Introduction
Asthma is influenced by a combination of host susceptibility and environmental factors, including viruses, pollutants, and allergens. Individuals with asthma often have allergic sensitization to allergens and for these individuals, identification and avoidance of relevant allergens is an established component of asthma management. However, non-atopic asthma contributes significantly to the worldwide and U.S. burden of disease, representing as much as 50% of the world's asthma, though atopic asthma likely predominates in children. Some evidence suggests that non-atopic asthma may confer a worse prognosis compared with atopic asthma1–5 but, because it is not considered an allergen-driven disease, environmental control recommendations are less well established.
Air pollutants are among the likely candidates of the possible environmental triggers for non-atopic asthma. Previous studies have suggested that air pollutants, such as sulfur dioxide, nitrogen dioxide, carbon monoxide and benzene, have a stronger effect in non-atopic asthma than atopic asthma.6, 7 Particulate Matter (PM) is a common air pollutant that has known detrimental health effects, especially for those with asthma. PM has both outdoor sources, including products of combustion and crustal materials, and indoor sources, including smoking, cooking, and cleaning activities.8,9 Increases in ambient PM have been associated greater morbidity in asthma and greater mortality in the general population.10–13 However, the effect on non-atopic asthma has not been isolated.
Although Americans spend most of their time indoors (>80%) and indoor PM concentrations can exceed those measured outdoors, less is known about the health effect of indoor PM exposure. Previous studies of health effects of indoor PM have linked indoor PM exposure to increases in respiratory symptoms and decreases in pulmonary function but have not evaluated differential health effects of pollutants between atopic and non-atopic asthmatics.11, 14–16 To better understand the etiologic mechanism and to inform recommendations for improving health of those with non-atopic asthma, we first need to provide evidence of the link between exposures and exacerbation of disease. The present study focuses on inner-city pre-school age children with asthma, a group known to have a high burden of disease and to be at risk for increased exposure to environmental pollutants. Using a cohort of well-characterized children with asthma14,17, we examined the response to indoor PM exposure in those with non-atopic and atopic asthma.
Methods
Study Design
The Johns Hopkins Medical Institutional Review Board approved the study and all participants provided written informed consent prior to beginning the study. Children participating in this longitudinal study17 were evaluated at baseline, 3, and 6 months. At each time interval, environmental monitoring occurred for 3 consecutive days and health outcomes were assessed through caregiver report.
Participants
Children were recruited from health systems that provide care to most East Baltimore residents. Inclusion criteria were (1) age 2–6 years (2) residence in one of nine contiguous zip codes within East Baltimore (3) physician diagnosis of asthma, and (4) asthma symptoms and/or medication use in previous 6 months.
Air Quality Assessment
Environmental monitoring methods are described elsewhere.17 At baseline, 3, and 6 months, integrated air sampling was performed in the child's bedroom over 3 days using PM10 and PM2.5 samples collected with personal environmental monitors (SKC, Inc. Eighty Four, PA) which had been loaded with 37mm Teflo® filters, (Pall-Gelman, Ann Arbor, MI). Coarse PM fraction was calculated as PM10 −PM2.5. 18Inlet flow rates were calibrated at the beginning and end of sampling periods using primary standards (BIOS DryCal™, Bios International Corporation, Butler, NJ). PM gravimetric analysis was conducted on a Metler T5 microbalance. Ambient PM for the study was measured at a central site within the study area using a PM2.5 Partisol- Plus model 2025 FRM Sequential Air Sampler and the PM10 tapered element oscillating microbalance, TEOM 1400,(Rupprecht & Patashnick Co. Inc., Albany, NY). When ambient values were missing (<10% missing), values were supplemented from Maryland Department of Environment Station that is within one mile of the central monitoring site.19
Clinical Evaluation
Each child underwent baseline skin prick testing (Multi-Test II, Lincoln Diagnostics, Decatur, IL) to a standard mix of 14 aeroallergens. Atopy was defined as at least 1 positive skin test, defined as wheal size at least 2 mm greater than the negative control, as in previous childhood asthma studies.20–22 At baseline, 3, and 6 months, caregivers completed questionnaires that included closed-ended questions from International Study of Asthma and Allergies in Childhood23 and Children's Health Survey for Asthma24. Questions included rescue medication use (short-acting beta agonist) and symptoms in the previous 2 weeks, including 1) wheezing, coughing, or chest tightness; 2) the need to slow down/stop activities; 3) wheezing so badly that the child could only speak one or two words between breaths; 4) symptoms with exercise; and 5) nocturnal symptoms. Symptoms were quantified as number of days present in the previous two weeks (0–14 days). Participants completed a daily activity diary during each 3-day environmental monitoring period and this included an account of time spent in the room where monitoring occurred.
Statistical analysis
Summary statistics, such as means or medians were generated. Comparisons of baseline demographic characteristics were made using χ2 test for proportions and Student's t-test or Wilcoxon signed-rank test for continuous data. Negative binomial regression models were fit using generalized estimating equations25 to model the relationship between PM and repeated measures of days of symptoms or rescue medication use. Multivariate models were constructed to account for potential confounders identified based on known relationships with asthma, atopy, or PM or statistically significant associations in bivariate models of PM and symptom outcomes. An interaction term for atopy and PM exposure was also tested. Analyses were performed with Stata statistical software, version 8.0 (Stata Corp, College Station, TX). Statistical significance was defined as p<0.05.
Results
Participant characteristics
The 150 pre-school children enrolled in this longitudinal cohort study were predominantly African American from lower income households (Table 1). Of the 133 who completed allergy skin testing, 31% were classified as non-atopic and 69% as atopic. Non-atopic children were slightly younger with a mean age of 3.9 years compared to atopic children who had a mean age of 4.6 years (p=0.01). There were no significant differences between the groups with respect to race, gender, or socioeconomic status. Both the atopic and non-atopic children had evidence of active asthma with similar measures of morbidity (Table 1). Half of participants reported symptoms and use of rescue medications over the past 2 weeks, and about one-third reported the need for evaluation in an acute health care setting in the previous 3 months. Children spent about half of each 24-hour day in their own home and most of this time was spent in the bedroom where environmental monitoring was conducted (Table 1). The majority of homes were rowhomes in close proximity to the roadway, and these characteristics did not differ by atopic status.
Table 1.
Participant Characteristics
| Baseline Characteristics | Non-Atopic N=41 | Atopic N=92 | P-value |
|---|---|---|---|
| Race (% African American) | 85 | 92 | 0.20 |
| Gender (% Male) | 59 | 59 | 0.98 |
| Age (years) Mean(SD) | 3.9 (1.3) | 4.6 (1.5) | 0.01 |
| Household Income (%)* | |||
| <$15,000 | 62 | 49 | 0.60 |
| $15,000–30,000 | 24 | 34 | |
| >$30,000 | 14 | 17 | |
| Caregiver education (%) | |||
| 8th grade/some high school | 44 | 36 | 0.06 |
| High School | 49 | 39 | |
| Some college | 7 | 25 | |
| Time (hours/day) Mean(SD) | |||
| Home | 12.4 (6.8) | 12.6 (6.2) | 0.88 |
| Bedroom with monitor | 7.0 (4.4) | 6.5 (3.9) | 0.49 |
| Parental history of asthma (%) | 80 | 65 | 0.11 |
| Symptoms in previous 2 weeks (%) | 52 | 41 | 0.25 |
| Rescue medications in previous 2 weeks (%) | 53 | 53 | 0.96 |
| Acute health care use in previous 3 months (%) | |||
| ED visits | 27 | 23 | 0.62 |
| Hospitalizations | 2 | 3 | 0.80 |
| Unscheduled doctor visits | 15 | 18 | 0.59 |
for those who responded. 20% of participants did not respond to this question.
Indoor Air Quality in Homes of Children with Atopic Versus Non-Atopic Asthma
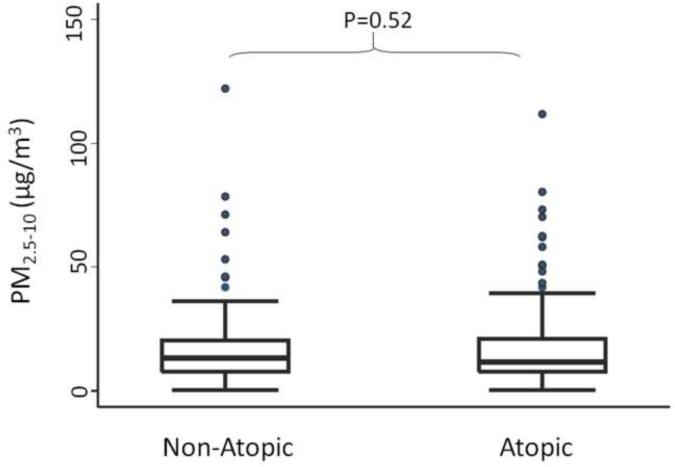
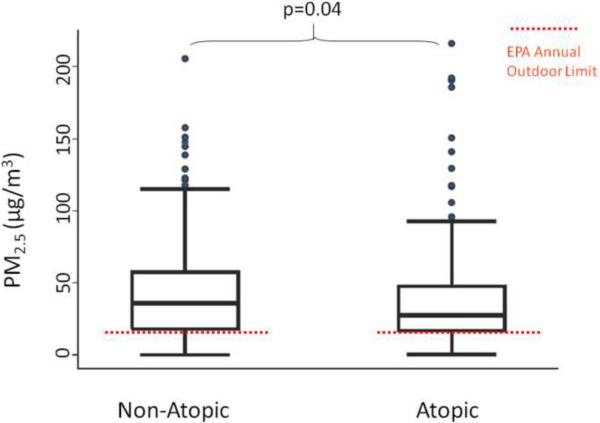
The median (IQR) indoor PM2.5–10 concentrations were similar among children with non-atopic and atopic asthma, with concentrations of 13.4 (13.2) μg/m3 and 11.6 (13.2) μg/m3, respectively (p=0.52) (Figure 1). Fine PM concentrations were elevated with over 75% of homes exceeding the EPA National Ambient Air Quality Standards annual limit for ambient PM2.5 concentrations (Figure 2).26 The indoor PM2.5 concentrations were higher in the homes of children with non-atopic asthma compared to those with atopic asthma, 35.7 (39.4) μg/m3 and 27.6 (30.7) μg/m3, respectively (p=0.04).
Figure 1.
Indoor coarse PM concentrations in the homes of non-atopic and atopic children. Boxes show the interquartile range (IQR) and the heavy dark lines are the median values. Whiskers represent the closest value within 1.5 times the IQR. Indoor concentrations of coarse PM did not significantly differ between non-atopic and atopic children.
Figure 2.
Indoor fine PM concentrations in the homes of non-atopic and atopic children. Over 75% of homes had indoor fine PM concentrations that exceeded the EPA annual outdoor limit,26 demonstrated by the dashed red line. Indoor concentrations of fine PM were greater in the homes of non-atopic children compared with atopic children.
Effect of Indoor Coarse PM on Asthma Morbidity by Atopic Status
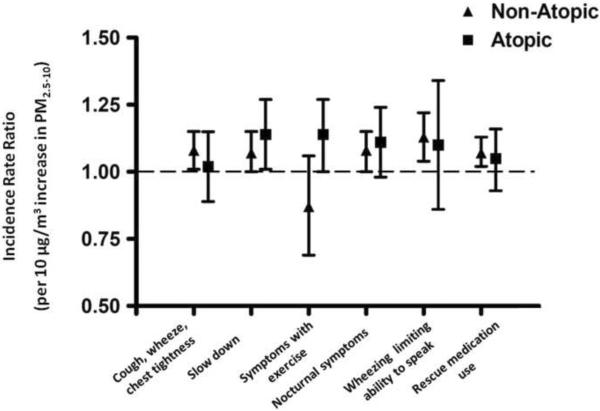
Higher concentrations of indoor coarse PM were associated with increases in asthma symptoms and the need for rescue medications for both the non-atopic and atopic groups (Table 2a, 2b, Figure 3). For the non-atopic group, nearly all associations were statistically significant with a 7 [95% CI, 0,15] − 13 [95% CI 4,22]% higher incidence of symptoms per 10μg/m3, after adjusting for age, race, sex, socioeconomic status, season, indoor fine and ambient fine and coarse PM concentrations. The magnitude of the associations was similar among the atopic children. There was a statistically significant interaction between atopic status and the association between coarse PM and symptoms with running (p= 0.04 for interaction term) in the multivariate model (Table 2b excludes overall results for this outcome). For children with atopic asthma, there was a 14% [95% CI, 0, 27] increase in the incidence of symptoms with exercise for every 10 μg/m3 increase in PM2.5–10 (p=0.01), after adjustment for potential confounders, but this increase was not found in non-atopic children.
Table 2a.
Indoor coarse PM concentrations and asthma morbidity by atopic status: bivariate models*
| Non-atopic coarse | Atopic coarse | |||||
|---|---|---|---|---|---|---|
| IRR | 95% CI | p-value | IRR | 95% CI | p-value | |
| Cough, wheezing, chest tightness | 1.06 | (1.0, 1.13) | 0.06 | 1.02 | (0.92, 1.13) | 0.68 |
| Slow down | 1.07 | (1.0, 1.13) | 0.05 | 1.14 | (1.04, 1.25) | <0.01 |
| Symptoms with running | 0.73 | (0.56, 0.90) | <0.01 | 1.12 | (1.01, 1.23) | 0.02 |
| Nocturnal symptoms | 1.08 | (1.02, 1.15) | 0.01 | 1.03 | (0.93, 1.14) | 0.55 |
| Limited speech | 1.11 | (1.04, 1.18) | <0.01 | 1.14 | (0.99, 1.30) | 0.07 |
| Beta Agonist use | 1.07 | (1.01, 1.13) | 0.02 | 1.06 | (0.96, 1.15) | 0.24 |
Incidence rate ratios (IRR) are presented per 10 μg/m3 increase in PM concentration.
Models were adjusted for age, race, gender, parent education, season, indoor PM2.5, outdoor PM2.5, and outdoor PM2.5–10
Table 2b.
Indoor coarse PM concentrations and asthma morbidity by atopic status: multivariate models*
| Non-atopic coarse | Atopic coarse | |||||
|---|---|---|---|---|---|---|
| IRR | 95% CI | p-value | IRR | 95% CI | p-value | |
| Cough, wheezing, chest tightness | 1.08 | (1.0, 1.15) | 0.02 | 1.02 | (0.89, 1.15) | 0.73 |
| Slow down | 1.07 | (1.0, 1.15) | 0.05 | 1.14 | (1.01, 1.27) | 0.04 |
| Symptoms with running | 0.87 | (0.69, 1.06) | 0.18 | 1.14 | (1.00, 1.27) | 0.04 |
| Nocturnal symptoms | 1.08 | (1.00, 1.15) | 0.04 | 1.11 | (0.98, 1.24) | 0.11 |
| Limited speech | 1.13 | (1.04, 1.22) | <0.01 | 1.10 | (0.86, 1.34) | 0.41 |
| Beta Agonist use | 1.07 | (1.02, 1.13) | 0.01 | 1.05 | (0.93, 1.16) | 0.43 |
Incidence rate ratios (IRR) are presented per 10 μ/m3 increase in PM concentration.
Models were adjusted for age, race, gender, parent education, season, indoor PM2.5, outdoor PM2.5, and outdoor PM2.5–10
Figure 3.
Multivariate analysis of the effect of indoor coarse PM on asthma morbidity. Incidence rate ratios are displayed as point estimates and 95% confidence intervals for the effect of indoor coarse PM2.5–10 on asthma symptom outcomes and rescue medication use. Models were adjusted for age, race, gender, parent education, season, indoor PM2.5, outdoor PM2.5, and outdoor PM2.5–10. With the exception of symptoms with exercise, there was an increase in the incidence of asthma morbidity outcomes for every 10μg/m3 increase in PM2.5–10 among both the non-atopic and atopic children with narrower confidence intervals for non-atopic asthma.
Effect of Indoor Fine PM on Asthma Morbidity by Atopic Status
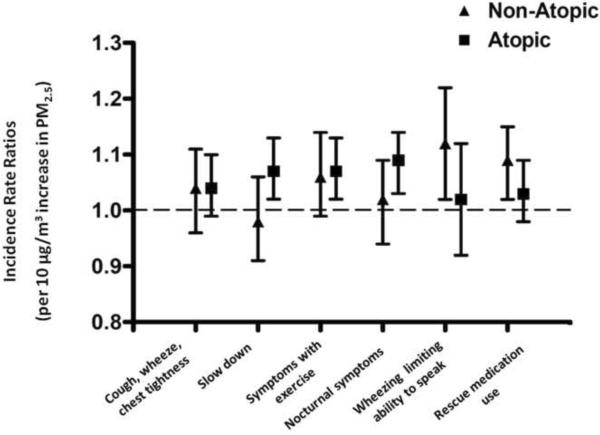
Higher concentrations of fine PM measured indoors were associated with increases in asthma symptoms and the need for rescue medications in both the non-atopic and the atopic subgroups. The magnitude of the effect was similar between groups in both the bivariate and multivariate models (Table 3a, 3b; Figure 3). There was no significant interaction between atopic status and the effect of fine PM on respiratory symptoms or rescue medication use.
Table 3a.
Indoor fine PM concentrations and asthma morbidity by atopic status: bivariate models*
| Non-atopic fine | Atopic fine | |||||
|---|---|---|---|---|---|---|
| IRR | 95% CI | p-value | IRR | 95% CI | p-value | |
| Cough, wheezing, chest tightness | 1.03 | (0.97, 1.09) | 0.31 | 1.02 | (0.97, 1.07) | 0.36 |
| Slow down | 0.99 | (0.92, 1.05) | 0.73 | 1.03 | (0.98, 1.07) | 0.28 |
| Symptoms with running | 1.05 | (0.99, 1.11) | 0.12 | 1.04 | (1.0, 1.08) | 0.11 |
| Nocturnal symptoms | 0.98 | (0.91, 1.05) | 0.59 | 1.04 | (0.99, 1.08) | 0.12 |
| Limited speech | 1.05 | (0.97, 1.13) | 0.26 | 0.97 | (0.88, 1.06) | 0.52 |
| Beta Agonist use | 1.08 | (1.02, 1.14) | <0.01 | 1.02 | (0.97, 1.06) | 0.43 |
Incidence rate ratios (IRR) are presented per 10 μ/m3 increase in PM concentration.
Models were adjusted for age, race, gender, parent education, season, indoor PM2.5, outdoor PM2.5, and outdoor PM2.5–10. Models were adjusted for age, race, gender, parent education, season, indoor PM2.5–10, outdoor PM2.5, and outdoor PM2.5–10
Table 3b.
Indoor fine PM concentrations and asthma morbidity by atopic status: multivariate models*
| Non-atopic fine | Atopic fine | |||||
|---|---|---|---|---|---|---|
| IRR | 95% CI | p-value | IRR | 95% CI | p-value | |
| Cough, wheezing, chest tightness | 1.04 | (0.96, 1.11) | 0.34 | 1.04 | (0.99, 1.10) | 0.14 |
| Slow down | 0.98 | (0.91, 1.06) | 0.70 | 1.07 | (1.02, 1.13) | 0.01 |
| Symptoms with running | 1.06 | (0.99, 1.14) | 0.12 | 1.07 | (1.02, 1.13) | 0.01 |
| Nocturnal symptoms | 1.02 | (0.94, 1.09) | 0.66 | 1.09 | (1.03, 1.14) | <0.01 |
| Limited speech | 1.12 | (1.02, 1.22) | 0.02 | 1.02 | (0.92, 1.12) | 0.70 |
| Beta Agonist use | 1.09 | (1.02, 1.15) | 0.01 | 1.03 | (0.98, 1.09) | 0.21 |
Incidence rate ratios (IRR) are presented per 10 μg/m3 increase in PM concentration.
Models were adjusted for age, race, gender, parent education, season, indoor PM2.5, outdoor PM2.5, and outdoor PM2.5–10. Models were adjusted for age, race, gender, parent education, season, indoor PM2.5–10, outdoor PM2.5, and outdoor PM2.5–10
Discussion
We found that in-home particle concentrations were associated with asthma morbidity, including symptoms and rescue medication use, among not only atopic but also non-atopic children. Although there were fewer non-atopic (n=41) than atopic children (n=92) in this inner-city, predominantly African American cohort, we found substantial, statistically significant relationships between in-home PM concentrations and asthma outcomes in this group. The magnitude of the response to PM was similar in non-atopic and atopic children. To our knowledge, this is the first study to focus on the relationship between atopic status and the health effects of indoor PM.
There has been relatively little attention paid to environmental triggers of non-atopic asthma. Of the few studies that have examined the effect of indoor PM on children with asthma, most have not evaluated susceptibility among non-atopic asthmatics. Studies of children in Seattle found that higher indoor and outdoor PM concentrations were associated with lower maximal midexpiratory flows among a subgroup of 11 children who were not taking anti-inflammatory medications but the atopic status of participants was not assessed.15, 16 In a study based in Southern California, FEV1 was inversely associated with personal and indoor PM concentrations among 19 children with asthma11 In a subset of 12 male children in this study, an analysis of the influence of atopic status on the susceptibility to PM exposure revealed mixed results. Atopic boys showed stronger inverse associations between personal PM and FEV1, but weaker associations between stationary-site PM and FEV1, compared to non-atopic boys, (though this latter difference was not significant). In our larger present study, we found evidence that both atopic and non-atopic children were similarly adversely impacted by indoor airborne PM exposure.
In addition to PM, several other indoor pollutants have been shown to impact those with non-atopic asthma and may even disproportionately affect non-atopic as compared to atopic asthma. For example, some studies of secondhand smoke exposure have shown a stronger effect in terms of the incidence and disease severity among non-atopic children with asthma compared to those with atopy.27–30 Based on previous work, we have determined that penetration of outdoor air into indoor space and indoor smoking, cooking, and cleaning activities contributed to elevated in-home PM concentrations.8 Secondhand smoke is likely to contribute to the asthmatic response that is associated with indoor PM exposure in our study. Increased levels of NO2 were associated with increased asthma symptoms and decreased peak flows only among non-atopic asthmatic children in one study,6 and in our inner city Baltimore cohort, indoor NO2 levels were associated with increased asthma morbidity, independent of atopic status.31 These findings suggest that environmental controls aimed at pollutants may be especially important to the non-atopic asthmatic.
Studies have not only suggested that pollutant exposure exacerbates existing non-atopic asthma but also that pollutant exposure may increase susceptibility to the development of non-atopic asthma, though these results have been inconsistent. In a study that investigated susceptibility to the risk of childhood asthma and wheeze with exposure to traffic, living within 75 meters of a major road was associated with a more than 2-fold increased risk of lifetime asthma, prevalent asthma, or current wheeze among children without allergic symptoms but not among those with allergic symptoms.32 Another recent study suggested that traffic-related pollution exposure increased the risk of incident asthma and of asthma-related symptoms and that this effect may be limited to non-atopic asthma but, according the study authors, the small sample size limited their ability to interpret this finding.33 However, other studies have yielded different conclusions, supporting stronger responses to traffic-related pollutant exposure among atopic children.34, 35
Evidence suggests that the cellular response is similar between non-atopic and atopic asthma36–38 but that the allergens and antigens that “trigger” asthmatic responses may differ. Exposure to allergen and a subsequent allergic inflammatory response with associated bronchial hyperreactivity is associated with exacerbation of allergic asthma. For allergic asthma, pollutants may provoke asthma through various mechanisms and hypotheses propose that particulate pollution can directly stimulate an inflammatory response or it can serve as a vehicle for carrying allergen and therefore provoke asthma through atopic pathways. Laboratory evidence supports this concept and suggests that air pollution exposure enhances the effect of allergens on asthma.39–41 Interestingly, exposure to PM collected outdoors in Baltimore has also been shown to directly induce airway hyperresponsiveness and airway inflammation in mice in the absence of exogenous exposure to allergens in a T cell dependent manner.42 Although no known protein allergens have been found in these samples of outdoor Baltimore PM, it is possible previously unrecognized allergens are present or that PM induces cellular damage and leads to modification of self-proteins leading to T cell activation in the absence of atopy. In support of the former hypothesis, Burney and colleagues reported that in human studies exacerbations of asthma were associated with increases in the patient's IgE binding to outdoor airborne particles collected during the weekend preceding the exacerbation as compared to control weekends in both non-atopic and atopic asthmatics.43 Taken together studies suggest that non-atopic patients can respond to previously unrecognized airborne antigens in a manner similar to atopic asthmatics, but in the absence of atopy.
There are limitations to our study, including that we do not have biologic measures to investigate mechanistic differences between atopic and non-atopic responses to indoor pollutant exposure. A study currently underway44, 45 is examining potential differences in the inflammatory and oxidative stress responses between non-atopic and atopic asthmatics. While it is challenging to assess exposure in all microenvironments, our measurement of indoor PM was performed in the home where children spent about half of their time (average of 12 hours) and most of this time was spent in the bedroom where the environmental equipment was placed. We were also able to adjust for ambient PM concentrations in our models. While we were not able to account for additional potential co-pollutants, we were able to adjust for ambient PM concentrations in our models. The classification of atopic status represents a single point in time during pre-school age and we acknowledge that atopic status may change in these children over time. However, we were able to perform skin testing using a comprehensive panel of allergens that represent common environmental exposures in this community. The size of the present study is a strength, providing an evaluation of atopic status in a large, well characterized cohort of patients with extensive environmental monitoring, overcoming some limitations of previous studies of indoor PM that included sample sizes of less than 20 subjects.11, 12, 15, 16
Guideline recommendations for the management of asthma include environmental control practices that focus largely on allergen avoidance for those with atopic asthma.46, 47 There are very few environmental control practice recommendations that address airborne pollutants, mainly due to the lack of strong evidence supporting a beneficial health effect of pollutant reduction. The present study demonstrates that exposure to indoor PM is associated with increased asthma morbidity among both atopic and non-atopic children. As there are relatively fewer competing causes for exacerbations of non-atopic asthma, environmental control practices that decrease exposure to pollutants may confer an even greater health benefit for this group. In the few major asthma intervention trials48, 49, non-atopic children have sometimes been excluded from participation.49 The inclusion of non-atopic children should be emphasized in future studies and comparison of responses to environmental control practices between non-atopic and atopic should be reported.
Conclusions
In -home PM concentrations were associated with increased asthma symptoms and the need for rescue medication among both non-atopic and atopic pre-school children living in inner-city Baltimore. This finding may be especially important for non-atopic asthmatics, as there are fewer alternative triggers compared to those with atopy. Future studies investigating the impact of interventions to reduce indoor PM and other indoor pollutants may be critical to better understanding the etiologic mechanism of non-atopic asthma and to providing evidence to support environmental modification recommendations for future iterations of current national and international asthma guidelines. In the meantime, strategies to reduce and eliminate sources of indoor particulate matter pollution46, 47 should be considered a priority in the management of non-atopic asthma.
Figure 4.
Multivariate analysis of the effect of indoor fine PM on asthma morbidity. Incidence rate ratios are displayed as point estimates and 95% confidence intervals for the effect of indoor PM2.5 on asthma symptom outcomes and rescue medication use. Models were adjusted for age, race, gender, parent education, season, indoor PM2.5–10, outdoor PM2.5, and outdoor PM2.5–10. There was an increase in the incidence of asthma morbidity outcomes for every 10μg/m3 increase in PM2.5 for most symptom outcomes and for rescue medication use among both non-atopic and atopic children.
Acknowledgments
This research was supported by the NIEHS (K23 ES 016819; PO1 ES 09606; P50 ES 015903), NIAID (R01 AI070630) and U.S. EPA (PO1 R-826724), and the Johns Hopkins NIEHS Center in Urban Environmental Health (P30 ES 03819).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beasley R, Pekkanen J, Pearce N. Has the role of atopy in the development of asthma been over-emphasized? Pediatr Pulmonol. 2001;(Suppl 23):149–150. [PubMed] [Google Scholar]
- 2.Pearce N, Pekkanen J, Beasley R. How much asthma is really attributable to atopy? Thorax. 1999;54(3):268–272. doi: 10.1136/thx.54.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostergaard PA. Non-IgE-mediated asthma in children. Acta Paediatr Scand. 1985;74(5):713–719. doi: 10.1111/j.1651-2227.1985.tb10019.x. [DOI] [PubMed] [Google Scholar]
- 4.Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Longo G, Panontin E, Ventura G. Non-atopic persistent asthma in children. Thorax. 2009;64(5):459. doi: 10.1136/thx.2007.084814corr1. [DOI] [PubMed] [Google Scholar]
- 6.Kattan M, Gergen PJ, Eggleston P, Visness CM, Mitchell HE. Health effects of indoor nitrogen dioxide and passive smoking on urban asthmatic children. J Allergy Clin Immunol. 2007;120(3):618–624. doi: 10.1016/j.jaci.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch T, Weiland SK, von ME, et al. Inner city air pollution and respiratory health and atopy in children. Eur Respir J. 1999;14(3):669–677. doi: 10.1034/j.1399-3003.1999.14c29.x. [DOI] [PubMed] [Google Scholar]
- 8.McCormack MC, Breysse PN, Hansel NN, et al. Common household activities are associated with elevated particulate matter concentrations in bedrooms of inner-city Baltimore pre-school children. Environ Res. 2008;106(2):148–155. doi: 10.1016/j.envres.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace LA, Mitchell H, O'Connor GT, et al. Particle concentrations in inner-city homes of children with asthma: the effect of smoking, cooking, and outdoor pollution. Environ Health Perspect. 2003;111(9):1265–1272. doi: 10.1289/ehp.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med. 2000;343(24):1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 11.Delfino RJ, Quintana PJ, Floro J, et al. Association of FEV1 in asthmatic children with personal and microenvironmental exposure to airborne particulate matter. Environ Health Perspect. 2004;112(8):932–941. doi: 10.1289/ehp.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mar TF, Larson TV, Stier RA, Claiborn C, Koenig JQ. An analysis of the association between respiratory symptoms in subjects with asthma and daily air pollution in Spokane, Washington. Inhal Toxicol. 2004;16(13):809–815. doi: 10.1080/08958370490506646. [DOI] [PubMed] [Google Scholar]
- 13.McConnell R, Berhane K, Gilliland F, et al. Prospective study of air pollution and bronchitic symptoms in children with asthma. Am J Respir Crit Care Med. 2003;168(7):790–797. doi: 10.1164/rccm.200304-466OC. [DOI] [PubMed] [Google Scholar]
- 14.McCormack MC, Breysse PN, Matsui EC, et al. In-home particle concentrations and childhood asthma morbidity. Environ Health Perspect. 2009;117(2):294–298. doi: 10.1289/ehp.11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koenig JQ, Mar TF, Allen RW, et al. Pulmonary effects of indoor- and outdoor-generated particles in children with asthma. Environ Health Perspect. 2005;113(4):499–503. doi: 10.1289/ehp.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trenga CA, Sullivan JH, Schildcrout JS, et al. Effect of particulate air pollution on lung function in adult and pediatric subjects in a Seattle panel study. Chest. 2006;129(6):1614–1622. doi: 10.1378/chest.129.6.1614. [DOI] [PubMed] [Google Scholar]
- 17.Diette GB, Hansel NN, Buckley TJ, et al. Home indoor pollutant exposures among inner-city children with and without asthma. Environ Health Perspect. 2007;115(11):1665–1669. doi: 10.1289/ehp.10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunekreef B, Forsberg B. Epidemiological evidence of effects of coarse airborne particles on health. Eur Respir J. 2005;26(2):309–318. doi: 10.1183/09031936.05.00001805. [DOI] [PubMed] [Google Scholar]
- 19.U.S.EPA (Environmental Protection Agency) [accessed 25 August 2006];Air Quality System Data Mart [database] 2006 Available: http://www.epa.gov/ttn/airs/aqsdatamart.
- 20.Gruchalla RS, Pongracic J, Plaut M, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115(3):478–485. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Matsui EC, Eggleston PA, Buckley TJ, et al. Household mouse allergen exposure and asthma morbidity in inner-city preschool children. Ann Allergy Asthma Immunol. 2006;97(4):514–520. doi: 10.1016/S1081-1206(10)60943-X. [DOI] [PubMed] [Google Scholar]
- 22.Phipatanakul W, Eggleston PA, Wright EC, Wood RA. Mouse allergen. II. The relationship of mouse allergen exposure to mouse sensitization and asthma morbidity in inner-city children with asthma. J Allergy Clin Immunol. 2000;106(6):1075–1080. doi: 10.1067/mai.2000.110795. [DOI] [PubMed] [Google Scholar]
- 23.Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8(3):483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 24.Asmussen L, Olson LM, Grant EN, Fagan J, Weiss KB. Reliability and validity of the Children's Health Survey for Asthma. Pediatrics. 1999;104(6):e71. doi: 10.1542/peds.104.6.e71. [DOI] [PubMed] [Google Scholar]
- 25.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 26.U.S. EPA (Environmental Protection Agency) National ambient air quality standards for particulate matter. Fed Reg. 1997;62(138):11. [Google Scholar]
- 27.Strachan DP, Cook DG. Health effects of passive smoking. 6. Parental smoking and childhood asthma: longitudinal and case-control studies. Thorax. 1998;53(3):204–212. doi: 10.1136/thx.53.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strachan DP, Butland BK, Anderson HR. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. BMJ. 1996;312(7040):1195–1199. doi: 10.1136/bmj.312.7040.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kershaw CR. Passive smoking, potential atopy and asthma in the first five years. J R Soc Med. 1987;80(11):683–688. doi: 10.1177/014107688708001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S. Department of Health and Human Services . The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2006. [Google Scholar]
- 31.Hansel NN, Breysse PN, McCormack MC, et al. A longitudinal study of indoor nitrogen dioxide levels and respiratory symptoms in inner-city children with asthma. Environ Health Perspect. 2008;116(10):1428–1432. doi: 10.1289/ehp.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McConnell R, Berhane K, Yao L, et al. Traffic, susceptibility, and childhood asthma. Environ Health Perspect. 2006;114(5):766–772. doi: 10.1289/ehp.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corrigan C. Mechanisms of intrinsic asthma. Curr Opin Allergy Clin Immunol. 2004;4(1):53–56. doi: 10.1097/00130832-200402000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Humbert M, Menz G, Ying S, et al. The immunopathology of extrinsic (atopic) and intrinsic (non-atopic) asthma: more similarities than differences. Immunol Today. 1999;20(11):528–533. doi: 10.1016/s0167-5699(99)01535-2. [DOI] [PubMed] [Google Scholar]
- 35.Jayaratnam A, Corrigan CJ, Lee TH. The continuing enigma of non-atopic asthma. Clin Exp Allergy. 2005;35(7):835–837. doi: 10.1111/j.1365-2222.2005.02283.x. [DOI] [PubMed] [Google Scholar]
- 36.Gehring U, Wijga AH, Brauer M, et al. Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life. Am J Respir Crit Care Med. 2010;181(6):596–603. doi: 10.1164/rccm.200906-0858OC. [DOI] [PubMed] [Google Scholar]
- 37.Janssen NA, Brunekreef B, van VP, et al. The relationship between air pollution from heavy traffic and allergic sensitization, bronchial hyperresponsiveness, and respiratory symptoms in Dutch schoolchildren. Environ Health Perspect. 2003;111(12):1512–1518. doi: 10.1289/ehp.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zmirou D, Gauvin S, Pin I, et al. Traffic related air pollution and incidence of childhood asthma: results of the Vesta case-control study. J Epidemiol Community Health. 2004;58(1):18–23. doi: 10.1136/jech.58.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D'Amato G, Liccardi G, D'Amato M, Cazzola M. Respiratory allergic diseases induced by outdoor air pollution in urban areas. Monaldi Arch Chest Dis. 2002;57(3–4):161–163. [PubMed] [Google Scholar]
- 40.Diaz-Sanchez D, Rumold R, Gong H., Jr. Challenge with environmental tobacco smoke exacerbates allergic airway disease in human beings. J Allergy Clin Immunol. 2006;118(2):441–446. doi: 10.1016/j.jaci.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 41.Takano H, Yanagisawa R, Ichinose T, et al. Diesel exhaust particles enhance lung injury related to bacterial endotoxin through expression of proinflammatory cytokines, chemokines, and intercellular adhesion molecule-1. Am J Respir Crit Care Med. 2002;165(9):1329–1335. doi: 10.1164/rccm.2108122. [DOI] [PubMed] [Google Scholar]
- 42.Saunders V, Breysse P, Clark J, Sproles A, Davila M, Wills-Karp M. Particulate Matter Induced Airway Hyperresponsiveness is Lymphocyte Dependent. Environ Health Perspect. 2010;118(5):640–6. doi: 10.1289/ehp.0901461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burney PG, Newson RB, Burrows MS, Wheeler DM. The effects of allergens in outdoor air on both atopic and nonatopic subjects with airway disease. Allergy. 2008;63(5):542–546. doi: 10.1111/j.1398-9995.2007.01596.x. [DOI] [PubMed] [Google Scholar]
- 44.Breysse PN. Center for Childhood Asthma in the Urban Environment: Domestic Indoor PM and Childhood Asthma Morbidity. The Johns Hopkins University School of Public Health [Awarded] 2007 Grant No. P50 ES015903. [Google Scholar]
- 45.McCormack MC. The Johns Hopkins University [Awarded] National Institute of Environmental Health Sciences; 2008. The Impact of Indoor Particulate Matter Exposure on Non-allergic Asthma. Grant No. K23 ES016819. [Google Scholar]
- 46.Global Initiative for Asthma GINA workshop report. [accessed May 4, 2010];Global strategy for asthma management and prevention. revised 2006, available at http://www.ginasthma.com/
- 47.National Heart, Lung, and Blood Institute, National Asthma Eduation and Prevention Program . Full Report 2007. National Institutes of Health, U.S. Department of Health and Human Services; Bethesda, MD: 2007. [accessed March 25 2010]. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. NIH Publication No. 07–4051. Available: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. [Google Scholar]
- 48.Eggleston PA, Butz A, Rand C, et al. Home environmental intervention in inner-city asthma: a randomized controlled clinical trial. Ann Allergy Asthma Immunol. 2005;95(6):518–524. doi: 10.1016/S1081-1206(10)61012-5. [DOI] [PubMed] [Google Scholar]
- 49.Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]