0126 untreated animals, ICAM-1 induction was reduced by hypothermia. But after U0126 treatment, ICAM-1 induction was not suppressed by hypothermia (Figure 2(b)).
3.3. STAT3 Phosphorylation Was Induced by Ischemia and Attenuated by Hypothermia
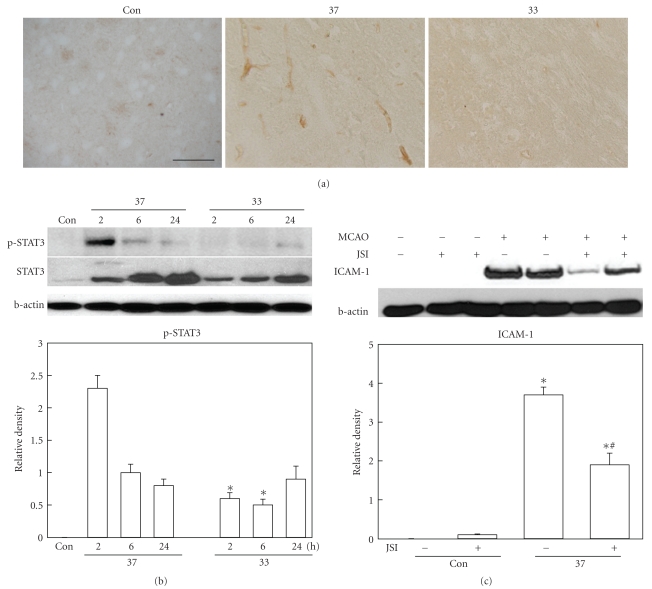
Even though hyperactivation of ERK1/2 seems to suppress ICAM-1 induction, the association between ERK1/2 phosphorylation and ICAM-1 expression is not clearly reported. So we first investigate the signal pathways implicated in the regulation of ICAM-1 expression in our model. During ischemia and until few hours after reperfusion initiation, phosphorylated STAT3 was observed in the ischemic brain, especially in the vessels (Figure 3(a)). At 24 hours after ischemia, STAT3 was not observed in the vessels. Western blot analysis demonstrated that ischemia increased STAT3 phosphorylation at 2 and 6 hours and it was declined to the basal level at 24 hours. Hypothermic attenuation of STAT3 phosphorylation was observed both in immunohistochemically stained tissues and Western blotted gel images (Figures 3(a) and 3(b)).
Figure 3.
(a) STAT3 phosphorylation in the cerebral cortex of the ischemic brain. Photomicrograph illustrates the presence of phosphorylated STAT3 in the vessels of the ischemic brain at 2 hours after ischemic insult. Positive immunoreactivity is not observed in nonischemic brain (sham), and hypothermia (33) reduced the number of phosphorylated STAT3 positive cell compared with normothermic group (37). Scale bar: 100 μm. (b) Western blot analysis shows that ischemia-induced STAT3 phosphorylation at 2 and 6 hours after MCAO onset in normothermic brain (37, n = 6) is reduced in hypothermia group (33, n = 6). *P < .05 versus normothermia. (c) Western blot analysis demonstrates that ICAM-1 induction at 24 hours after MCAO is reduced by STAT3 inhibitor treatment (JSI, n = 4). *P < .05 versus sham; #P < .05 versus ischemia without JSI-124.
3.4. STAT3 Inhibitor Attenuated ICAM-1 Induction
The presence of phosphorylated STAT3, which acts as a transcription factor, in the vessels and its suppression by hypothermia imply that STAT3 is important in ICAM-1 expression. To get more evidence of STAT3's role in ICAM-1 induction, we treated animals with JSI-124, an inhibitor of STAT3, at 1 hour before MCAO. The protein level of ICAM-1 was compared between the STAT3 inhibitor-treated and vehicle-treated groups. ICAM-1 induction at 24 hours after ischemia was significantly inhibited by JSI-124 treatment (Figure 3(c)).
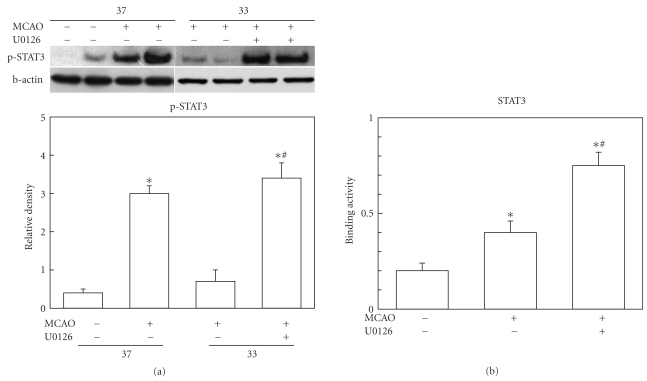
3.5. ERK1/2 Inhibition Enhanced STAT3 Phosphorylation and DNA Binding Activity
Based on the data so far, it seems that enhanced ERK1/2 activation and reduced STAT3 activation are the key role players of hypothermic suppression of ICAM-1 induction. To investigate whether these two factors are working dependently or not, we treated U0126 and observed phosphorylation and DNA binding activity of STAT3. Under hypothermic condition, STAT3 activation at 2 hours after MCAO was reduced. But when U0126 was treated to the hypothermic group, phosphorylated STAT3 was increased to the level of normothermia condition (Figure 4(a)). Since activity of STAT3 as a transcription factor can be indirectly estimated using the binding ability of STAT3 to its consensus sequence in the promoter area, we measured DNA binding activity of STAT3 as well. Tissue lysates from ischemic brain tissue of the hypothermia group were taken from the animals treated with or without ERK1/2 inhibition. U0126 treatment significantly enhanced the binding activity of STAT3 (Figure 4(b)).
Figure 4.
Effect of ERK1/2 inhibition on the STAT3 phosphorylation and DNA binding activity. (a) Western blot analysis demonstrates that STAT3 activation at 2 hours after ischemic insult is increased in normothermic group (37, n = 6). This increase was reduced by hypothermia (33, n = 6). In hypothermic group, U0126 treatment (33, n = 4) enhanced STAT3 phosphorylation to the level of normothermia group (37, n = 6). *P < .05 versus sham; #P < .05 versus hypothermic ischemia without U0126. (b) DNA binding activity assay of STAT3 using nuclear fraction from ischemic brain under hypothermia condition shows increased binding activity of STAT3 at 2 hours after MCAO (n = 4). STAT3 binding was further augmented by U0126 treatment (n = 4). *P < .05 versus sham; #P < .05 versus ischemia without U0126.
4. Discussion
This study is designed to investigate the mechanism of hypothermic suppression of ICAM-1 induction following brain ischemia. Our data are summarized as follows. ICAM-1 induction is suppressed by hypothermia after stroke. Stroke activates ERK1/2 mildly under normothermic condition and hypothermia potentiates ERK1/2 activation robustly. Stroke activates STAT3 under normothermic group and hypothermia attenuates this. ERK1/2 inhibition increases STAT3 activation and attenuates hypothermic effect. STAT3 inhibitor attenuates ICAM-1 induction. Based on these results, we suggest that hypothermia enhances ERK1/2 activation, inhibits STAT3 activation, and then leads to suppression of ICAM-1 induction.
Our first finding is potentiation of ERK1/2 activation by hypothermia. Even though it is well accepted that most of the metabolic and enzymatic pathways are down-regulated by hypothermia, increased activity of ERK1/2 by hypothermia was reported by many studies [25–29]. Since phosphorylated ERK1/2 from the brain tissue is a sum from mixed cell types, it cannot clearly indicate pure endothelial component. Our in vitro work (Supplementary Figure 2 available online at doi:10.4061/2011/846716) which demonstrated hypothermic enhancement of ERK1/2 activation in cultured mouse brain endothelial cells after oxygen-glucose deprivation should be a strong support for the hypothermic effect in the brain vascular endothelium. Roberts and colleagues also reported hypothermic activation of ERK1/2 in endothelial cells [30]. When hypothermia was applied without ischemic stimuli, it was not strong enough to activate ERK1/2. There was no difference between normothermia and hypothermia in nonischemic brain. Cultured brain endothelial cells showed the same result (Supplementary Figure 1). Even though we do not have a clear idea, it seems that hypothermia potentiates ERK1/2 activation when ERK1/2 activation is triggered first by ischemia. Hypothermia may have a direct influence on ERK1/2 itself or can affect one of the upstream factors of ERK1/2 pathway. Even though we did not investigate the upstream factors, we can draw valuable clues from 3 reports [25, 28, 29]. Chan and colleagues showed that hypothermic stress leads to activation of Ras in rat fibroblasts, and Raf-Mek-ERK cascade is rapidly activated when hypothermic cells are returned to physiologic temperature [28]. Sakurai and colleagues reported that mild hypothermia protects cells from TNF-alpha-induced apoptosis, at least partly, via induction of cold-inducible RNA-binding protein (CIRP), and that CIRP protects cells by activating the ERK pathway [25]. At our hands, CIRP gene expression was detected a few hours later than ERK1/2 activation in response to hypothermia (unpublished data). This implies that CIRP cannot be in the upstream of ERK1/2 pathway in our model. Atkins and colleagues demonstrated ERK activation in a traumatic brain model using similar hypothermia model as ours [29]. So we speculate that hypothermia acts on Raf-Mek-ERK cascade. Since the role of p38 or c-Jun N-terminal kinases (JNK) in the inflammation is well known, we also investigated p38 and JNK activation in the preliminary experiment. Active form of JNK was not detected in the vessel at the earlier time period after stroke, and phosphorylated p38 was observed in the vessels almost at the time window of ERK1/2 but showed no difference between normothermic and hypothermic conditions (unpublished data).
To elucidate the transcription factor which might be down-regulated by hypothermia and ERK1/2 signal, we searched references on ICAM-1 expression regulatory system. Studies on ICAM-1 promoter demonstrated the presence of STAT1/3 binding motif sequence, interferon response element (IRE) [31, 32], activator protein 1 (AP-1), nuclear factor kappa B (NFkappaB), Ets, CCAAT/enhancer binding protein (c/EBP), and SP1 [33, 34]. Among these transcription factors, we need to find the candidate which is the key factor of ICAM-1 induction in our model. In the preliminary experiment, we investigated a couple of transcription factors using immunohistochemistry and Western blot analysis. It is well known that NFkappaB system is the major pathway of inflammation [35, 36] and our previous study [37] also demonstrated that hypothermic suppression of NFkappaB system led to suppression of inflammation in stroke. Even though the role of NFkappaB in ICAM-1 induction was reported in many studies [38–40], Wen and colleagues [41] showed that nuclear translocation of NFkappaB observed in the ischemic area was mostly in the neurons and astrocytes. Our preliminary data also showed no NFkappaB translocation in the vessels during the early period ahead of ICAM-1 induction. Therefore, we tried other candidates. During ischemia and few hours after reperfusion initiation, c-Fos and phosphorylated STAT3 were observed in the ischemic brain. Phosphorylated STAT3 localized exclusively in the vessels while c-Fos were found in vessels and other cell types as well. When hypothermia was applied, there was no temperature difference in c-Fos (data not shown) but STAT3 phosphorylation was reduced by hypothermia. This suggests that STAT3 is a temperature sensitive transcription factor of ICAM-1. Yang and colleagues showed the evidence of STAT3 as a transcription factor of ICAM-1 in renal ischemia/reperfusion model [42]. By blocking Janus kinase (JAK)/STAT signal with selective JAK2 inhibitor tyrphostin AG490, expression of ICAM-1 was significantly inhibited, renal function was improved, and histological lesions and apoptosis were reduced [42]. In our stroke model, we also observed that STAT3 inhibitor JSI-124 effectively reduced ICAM-1 induction. These data might support that STAT3 plays as a key transcription factor of ICAM-1 expression in our system.
To evaluate whether STAT3 is regulated by ERK1/2 in addition to hypothermia, we tested the effect of ERK1/2 inhibition. When ERK1/2 activity was reduced, STAT3 phosphorylation and DNA binding activity were enhanced. Even though ERK1/2's role as the upstream regulator of STAT3 can be expected by ERK1/2 inhibition, it is not clear how ERK1/2 regulates STAT3 pathway. We just speculate that ERK1/2 might inhibit STAT3 directly or indirectly via one of the factors located in the upstream of STAT3 pathway. Even though JSI-124 effectively blocked the ICAM-1 induction, infarction size was not prevented or reduced in contrast to our expectation. We assume that there is a reason to explain this disappointing result. Many investigators have suggested STAT3 signal pathway as a neuroprotection mechanism. Shyu and colleagues [43] showed that secretoneurin's effect on reduced cerebral infarction improved motor performance, and increased brain metabolic activity in MCAO model was mediated via the JAK2/STAT3 pathway. Yamashita and colleagues [44] found that endogenous IL-6 plays a critical role in the neuroprotection, and its role may be mediated by STAT3 activation. In general it seems that STAT1 activation is related to cell death, whereas STAT3 activation is often associated with cell survival [45]. We also observed STAT3 immunoreactivity in neuron at 6 and 24 hours after MCAO while in the vessels at 2 and 6 hours. In spite of the immunohistochemistry data, we tried to confirm STAT3 phosphorylation in the endothelial cells. By repeating in vivo experimental conditions in the cultured brain endothelial cell system, we could demonstrate the hypothermic effect on STAT3 phosphorylation and effect of U0126 on STAT3 phosphorylation (Supplementary Figure 3). Based on these facts, STAT3 might have two opposite roles, promotion of ICAM-1 induction in the vessel and protection of cell in the neurons. This can explain why STAT3 inhibitor is not protective in our model. Even though STAT3 inhibitor used in this study was not protective against stroke, the better outcome can be expected when the endothelium-specific STAT3 inhibitor is developed.
5. Conclusions
We demonstrated that mild hypothermia has a robust suppressive effect on induction of ICAM-1 through regulation of ERK1/2 and STAT3. Even though mild hypothermia has been shown to have clinical efficacy in some settings, routine cooling of stroke victims may not always be practical or feasible. For those cases, ERK1/2 and STAT3 can be the good target of pharmaceutical treatment.
Supplementary Material
Supplementary figure 1. Effect of hypothermia on ERK1/2 phosphorylation in the non-ischemic control brains and cultured endothelial cells. When hypothermia was applied to the animals and cultured endothelial cells with the same protocol done in the ischemic models, ERK1/2 phosphorylation was not changed significantly in the non-ischemic control brain (left) and endothelial cells (right) compared with normothermia conditions.
Supplementary figure 2. Effect of oxygen glucose deprivation (OGD) on ERK1/2 activation under normothermia (37oC) or hypothermia (33oC) in the cultured endothelial cells. OGD enhanced ERK1/2 phosphorylation both in normothermia (left) and hypothermia (right) conditions. Activation of ERK1/2 was higher in hypothermia condition compared with normothermia.
Supplementary figure 3. Effect of U0126 on STAT3 phosphorylation in the ischemic brain (lower) and cultured endothelial cells (upper). Ischemic stimulation induced STAT3 phosphorylation in the brain and endothelial cells under normothermic condition (37oC). Under hypothermic condition, STAT3 phosphorylation was attenuated. Inhibition of ERK1/2 activation with U0126 reversed the hypothermic suppression of STAT3 phosphorylation.
Conflict of Interests
The authors declare that they have no conflict of interests.
Acknowledgment
This work was supported by a grant of the Korea Healthcare technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A100870) and Industrial Strategic technology development program (10035197) funded by the Ministry of Knowledge Economy (MKE, Korea).
Abbreviations
- ICAM-1:
Intercellular adhesion molecule-1
- ERK1/2:
Extracellular signal-regulated kinase-1/2
- STAT3:
Signal transducer and activator of transcription 3
- MCAO:
Middle Cerebral Artery Occlusion
- TTC:
2,3,5-triphenyl tetrazolium chloride
- DAB:
Diaminobenzidine
- JAK:
Janus kinase
- CIRP:
Cold-inducible RNA-binding protein
- JNK:
c-Jun N-terminal kinases
- IRE:
Interferon response element
- AP-1:
Activator protein 1
- NFkappaB:
Nuclear factor kappa B
- c/EBP:
CCAAT/enhancer-binding protein.
References
- 1.Dunn SM, Hillam AJ, Stomski F, et al. Leukocyte adhesion molecules involved in inflammation. Transplantation Proceedings. 1989;21(1, part 1):31–34. [PubMed] [Google Scholar]
- 2.Springer TA, Lasky LA. Sticky sugars for selections. Nature. 1991;349(6306):196–197. doi: 10.1038/349196a0. [DOI] [PubMed] [Google Scholar]
- 3.Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990;62(1):3–6. doi: 10.1016/0092-8674(90)90230-c. [DOI] [PubMed] [Google Scholar]
- 4.Stoolman LM. Adhesion molecules controlling lymphocyte migration. Cell. 1989;56(6):907–910. doi: 10.1016/0092-8674(89)90620-x. [DOI] [PubMed] [Google Scholar]
- 5.Bourdillon MC, Poston RN, Covacho C, Chignier E, Bricca G, McGregor JL. ICAM-1 deficiency reduces atherosclerotic lesions in double knock-out mice fed a fat or a chow diet. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20(12):2630–2635. doi: 10.1161/01.atv.20.12.2630. [DOI] [PubMed] [Google Scholar]
- 6.Haim M, Tanne D, Boyko V, et al. Soluble intercellular adhesion molecule-1 and long-term risk of acute coronary events in patients with chronic coronary heart disease: data from the Bezafibrate Infarction Prevention (BIP) study. Journal of the American College of Cardiology. 2002;39(7):1133–1138. doi: 10.1016/s0735-1097(02)01728-x. [DOI] [PubMed] [Google Scholar]
- 7.Del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathology. 2000;10(1):95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanne D, Haim M, Boyko V, et al. Soluble intercellular adhesion molecule-1 and risk of future ischemic stroke: a nested case-control study from the Bezafibrate Infarction Prevention (BIP) study cohort. Stroke. 2002;33(9):2182–2186. doi: 10.1161/01.str.0000029007.32244.40. [DOI] [PubMed] [Google Scholar]
- 9.Del Zoppo GJ, Hallenbeck JM. Advances in the vascular pathophysiology of ischemic stroke. Thrombosis Research. 2000;98(3):V73–V81. doi: 10.1016/s0049-3848(00)00218-8. [DOI] [PubMed] [Google Scholar]
- 10.Loddick SA, Rothwell NJ. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. Journal of Cerebral Blood Flow and Metabolism. 1996;16(5):932–940. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Krieger DW, Yenari MA. Therapeutic hypothermia for acute ischemic stroke: what do laboratory studies teach us? Stroke. 2004;35(6):1482–1489. doi: 10.1161/01.STR.0000126118.44249.5c. [DOI] [PubMed] [Google Scholar]
- 12.Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. Journal of Cerebral Blood Flow and Metabolism. 1999;19(8):819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Yenari MA, Kunis D, Sun GH, et al. Hu23F2G, an antibody recognizing the leukocyte CD 11/CD 18 integrin, reduces injury in a rabbit model of transient focal cerebral ischemia. Experimental Neurology. 1998;153(2):223–233. doi: 10.1006/exnr.1998.6876. [DOI] [PubMed] [Google Scholar]
- 14.Han HS, Qiao Y, Karabiyikoglu M, Giffard RG, Yenari MA. Influence of mild hypothermia on inducible nitric oxide synthase expression and reactive nitrogen production in experimental stroke and inflammation. Journal of Neuroscience. 2002;22(10):3921–3928. doi: 10.1523/JNEUROSCI.22-10-03921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toyoda T, Suzuki S, Kassell NF, Lee KS. Intraischemic hypothermia attenuates neutrophil infiltration in the rat neocortex after focal ischemia-reperfusion injury. Neurosurgery. 1996;39(6):1200–1205. doi: 10.1097/00006123-199612000-00024. [DOI] [PubMed] [Google Scholar]
- 16.Maier CM, Ahern KVB, Cheng ML, Lee JE, Yenari MA, Steinberg GK. Optimal depth and duration of mild hypothermia in a focal model of transient cerebral ischemia: effects on neurologic outcome, infarct size, apoptosis, and inflammation. Stroke. 1998;29(10):2171–2180. doi: 10.1161/01.str.29.10.2171. [DOI] [PubMed] [Google Scholar]
- 17.Inamasu J, Suga S, Sato S, et al. Intra-ischemic hypothermia attenuates intercellular adhesion molecule-1 (ICAM-1) and migration of neutrophil. Neurological Research. 2001;23(1):105–111. doi: 10.1179/016164101101198217. [DOI] [PubMed] [Google Scholar]
- 18.Deng H, Han HS, Cheng D, Sun GH, Yenari MA. Mild hypothermia inhibits inflammation after experimental stroke and brain inflammation. Stroke. 2003;34(10):2495–2501. doi: 10.1161/01.STR.0000091269.67384.E7. [DOI] [PubMed] [Google Scholar]
- 19.Luan X, Li J, McAllister JP, et al. Regional brain cooling induced by vascular saline infusion into ischemic territory reduces brain inflammation in stroke. Acta Neuropathologica. 2004;107(3):227–234. doi: 10.1007/s00401-003-0802-2. [DOI] [PubMed] [Google Scholar]
- 20.Wang GJ, Deng HY, Maier CM, Sun GH, Yenari MA. Mild hypothermia reduces ICAM-1 expression, neutrophil infiltration and microglia/monocyte accumulation following experimental stroke. Neuroscience. 2002;114(4):1081–1090. doi: 10.1016/s0306-4522(02)00350-0. [DOI] [PubMed] [Google Scholar]
- 21.Kira S, Daa T, Kashima K, Mori M, Noguchi T, Yokoyama S. Mild hypothermia reduces expression of intercellular adhesion molecule-1 (ICAM-1) and the accumulation of neutrophils after acid-induced lung injury in the rat. Acta Anaesthesiologica Scandinavica. 2005;49(3):351–359. doi: 10.1111/j.1399-6576.2005.00593.x. [DOI] [PubMed] [Google Scholar]
- 22.Inamasu J, Suga S, Sato S, et al. Post-ischemic hypothermia delayed neutrophil accumulation and microglial activation following transient focal ischemia in rats. Journal of Neuroimmunology. 2000;109(2):66–74. doi: 10.1016/s0165-5728(00)00211-3. [DOI] [PubMed] [Google Scholar]
- 23.Han HS. Effect of mild hypothermia on the mitogen activated protein kinases in experimental stroke. Korean Journal of Physiology and Pharmacology. 2004;8(4):187–194. [Google Scholar]
- 24.Han HS, Yenari MA. Effect on gene expression of therapeutic hypothermia in cerebral ischemia. Future Neurology. 2007;2(4):435–440. [Google Scholar]
- 25.Sakurai T, Itoh K, Higashitsuji H, et al. Cirp protects against tumor necrosis factor-α-induced apoptosis via activation of extracellular signal-regulated kinase. Biochimica et Biophysica Acta. 2006;1763(3):290–295. doi: 10.1016/j.bbamcr.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 26.D’Cruz BJ, Logue ES, Falke E, DeFranco DB, Callaway CW. Hypothermia and ERK activation after cardiac arrest. Brain Research. 2005;1064(1-2):108–118. doi: 10.1016/j.brainres.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 27.D’Cruz BJ, Fertig KC, Filiano AJ, Hicks SD, DeFranco DB, Callaway CW. Hypothermic reperfusion after cardiac arrest augments brain-derived neurotrophic factor activation. Journal of Cerebral Blood Flow and Metabolism. 2002;22(7):843–851. doi: 10.1097/00004647-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Chan EYW, Stang SL, Bottorff DA, Stone JC. Hypothermic stress leads to activation of Ras-Erk signaling. Journal of Clinical Investigation. 1999;103(9):1337–1344. doi: 10.1172/JCI5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atkins CM, Oliva AA, Jr., Alonso OF, et al. Hypothermia treatment potentiates ERK1/2 activation after traumatic brain injury. European Journal of Neuroscience. 2007;26(4):810–819. doi: 10.1111/j.1460-9568.2007.05720.x. [DOI] [PubMed] [Google Scholar]
- 30.Roberts JR, Rowe PA, Demaine AG. Activation of NF-κB and MAP kinase cascades by hypothermic stress in endothelial cells. Cryobiology. 2002;44(2):161–169. doi: 10.1016/s0011-2240(02)00018-4. [DOI] [PubMed] [Google Scholar]
- 31.Caldenhoven E, Coffer P, Yuan J, et al. Stimulation of the human intercellular adhesion molecule-1 promoter by interleukin-6 and interferon-γ involves binding of distinct factors to a palindromic response element. Journal of Biological Chemistry. 1994;269(33):21146–21154. [PubMed] [Google Scholar]
- 32.Kim OS, Park EJ, Joe EH, Jou I. JAK-STAT signaling mediates gangliosides-induced inflammatory responses in brain microglial cells. Journal of Biological Chemistry. 2002;277(43):40594–40601. doi: 10.1074/jbc.M203885200. [DOI] [PubMed] [Google Scholar]
- 33.van de Stolpe A, van der Saag PT. Intercellular adhesion molecule-1. Journal of Molecular Medicine. 1996;74(1):13–33. doi: 10.1007/BF00202069. [DOI] [PubMed] [Google Scholar]
- 34.Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. Journal of Leukocyte Biology. 1999;66(6):876–888. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- 35.Celec P. Nuclear factor kappa B-molecular biomedicine: the next generation. Biomedicine and Pharmacotherapy. 2004;58(6-7):365–371. doi: 10.1016/j.biopha.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 36.Li Q, Verma IM. NF-κB regulation in the immune system. Nature Reviews Immunology. 2002;2(10):725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 37.Han HS, Karabiyikoglu M, Kelly S, Sobel RA, Yenari MA. Mild hypothermia inhibits nuclear factor-κB translocation in experimental stroke. Journal of Cerebral Blood Flow and Metabolism. 2003;23(5):589–598. doi: 10.1097/01.WCB.0000059566.39780.8D. [DOI] [PubMed] [Google Scholar]
- 38.Haddad O, Chotard-Ghodsnia R, Verdier C, Duperray A. Tumor cell/endothelial cell tight contact upregulates endothelial adhesion molecule expression mediated by NFkappaB: differential role of the shear stress. Experimental Cell Research. 2010;316(4):615–626. doi: 10.1016/j.yexcr.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Fritzenwanger M, Foerster M, Meusel K, Jung C, Figulla HR. Cardiotrophin-1 induces intercellular adhesion molecule-1 expression by nuclear factor κB activation in human umbilical vein endothelial cells. Chinese Medical Journal. 2008;121(24):2592–2598. [PubMed] [Google Scholar]
- 40.MacKenzie CJ, Ritchie E, Paul A, Plevin R. IKKα and IKKβ function in TNFα-stimulated adhesion molecule expression in human aortic smooth muscle cells. Cellular Signalling. 2007;19(1):75–80. doi: 10.1016/j.cellsig.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Wen Y, Yang S, Liu R, et al. Estrogen attenuates nuclear factor-kappa B activation induced by transient cerebral ischemia. Brain Research. 2004;1008(2):147–154. doi: 10.1016/j.brainres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Yang N, Luo M, Li R, et al. Blockage of JAK/STAT signalling attenuates renal ischaemia-reperfusion injury in rat. Nephrology Dialysis Transplantation. 2008;23(1):91–100. doi: 10.1093/ndt/gfm509. [DOI] [PubMed] [Google Scholar]
- 43.Shyu WC, Lin SZ, Chiang MF, et al. Secretoneurin promotes neuroprotection and neuronal plasticity via the Jak2/Stat3 pathway in murine models of stroke. Journal of Clinical Investigation. 2008;118(1):133–148. doi: 10.1172/JCI32723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamashita T, Deguchi K, Sawamoto K, Okano H, Kamiya T, Abe K. Neuroprotection and neurosupplementation in ischaemic brain. Biochemical Society Transactions. 2006;34(6):1310–1312. doi: 10.1042/BST0341310. [DOI] [PubMed] [Google Scholar]
- 45.Planas AM, Gorina R, Chamorro A. Signalling pathways mediating inflammatory responses in brain ischaemia. Biochemical Society Transactions. 2006;34(6):1267–1270. doi: 10.1042/BST0341267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1. Effect of hypothermia on ERK1/2 phosphorylation in the non-ischemic control brains and cultured endothelial cells. When hypothermia was applied to the animals and cultured endothelial cells with the same protocol done in the ischemic models, ERK1/2 phosphorylation was not changed significantly in the non-ischemic control brain (left) and endothelial cells (right) compared with normothermia conditions.
Supplementary figure 2. Effect of oxygen glucose deprivation (OGD) on ERK1/2 activation under normothermia (37oC) or hypothermia (33oC) in the cultured endothelial cells. OGD enhanced ERK1/2 phosphorylation both in normothermia (left) and hypothermia (right) conditions. Activation of ERK1/2 was higher in hypothermia condition compared with normothermia.
Supplementary figure 3. Effect of U0126 on STAT3 phosphorylation in the ischemic brain (lower) and cultured endothelial cells (upper). Ischemic stimulation induced STAT3 phosphorylation in the brain and endothelial cells under normothermic condition (37oC). Under hypothermic condition, STAT3 phosphorylation was attenuated. Inhibition of ERK1/2 activation with U0126 reversed the hypothermic suppression of STAT3 phosphorylation.