Abstract
The brain-derived neurotrophic factor (BDNF) gene contains 4 major 5′ promoters which generate alternate transcripts. Previously, we found that pan-BDNF mRNA and protein is reduced in the dorsolateral prefrontal cortex (DLPFC) from patients with schizophrenia. In this study, we determined which of the 4 most abundant BDNF alternate transcripts (I–IX, II–IX, IV–IX and VI–IX) are altered in schizophrenia. Using a cohort from the NIMH, USA, we found that BDNF II–IX mRNA was significantly reduced in the DLPFC of patients with schizophrenia, and we replicated this finding using a second cohort from Sydney, Australia. Moreover, we show that the mature BDNF protein is reduced in the DLPFC of patients with schizophrenia, confirming and replicating our previous findings. We next determined the regional specificity of these findings by measuring BDNF transcripts in the parietal cortex and hippocampus and found no significant changes in these two brain regions. Since patients with schizophrenia are often prescribed antidepressants which can up-regulate expression of BDNF, we investigated the relationship between antidepressant treatment and BDNF transcript expression. All four BDNF transcripts were significantly up-regulated in schizophrenics patients treated with antidepressants. When we removed cases with recorded use of antidepressants, this revealed significant reductions in BDNF II–IX and IV–IX in the parietal cortex and VI–IX in the hippocampus of patients with schizophrenia. This suggests that down-regulation of at least 1/4 major BDNF transcripts occurs in various brain regions of patients with schizophrenia, particularly in the DLPFC which appears to have the most robust BDNF deficit in schizophrenia.
Keywords: brain-derived neurotrophic factor, BDNF, postmortem, DLPFC, antidepressant, schizophrenic
Introduction
Schizophrenia is a devastating mental illness believed to arise from abnormal brain development. Mounting evidence suggests that a deficit in neurotrophin supply to cortical neurons may be an underlying factor in the pathophysiology of schizophrenia as adequate neurotrophic support is required for normal brain development, maturation and function (1–9). The neurotrophin, brain-derived neurotrophic factor (BDNF), a member of the nerve growth factor family of neurotrophins is most abundant in areas implicated in the pathology of schizophrenia, including the neocortex and hippocampus (10). In the prefrontal cortex of patients with schizophrenia, reductions in pan-BDNF mRNA and mature BDNF protein expression exist (11, 12). However, the molecular mechanism by which BDNF expression is altered in schizophrenia has yet to be described.
The BDNF gene contains multiple transcription start sites that produce alternative 5′ non-coding exons spliced onto one common 3′ exon encoding the preproBDNF protein (13, 14). These distinct alternate 5′ promoters enables up-regulation of BDNF during different developmental periods, under distinct environmental and regional demands and may allow for differential subcellular targeting (15, 16) Because only a subset of BDNF mRNAs are targeted to the dendrites, interneurons that synapse on dendrites, may rely more heavily upon particular BDNF transcripts for their differentiation, maintenance or survival. While reductions in BDNF expression may impact both glutamatergic and GABAergic cortical neurons, BDNF is synthesized by glutamatergic neurons in cortex (12, 17, 18) and deficits in BDNF supply negatively impact GABAergic neuron density, the level of GAD67 mRNA expression, the number of GABAergic terminals, and the expression levels of GABA neuron markers such as somatostanin and parvalbumin (19–23). For glutamatergic neurons, a reduction in cortical BDNF expression is known to decrease dendritic branching and weaken synaptic transmission (8, 24). Moreover, key pathological findings in schizophrenia, including reductions in GAD67, parvalbumin mRNA and somatostatin mRNA, reduced dendritic branching and reduced somal size of pyramidal neurons point to a deficit in BDNF signaling (11, 25). We predicted that alterations affecting one or more of the 4 major BDNF promoters (I, II, IV, VI) may underlie the decrease in pan-BDNF mRNA and mature BDNF protein expression in schizophrenia. Thus, in this current study, determined which of the 4 major BDNF transcripts (I–IX, II–IX, IV–IX, and VI–IX) generated from their respective promoters is/are altered in the dorsolateral prefrontal cortex (DLPFC) of patients with schizophrenia. To determine if changes occurring in the DLPFC were anatomically specific, we examined BDNF transcripts in the parietal cortex and hippocampus. The parietal cortex is a neocortical association area showing grey matter and functional deficits in patients with schizophrenia (26, 27). In schizophrenia, abnormalities in hippocampal structure and function, histology, morphometry, neurochemistry and gene expression have been demonstrated (28, 29). Moreover, reductions or no change in BDNF protein in the hippocampus of patients with schizophrenia has been reported, suggesting that further studies using complementary techniques to measure BDNF are warranted (30, 31).
Long-term treatment with antidepressants, including selective serotonin reuptake inhibitors (32–35), selective norepinephrine reuptake inhibitors (36, 37), tricyclic and tetracyclic antidepressants (35, 38, 39); and monoamine oxidase inhibitors (35, 40) have been shown to up-regulate pan-BDNF mRNA and mature BDNF protein in the prefrontal cortex and hippocampus of rodents and mice; moreover, the increase in pan-BDNF mRNA has been shown to occur via recruitment of specific 5′ promoter(s) (36, 41, 42). Thus, we hypothesized that alterations in BDNF transcript expression in the diseased state may be influenced by antidepressant usage.
Materials and Methods
Cohorts
The demographic data for each subject of the Section on Neuropathology of the Clinical Brain Disorders Branch (CBDB) at the National Institute of Mental Health, Bethesda, MD (herein the CBDB cohort) are listed in Table 1. Sixty eight cases of 34 normal controls and 34 patients with schizophrenia meeting DSM-IV criteria for schizophrenia (30)/schizoaffective (4) (Table S1) were obtained through the Offices of the Chief Medical Examiner of District of Columbia and of Northern Virginia. Brain collection and tissue preparation have been carried out as described previously by us (43).
Table 1.
Brain Cohort Demographics
| CBDB Cohort | TRC Cohort | |||
|---|---|---|---|---|
| Control | Schizophrenia | Control | Schizophrenia | |
| Age | 44.5 ± 2.51 | 45.8 ± 2.63 | 51 ± 2.40 | 51.3 ± 2.32 |
| Gender | 24M/10F | 23M/11F | 30M/7F | 24M/13F |
| Hemisphere | 27L/5R | 27L/6R | 14L/23R | 20L/17R |
| pH | 6.55 ± 0.05 | 6.51 ± 0.04 | 6.66 ± 0.05 | 6.61 ± 0.05 |
| PMI | 31.4 ± 2.00 | 32.8 ± 1.99 | 24.8 ± 1.80 | 28.8 ± 2.31 |
| DLPFC RIN | 6.50 ± 0.29 | 6.16 ± 0.33 | 7.30 ± 0.09 | 7.27 ± 0.10 |
| PC RIN | 7.65 ± 0.13 | 7.06 ± 0.17 | - | - |
| HP RIN | 5.33 ± 0.21 | 4.97 ± 0.24 | - | - |
| Age of Onset | - | 22.06 ± 1.18 | - | 23.7 ± 0.10 |
| DOI (days) | - | 7083.88 ± 711.34 | - | 10081.89 ± 829.22 |
| Daily (Mean) CPZ (mg) | - | 486.15 ± 50.96 | - | 691.64 ± 82.56 |
| Lifetime CPZ | - | 3702673.21 ± 534515.96 | - | 7610653.47 ± 1340016 |
| Last CPZ Dose (mg) | - | 546.22 ± 85.97 | - | 483.76 ± 66.20 |
Demographics are presented as mean values +/− SEM. M=male, F=female, L=left, R=right, DOI=duration of illness, CPZ=chlorpromazine PC=parietal cortex, HP=hippocampus, DLPFC=dorsolateral prefrontal cortex
The demographic data for each subject of the Tissue Resource Centre (TRC) cohort from Sydney, Australia are listed in Table 1. Seventy four cases of 37 non-psychiatric, substance abuse-free controls and 37 patients with schizophrenia meeting DSM-IV criteria for schizophrenia (30)/schizoaffective (7) (Table S1) were obtained from the New South Wales TRC. Brain collection, clinical chart review, agonal state determination and tissue preparation have been carried out as described previously by us (44).
For this study, we excluded cases from both cohorts if they were over 78 years of age, if brain pH was less than 5.7 and if the postmortem interval (PMI) was greater than 60 hours. This resulted in 34 cases for the CBDB cohort and 37 cases for the TRC cohort (see flow chart for reviewers only). For both the CBDB and TRC cohorts, schizophrenic cases were matched for demographic variables known to affect BDNF expression on a case by case basis in the following order of importance, subject age at death (within 10 years), brain pH (within 0.59 pH units) and PMI (within 22 hours). Diagnostic groups did not differ according to these variables (all p>0.20).
Anatomical Dissections
Anatomical dissections of the DLPFC, parietal cortex and hippocampus for the CBDB cohort and DLPFC for the TRC cohort are detailed in supplemental materials and methods.
Medication
Medication history was gathered in two ways, by toxicology analysis of blood and/or cerebellar samples and by examination of all recorded doses of psychiatric medications available in psychiatric/medical records of patients with schizophrenia. Neuroleptic dosages were converted to last, daily and lifetime chlorpromazine (CPZ) equivalent doses in milligrams, as previously described (45–47). Schizophrenic cases in the two cohorts were exposed to both typical and atypical antipsychotics (Table S2) and ~50% of cases were exposed to both classes of antipsychotics. Schizophrenic individuals were first coded as positive or negative for current antidepressant treatment at time of death based on quantitative data from toxicology screenings. While toxicology data provides a confirmation of acute antidepressant exposure, psychiatric records may provide a better measure of chronic antidepressant exposure. Thus, we tested for the effects of antidepressants in two ways: 1) grouping those individuals who were positive for antidepressants at the time of death based on toxicology alone versus those that were not and 2) grouping those individuals who were positive for antidepressants according to their medical records and police reports versus those who had no medical history of exposure to antidepressants. Indexing antidepressant by records alone has been found by our group and by others to increase pan-BDNF mRNA and mature BDNF protein levels (31, 48). Schizophrenic cases from both cohorts were exposed to several classes of antidepressants including selective serotonin reuptake inhibitors, selective norepinephrine reuptake inhibitors, tricyclic antidepressants and tetracyclic antidepressants (Table S2). Analyses were conducted on schizophrenic cases positive for antidepressants based on toxicology, positive for antidepressants based on medical history, and positive for antidepressants based on medical history or toxicology (this includes those few cases which were positive for antidepressant usage based on medical history but negative by toxicological screening and vice versa).
RNA Extraction and cDNA Synthesis
For all brain regions for both cohorts, total RNA was extracted from ~300mg of frozen tissue using a modified version of the TRIZOL Reagent method (Invitrogen, Carlsbad, CA) as previously described (49). RNA integrity (RIN) was assessed with high resolution capillary electrophoresis (Agilent Technologies, Palo Alto, CA). Three separate aliquots of 3μg RNA were reverse transcribed to synthesize cDNA by random hexamer priming using the SuperScript First-Strand Synthesis System according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA), pooled and diluted for RT-PCR.
Quantitative Real-Time PCR (qPCR)
BDNF mRNA levels from the 4 distinct transcripts in brain were measured by qPCR using an ABI Prism 7900HT Fast Real-Time PCR System with a 384-well format as previously described (50). Control probes or housekeeping genes (n=4) used to calculate the geometric means of the two cohorts have previously been described (43, 44). The geometric mean of the 4 housekeeping genes used was calculated as described previously (51). The transcripts arising from the 4 best characterized BDNF exons (13) were targeted by the following ABI probes: BDNF I–IX (Cat# hs00538277-m1), BDNF II–IX (Cat# hs00538278-m1), BDNF IV–IX (Cat# hs00380947-m1) and BDNF VI–IX (Cat# hs00156058-m1). Either 9ng of cDNA (I–IX, II–IX and IV–IX) or 90ng (VI–IX) of each sample were run in triplicate with an 8 point standard curve using serial dilutions of pooled cDNA (from all cases) under standard cycling conditions (50). Several no template controls were also included which produced no signal. Sequence Detector Software (SDS version 2.0, Applied Biosystems) software plotted real-time fluorescence intensity. The threshold was set within the linear phase of the amplicon profiles. None of the housekeeping genes varied between the diagnostic groups (p>0.05 for all housekeeping genes and geometric mean). The geometric mean was used for data normalization and the normalized levels of BDNF in the control group were set to 100%.
Western Blotting
Protein extraction and BDNF protein expression was measured as previously described (50). Briefly, samples of equal protein (10μg) were analyzed by SDS-PAGE on 12% Bis-Tris gels (BioRad). The primary antibody used to detect human BDNF (Santa Cruz Cat# sc-546, previously characterized for use on human postmortem brain tissue (52)).β-actin (Chemicon International Cat# MAB1501) was probed as a protein loading control. Bands were visualized on a Chemidoc Imaging System (BioRad) and quantitated by densitometry using the Quantity One 1-D Analysis Software v4.6.5 (BioRad).
Statistical Analysis
Statistical analyses were conducted using Statistica 7 (StatSoft Inc., 2000, STATISTICA for Windows). Tests for normality and homogeneity of variance were conducted and two-tailed or one-tailed unpaired t-tests (only for replication using the TRC cohort) were used to compare normalized BDNF mRNA levels between control and schizophrenics. To exclude measurement errors, we conducted outlier detection of the triplicates obtained from the qPCR raw data by determining the percent variance amongst each of the replicates. If one replicate showed greater than 50% variance, the replicate was removed and duplicate measures were used. If the duplicate measures still showed greater than 50% variance, the entire sample was removed from the cohort (for all regions in the CBDB cohort: on average 1–2 subjects/probe/diagnostic group were dropped; for the TRC cohort: on average 2–3 subjects/probe/diagnostic group were dropped). As a test for population outliers, cases greater than 2 standard deviations from the mean for that diagnostic group were excluded from analyses (no more than two subjects/diagnostic group were dropped. Pearson-Product Moment correlations were run to compare cohort characteristics (including CPZ equivalent in the schizophrenia group) and normalized BDNF mRNA expression. Where a relationship existed, analysis of covariance (ANCOVA) was also calculated. A two-tailed unpaired t-test or ANOVA was used to determine the influence of antidepressants and manner of death on the 4 major BDNF transcripts.
Results
Demographic Variables and mRNA Controls
Detailed description and analyses of the CBDB and TRC cohorts used in this study with regards to gene expression for the housekeeping genes and demographic variables can be referenced from Lipska et al. (43) and Weickert et al. (44) respectively. The geometric mean of the mRNAs used for normalization showed no significant difference between diagnostic groups (CBDB cohort: all brain regions t>−1.23, df>57, p>0.20; TRC cohort: t=0.45, df=72, p=0.66).
Alterations in BDNF Transcript II–IX Expression in the DLPFC of Schizophrenic Subjects
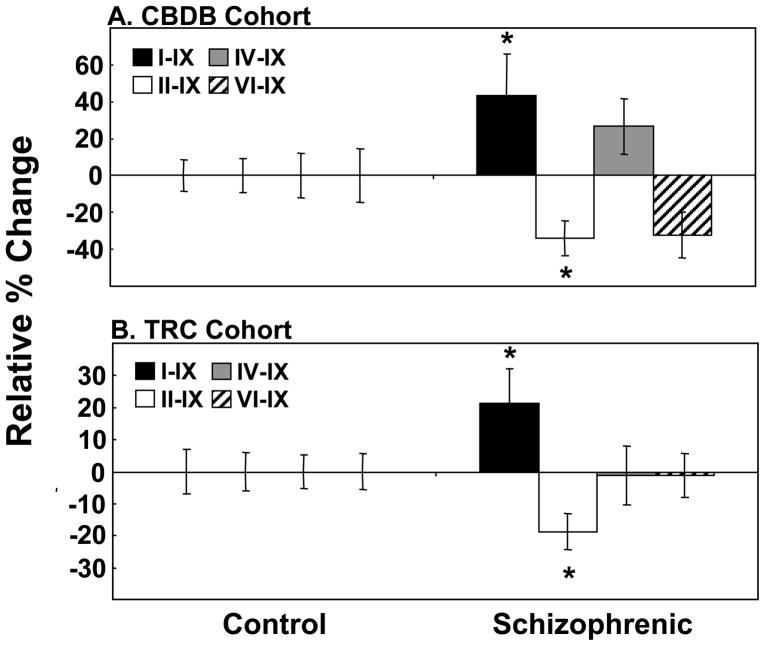
We found a robust reduction in BDNF transcript II–IX in schizophrenic cases compared to controls (34% decrease, t=−2.56, df=62, p=0.01) (Figure 1A). Unexpectedly, expression of BDNF transcript I–IX showed a slight elevation in schizophrenic cases although this did not reach statistical significance (43% increase, t=1.74, df=63, p=0.09) (Figure 1A). Expression of BDNF transcript IV–IX and VI–IX showed no significant change in schizophrenic cases (27% increase, t=1.39, df=63, p=0.17 and 32% decrease, t=−1.69, df=64, p=0.10, respectively).
Figure 1. BDNF Alternate Transcript Expression in the DLPFC of Patients with Schizophrenia.
Expression of the 4 major BDNF transcripts: I–IX (black bars), II–IX (white bars), IV–IX (grey bars) and VI–IX (hatched bars) were measured by qPCR. (A) CBDB cohort and (B) TRC cohort. Data is expressed as percent change relative to the control group and presented as mean −/+ SEM. Significance between groups was *p<0.05 or lower. Note: BDNF transcript I–IX was significant by ANCOVA- CBDB: F=4.59, df=1, 62, p=0.04 and TRC: F=4.42, df=1, 66, p=0.04.
In a second independent cohort of control and schizophrenic cases (TRC cohort) we observed a significant reduction in BDNF transcript II–IX (19% decrease, t=−2.29, df=63, p=0.02) and a trend towards an increase in BDNF transcript I–IX (21% increase, t=1.63, df=67, p=0.06) (Figure 1B) in the DLPFC. Expression of BDNF transcripts IV–IX and VI–IX showed no significant change between control and schizophrenic cases (both t<−0.11, df>59, p≥0.90).
Mature BDNF Protein is Decreased in the DLPFC of Schizophrenic Subjects
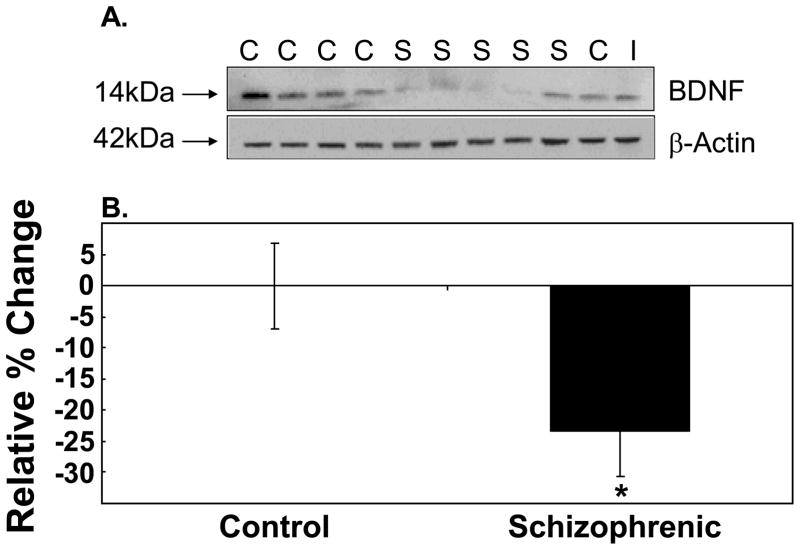
In this current study, we confirmed and replicated the reduction in the mature form of the BDNF protein in the DLPFC of patients with schizophrenia (12) using the TRC cohort. We found immunoreactive bands migrating at ~14kDa corresponding to the mature form of BDNF in both control and schizophrenic cases (Figure 2A). The overall band intensities from the schizophrenic cases were significantly reduced compared to controls, and quantitation revealed a 23% reduction in BDNF immunoreactive levels (t=−2.64, df=70, p=0.01, two tail) (Figure 2B). Levels of β-actin did not vary between control and schizophrenics (t=1.10, df=72, p=0.27). Expression of the mature BDNF protein was positively correlated with pH, and ANCOVA analysis with pH as a covariate retained the significance of the BDNF protein reduction in schizophrenia (F=6.29, df=1, 69, p=0.01). No significant correlation of BDNF protein expression with age, PMI, RIN or agonal state was observed (all p>0.05). Sex and brain hemisphere had no impact on BDNF protein levels in the DLPFC (all p>0.05).
Figure 2. Expression of the Mature BDNF protein in the DLPFC of Patients with Schizophrenia (TRC Cohort).
(A) A representative western blot containing both control and schizophrenic cases was immunoprobed for the ~14kDa mature BDNF protein. β-actin was probed as a loading control and an internal control (I) was used for normalization between western blots. (B) Bands were quantitated by densitometry. Expression of the mature BDNF protein is expressed as percent change relative to the control group and presented as mean −/+ SEM.
Alterations in BDNF Alternate Transcript Expression in Other Brain Regions in Schizophrenia
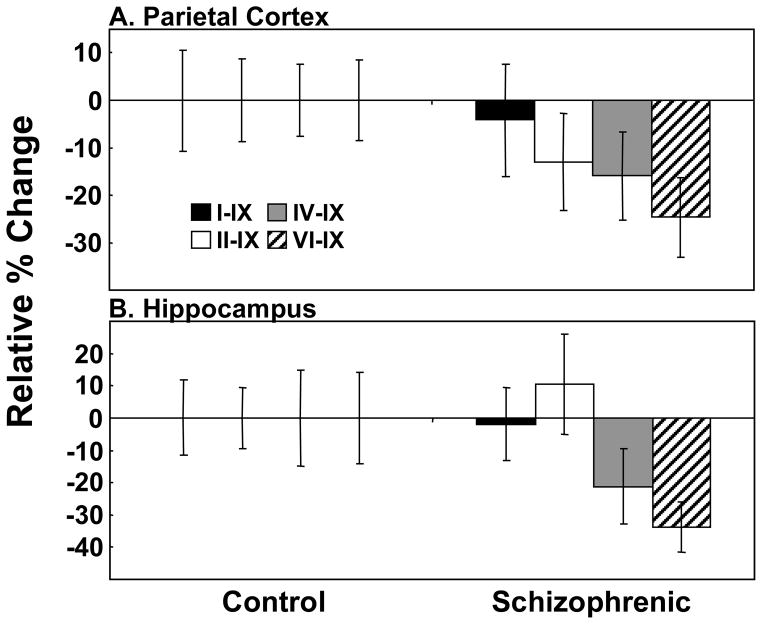
No significant changes were observed in any of the 4 major BDNF alternate transcripts in the parietal cortex (Figure 3A) and hippocampus (Figure 3B) in cases with schizophrenia. However, there was a trend towards reduced expression in BDNF transcripts VI–IX in both regions (parietal cortex: VI–IX: t=−1.90, df=56, p=0.06; hippocampus: VI–IX: t=−1.91, df=53, p=0.06).
Figure 3. BDNF Alternate Transcript Expression in the Parietal Cortex and Hippocampus of Patients with Schizophrenia (CBDB Cohort).
Expression of the 4 major BDNF transcripts: I–IX (black bars), II–IX (white bars), IV–IX (grey bars) and VI–IX (hatched bars) in (A) the parietal cortex and (B) hippocampus were measured by qPCR. Data is expressed as percent change relative to the control group and presented as mean −/+ SEM.
Effect of Antidepressants on BDNF Alternate Transcript Expression in Schizophrenia
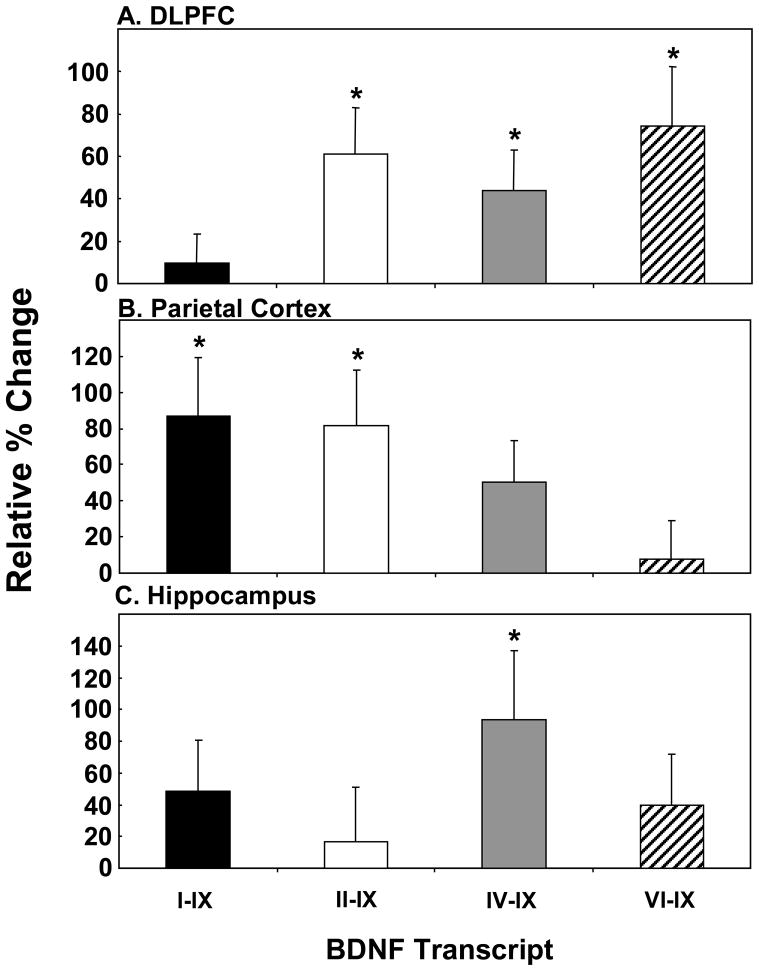
In the DLPFC, we found that antidepressant usage based on toxicology had no significant effect on BDNF alternate transcript expression in schizophrenia (Figure S1). In contrast, we found that antidepressant treatment based on medical history was associated with a robust increase in the expression of specific BDNF transcripts (Figure 4A). We found significant up-regulation of BDNF transcript II–IX (t=2.31, df=62, p=0.02), IV–IX (t=2.52, df=66, p=0.03) and VI–IX (t=3.70, df=63, p=0.03) in the DLPFC for those patients with recorded use of antidepressants. When schizophrenic cases positive for toxicology or history were combined, we observed the same significant up-regulation of BDNF transcripts II–IX, IV–IX and VI–IX (all p<0.05). This would suggest that chronic antidepressant usage gathered via psychiatric records spanning many years may better capture the cumulative lifetime effects of antidepressants on BDNF alternate transcript expression than using toxicology alone.
Figure 4. Effect of Antidepressants on BDNF Alternate Transcript Expression in the DLPFC, Parietal Cortex and Hippocampus of Schizophrenia Cases with Reported Use of Antidepressants.
Expression of the 4 major BDNF transcripts: I–IX (black bars), II–IX (white bars), IV–IX (grey bars) and VI–IX (hatched bars) in the (A) DLPFC, (B) parietal cortex and (C) hippocampus were measured by qPCR. For the DLPFC, data for the TRC and CBDB cohorts were pooled for analysis. For the parietal cortex and hippocampus, the CBDB cohort was used. Data is expressed as percent change relative to the control group and presented as mean + SEM. Significance between groups was *p<0.05 or lower.
We next examined the effect of chronic antidepressant treatment (based on history) on the parietal cortex and hippocampus. In the parietal cortex, we found significant elevations in BDNF transcripts I–IX (t=2.55, df=25, p=0.02) and II–IX (t=2.56, df=24, p=0.02) in schizophrenic cases positive for antidepressant treatment (Figure 4B). In the hippocampus, only BDNF transcript IV–IX was significantly increased (t=2.16, df=24, p=0.04) (Figure 4C) in those subjects with a positive history of antidepressant usage.
Since reductions in BDNF transcript expression in schizophrenia may be masked by chronic antidepressant treatment, we selected the subset of patients with schizophrenia having no evidence of antidepressant treatment to analyze. Removal of the cases who were antidepressant positive increased the statistical significance of the BDNF transcript II–IX down-regulation in the DLPFC of patients with schizophrenia compared to controls (t=−3.09, df=53, p=0.003) (Figure S2). In the parietal cortex, significant decreases in BDNF transcripts II–IX and IV–IX (II–IX: t=−2.17, df=44, p=0.04; IV–IX: t=−2.04, df=46, p=0.05); and a trend towards reduction of BDNF transcript VI–IX (t=1.78, df=46, p=0.08) was found. In the hippocampus a significant reduction in BDNF transcript VI–IX (t=−2.02, df=46, p=0.05) in patients with schizophrenia (compared to controls) was revealed.
Effect of Demographic Variables
TRC Cohort-DLPFC
Correlation analyses of BDNF measurements and cohort characteristics for the TRC cohort are detailed in Table S3A. With the exception of BDNF transcript I–IX, all other transcripts correlated negatively with age. Interestingly, all 4 major BDNF transcripts correlated positively with pH; however, only BDNF transcripts II–IX and IV–IX showed significant positive correlations with RIN, and only BDNF transcripts I–IX and VI–IX showed significant positive correlations with brain weight. BDNF transcript I–IX showed a positive correlation with PMI; whereas BDNF transcript II–IX was the only transcript to correlate negatively with agonal state.
We next examined whether changes observed in BDNF alternate transcript expression in schizophrenia were influenced by those demographic variables which correlated significantly. With the exception of BDNF transcript I–IX, changes in all other transcripts were not altered when co-varied with demographic variables which correlated significantly (Table S3A). BDNF transcript I–IX showed a significant increase in expression in schizophrenia cases when expression was co-varied for brain weight (F=4.42, df=1, 66, p=0.04) (Figure 1B). Interestingly, when we took diagnosis into account, the significance in BDNF transcript I–IX correlation with brain weight was observed only in cases with schizophrenia (r=0.48, p=0.004).
CBDB Cohort
Correlation analyses for the CBDB cohort are detailed in Table S3B. In the DLPFC, with the exception of BDNF transcript II–IX, BDNF transcripts showed significant negative correlations with age. BDNF transcript II–IX was the only transcript to correlate positively and significantly with pH in the DLPFC. In the parietal cortex, BDNF transcripts IV–IX and VI–IX were negatively correlated with age. While BDNF transcripts I–IX and IV–IX were positively correlated with pH and RIN, BDNF transcripts II–IX and VI–IX were only significantly correlated with RIN. In the hippocampus, with the exception of BDNF transcript IV–IX which showed a positive correlation with RIN, no significant association of the other BDNF transcripts with demographic variables was observed.
With the exception of BDNF transcript I–IX in the DLPFC, the significance of changes in BDNF alternate transcript expression in all three brain regions were not altered by co-variation with demographic variables. In the DLPFC, BDNF transcript I–IX showed a negative correlation with age. When age was co-varied, we observed a significant increase in BDNF I–IX mRNA expression in patients with schizophrenia (F=4.59, df=1, 62, p=0.04) (Figure 1A). When diagnosis was accounted for, we found that BDNF I–IX expression in both control and schizophrenia cases correlated negatively with age (control: r=−0.71, p=0.000006; schizophrenia: r=−0.41, p=0.02).
Relationship Between BDNF Expression and Clinical Variables
In the TRC cohort, BDNF protein expression in the DLPFC was not significantly correlated with duration of illness or any estimated measure of antipsychotic exposure (all p>0.05). BDNF transcripts II–IX, IV–IX and VI–IX showed highly significant negative correlations with duration of illness (II–IX: r=−0.64, p=0.00007; IV–IX: r=−0.50, p=0.002; VI–IX: r=−0.41, p=0.02). However, as duration of illness is a function of age, we controlled for the effect of age by partial correlation. Partial correlation with age removed the significance in correlation between BDNF transcripts II–IX, IV–IX and VI–IX with duration of illness. No antipsychotic medication exposure estimate (daily, lifetime, last CPZ) correlated with BDNF alternate transcript expression levels (all p>0.05).
In the CBDB cohort, in the DLPFC, only BDNF transcript I–IX was significantly correlated (r=−0.46, p=0.01; by partial correlation) with duration of illness. In the parietal cortex, none of the 4 major BDNF alternate transcripts correlated with duration of illness (all r<0.13, all p<0.37; partial correlation). BDNF alternate transcript expression in the hippocampus showed no correlation with duration of illness. Interestingly, only BDNF transcript I–IX showed a significant negative correlation with lifetime CPZ in the DLPFC (r=−0.37, p=0.04). However, this effect was lost when the effect of age was partialed for. No estimate of neuroleptic exposure correlated with any of the other BDNF transcripts in the DLPFC. In the parietal cortex and hippocampus, none of the 4 BDNF alternate transcripts correlated significantly with any measure of neuroleptic exposure. Moreover, schizophrenic cases positive for antipsychotics by toxicology at time of death showed no significant change in any BDNF transcript in any brain region as compared to those negative for antipsychotics by toxicology (all brain regions: t>−1.09, df>24, p>0.21).
Expression of BDNF has also been reported to be influenced by the manner of death (53, 54). Comparison of subjects with schizophrenia who had died by suicide to those subjects with schizophrenia who died by natural causes to controls revealed a significant change in BDNF transcript I–IX (F=3.13, df=2, 31, p=0.047) with suicide completers having significantly increased levels compared to controls (p=0.02). Similar trends were found for BDNF II–IX and IV–IX (p=0.06 and 0.07 respectively).
Discussion
In this current study, we report several major findings. We replicated the reduction in mature BDNF protein expression in the DLPFC of patients with schizophrenia (12) and extended this by examining the 4 major BDNF alternate transcripts. BDNF transcript II–IX was significantly reduced in the DLPFC of patients with schizophrenia compared to controls in two independent cohorts. This decrease in BDNF II–IX initially appeared specific to the DLPFC as examination of the parietal cortex and hippocampus showed no significant diagnostic difference in any of the 4 major transcripts. However, significant reductions in BDNF transcripts in the parietal cortex (II–IX and IV–IX) and hippocampus (VI–IX) in the subset of patients with schizophrenia without history of antidepressant treatment were found. The attenuation of transcription from promoter VI was observed in all 3 brain regions, reaching significance only in the hippocampus, but suggesting diminished transcription from this stress-sensitive promoter may be ubiquitous (55).
While we observe disease-associated changes in BDNF alternate transcript expression in schizophrenia, our study, like others, is limited as changes in BDNF expression associated with specific pathological changes in schizophrenia is inevitably confounded by epiphenomena including nicotine, environmental deprivation and repeated stress. Thus, while we were able to determine specific changes in BDNF alternate transcript expression between control and schizophrenics as well as separate out the effects of antidepressants, and to an extent, manner of death and antipsychotic treatment, we acknowledge that other factors linked to schizophrenia rather than just the underlying cause of schizophrenia may be influencing the changes in BDNF expression we detect.
Implications for Reduced Expression of Specific BDNF Transcripts in Schizophrenia
A reduction in neurotrophic support, particularly BDNF II–IX, may be an upstream event in the pathophysiology of schizophrenia. Alterations in specific BDNF promoters would impact neuronal survival and maturation during the critical phases of brain development. In the human prefrontal cortex, we have previously shown that expression of the 4 alternative BDNF transcripts are regulated in a developmental-specific manner and that expression is highest in cortical layers V and VI, and as the cortex matures with age, the levels of BDNF mRNA increases in layer IV especially for BDNF transcript II–IX (50). Recently, different transcripts have been shown to be targeted to particular sub-cellular regions of neurons in the rodent hippocampus (15, 56). The BDNF mRNAs with long 3′ UTRs are targeted to the dendrites where they help to maintain dendritic spines; and the BDNF with short 3′ UTRs are biased towards somal localization (56). Exon II containing BDNF mRNAs were reported to be significantly enriched in the long dendrite-targeting form whereas exons IV and VI containing BDNF mRNAs were reported to be enriched in the short form (56). Thus, together, our findings of decreased BDNF II–IX expression in the DLPFC and parietal cortex of patients with schizophrenia would imply that dendrite-targeting of BDNF mRNA may be compromised in the pyramidal cortical neurons in schizophrenia. Similarly, the reduction in BDNF transcripts IV–IX in the parietal cortex and transcript VI–IX in the hippocampus suggest that somal targeted BDNF, and hence neurons targeting the cell body of pyramidal neurons in these brain regions may be compromised in schizophrenia, especially in those individuals not prescribed antidepressants.
Effect of Medication
Neuroleptics
We found that in general the 4 major BDNF alternate transcripts did not correlate significantly with estimates of antipsychotic exposure nor were significant changes observed in patients with schizophrenia positive or negative for antipsychotic treatment by toxicology at time of death. This is consistent with previous studies by us and by others (11, 12, 31) showing no correlation between estimated antipsychotic exposure and BDNF expression. However, other studies have reported differential effects in BDNF mRNA expression following antipsychotic exposure (for review see (57)). In animals, acute and chronic treatment with both clozapine and haloperidol has been shown to significantly reduce expression of BDNF in the hippocampus, but not in the prefrontal cortex (58, 59). In contrast, others have reported that chronic treatment with the atypical antipsychotics, clozapine and olanzapine is associated with increased hippocampal BDNF expression (60, 61). This modulatory effect of atypical antipsychotics on BDNF mRNA may depend on the dose of antipsychotic administered as atypical antipsychotics supplied at high and low dose exhibit differential effects on the 5-HT2-D2 receptor pathways which have been shown to affect BDNF expression (58). Our finding that antipsychotic medications have no significant effect on BDNF expression in both cohorts may have resulted from reciprocal effects of typical vs. atypical antipsychotics on BDNF expression. However, we cannot be certain that the changes we detect in BDNF were not due at least in part to ante-mortem antipsychotic exposure.
Antidepressants
A major finding we report is that transcription at specific 5′ BDNF promoters in the DLPFC, parietal cortex and hippocampus are increased in postmortem brain tissue from patients with schizophrenia with chronic antidepressant treatment based on history. The majority of studies in humans and animals have shown that pan-BDNF transcript expression is increased with chronic antidepressant treatment (31, 35, 36, 39, 42, 62–69). We find that these reported increases in BDNF expression may be due to increased activity at specific human BDNF promoters depending on the brain region. Our comparison of the effects of antidepressants on BDNF alternate transcript expression based on toxicology and medical records showed that transcript expression was influenced moreso by documented chronic history of antidepressant treatment, congruent with our previous findings (31, 48).
In rodents, antidepressants were found to increase transcription at promoters I, III (human: IV) and IV (human: VI) in the frontal and parietal cortices, whereas promoter I, II and IV (human: VI) have been shown to be up-regulated by antidepressants in the hippocampus (36, 70). These are consistent with our current findings in humans as we observed increased expression of BDNF transcripts I–IX, II–IX and VI–IX in the DLPFC and/or parietal cortex for those schizophrenic cases exposed to antidepressants. This would suggest that there is molecular conservation of transcriptional control across species and that the ability of patients with schizophrenia to up-regulate BDNF mRNA through the pathways activated by antidepressants in the cortex is intact. However, we failed to find a significant up-regulation of BDNF transcripts I, II or IV in the hippocampus of patients with schizophrenia with a history of antidepressant usage as would be predicted from rodent studies. This would suggest that antidepressants may be more variable in their effect in the hippocampus of patients with schizophrenia. Taken together, our findings suggest that BDNF expression in the human brain may be up-regulated by antidepressant treatment in patients with schizophrenia and that antidepressants may be able to stimulate particular human BDNF promoters depending on the brain region.
Considerations
In this study, we observed a reduction in specific BDNF transcripts in the brains of patients with schizophrenia. However, it should be noted that reductions in BDNF transcript expression is also present in other psychiatric illnesses. Alterations in BDNF expression has been suggested to play a contributing role in the pathophysiology of depression and suicide (reviewed in (54)). Numerous studies have demonstrated reduced expression of BDNF in depressed and suicidal patients, and in postmortem human brain studies, reductions in BDNF mRNA and protein expression in the hippocampus and DLPFC have been shown (53, 54). Moreover, the reduction in BDNF expression appeared specific to these brain regions as examination of the entorhinal cortex showed no significant difference between patients and normal controls (52). These findings are in contrast to our current findings as we observed an up-regulation of BDNF transcripts in the DLPFC in those subjects with schizophrenia who committed suicide. However, it should be noted that in both the cohorts used in this study, almost half the schizophrenic subjects who had died by suicide were also positive for antidepressant usage (44%) such that the impact of medication and manner of death can not be adequately analyzed separately.
Summary
In this current study, we have robustly demonstrated that frontal cortical reductions in pan-BDNF mRNA and mature BDNF protein expression in schizophrenia may be explained by reduced expression of at least one specific BDNF transcript (II–IX and with some contribution from VI–IX) in the diseased state. As all transcript variants of BDNF give rise the same protein, our finding of reduced protein and consistent reduction in BDNF transcript II–IX may suggest that this variant is the main transcript contributing to the BDNF protein reduction in the DLPFC of patients with schizophrenia. While we note that multiple contributing factors including suicide, medication, and even brain region may alter BDNF protein expression by modulating transcript expression at the different 5′ promoters, the consistency of reduced mRNA (II–IX) after controlling for medication and manner of death, indicate that reductions in cortical BDNF expression may be an underlying pathological feature in schizophrenia. This suggests that future work could be focused on developing a more detailed understanding of the molecular control of BDNF gene regulation at these specific promoters in patients with schizophrenia. As BDNF is critical for neuronal survival, development and maturation, reduced activity at specific BDNF 5′ promoters may have functional consequences on the targeted availability of BDNF to brain regions and neurons which require adequate BDNF supply. Our finding that antidepressant treatment can up-regulate the BDNF transcripts which are reduced in schizophrenia suggests that use of antidepressants may be of benefit in reversing the deficit in BDNF supply in schizophrenia. However, it should be noted that treatment with antidepressants does not appear to normalize the most deficient BDNF transcript (II–IX) within the DLPFC, although it may be more effective at normalizing BDNF transcripts in other brain regions, such as the parietal cortex and hippocampus.
Supplementary Material
Acknowledgments
Debora Rothmond, Shan Yuan-Tsai, Duncan Sinclair and Alice Rothwell for advice and technical support; Dr Barbara Lipska and Dr Mary Herman for their contribution. This work was supported by the NIMH IRP, Schizophrenia Research Institute, New South Wales Health, Macquarie Group Foundation, Prince of Wales Medical Research Institute, and the University of New South Wales. JW is supported by the National Health and Medical Research Council Postdoctoral Training Fellowship (568884).
References
- 1.McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 2.Thome J, Foley P, Riederer P. Neurotrophic factors and the maldevelopmental hypothesis of schizophrenic psychoses. Review article. J Neural Transm. 1998;105:85–100. doi: 10.1007/s007020050040. [DOI] [PubMed] [Google Scholar]
- 3.Hyman C, Juhasz M, Jackson C, Wright P, Ip NY, Lindsay RM. Overlapping and distinct actions of the neurotrophins BDNF, NT-3, and NT-4/5 on cultured dopaminergic and GABAergic neurons of the ventral mesencephalon. J Neurosci. 1994;14:335–347. doi: 10.1523/JNEUROSCI.14-01-00335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larkfors L, Lindsay RM, Alderson RF. Characterization of the responses of Purkinje cells to neurotrophin treatment. J Neurochem. 1996;66:1362–1373. doi: 10.1046/j.1471-4159.1996.66041362.x. [DOI] [PubMed] [Google Scholar]
- 5.Spenger C, Hyman C, Studer L, Egli M, Evtouchenko L, Jackson C, et al. Effects of BDNF on dopaminergic, serotonergic, and GABAergic neurons in cultures of human fetal ventral mesencephalon. Exp Neurol. 1995;133:50–63. doi: 10.1006/exnr.1995.1007. [DOI] [PubMed] [Google Scholar]
- 6.Seil FJ. BDNF and NT-4, but not NT-3, promote development of inhibitory synapses in the absence of neuronal activity. Brain Res. 1999;818:561–564. doi: 10.1016/s0006-8993(98)01304-3. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh A, Carnahan J, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- 8.Gorski JA, Zeiler SR, Tamowski S, Jones KR. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci. 2003;23:6856–6865. doi: 10.1523/JNEUROSCI.23-17-06856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng B, Mattson MP. NT-3 and BDNF protect CNS neurons against metabolic/excitotoxic insults. Brain Res. 1994;640:56–67. doi: 10.1016/0006-8993(94)91857-0. [DOI] [PubMed] [Google Scholar]
- 10.Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, et al. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25:372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2003;8:592–610. doi: 10.1038/sj.mp.4001308. [DOI] [PubMed] [Google Scholar]
- 13.Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, et al. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- 14.Pruunsild P, Kazantseva A, Aid T, Palm K, Timmusk T. Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. Genomics. 2007;90:397–406. doi: 10.1016/j.ygeno.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiaruttini C, Sonego M, Baj G, Simonato M, Tongiorgi E. BDNF mRNA splice variants display activity-dependent targeting to distinct hippocampal laminae. Mol Cell Neurosci. 2008;37:11–19. doi: 10.1016/j.mcn.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Pattabiraman PP, Tropea D, Chiaruttini C, Tongiorgi E, Cattaneo A, Domenici L. Neuronal activity regulates the developmental expression and subcellular localization of cortical BDNF mRNA isoforms in vivo. Mol Cell Neurosci. 2005;28:556–570. doi: 10.1016/j.mcn.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Huntley GW, Benson DL, Jones EG, Isackson PJ. Developmental expression of brain derived neurotrophic factor mRNA by neurons of fetal and adult monkey prefrontal cortex. Brain Res Dev Brain Res. 1992;70:53–63. doi: 10.1016/0165-3806(92)90103-4. [DOI] [PubMed] [Google Scholar]
- 18.Wetmore C, Ernfors P, Persson H, Olson L. Localization of brain-derived neurotrophic factor mRNA to neurons in the brain by in situ hybridization. Exp Neurol. 1990;109:141–152. doi: 10.1016/0014-4886(90)90068-4. [DOI] [PubMed] [Google Scholar]
- 19.Villuendas G, Sanchez-Franco F, Palacios N, Fernandez M, Cacicedo L. Involvement of VIP on BDNF-induced somatostatin gene expression in cultured fetal rat cerebral cortical cells. Brain Res Mol Brain Res. 2001;94:59–66. doi: 10.1016/s0169-328x(01)00177-2. [DOI] [PubMed] [Google Scholar]
- 20.Glorioso C, Sabatini M, Unger T, Hashimoto T, Monteggia LM, Lewis DA, et al. Specificity and timing of neocortical transcriptome changes in response to BDNF gene ablation during embryogenesis or adulthood. Mol Psychiatry. 2006;11:633–648. doi: 10.1038/sj.mp.4001835. [DOI] [PubMed] [Google Scholar]
- 21.Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 22.Cotrufo T, Viegi A, Berardi N, Bozzi Y, Mascia L, Maffei L. Effects of neurotrophins on synaptic protein expression in the visual cortex of dark-reared rats. J Neurosci. 2003;23:3566–3571. doi: 10.1523/JNEUROSCI.23-09-03566.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arango-Gonzalez B, Cellerino A, Kohler K. Exogenous brain-derived neurotrophic factor (BDNF) reverts phenotypic changes in the retinas of transgenic mice lacking the BDNF gene. Invest Ophthalmol Vis Sci. 2009;50:1416–1422. doi: 10.1167/iovs.08-2244. [DOI] [PubMed] [Google Scholar]
- 24.Stoop R, Poo MM. Synaptic modulation by neurotrophic factors: differential and synergistic effects of brain-derived neurotrophic factor and ciliary neurotrophic factor. J Neurosci. 1996;16:3256–3264. doi: 10.1523/JNEUROSCI.16-10-03256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. The American Journal of Psychiatry. 2009 doi: 10.1176/appi.ajp.2010.09060784. Manuscript Submitted. [DOI] [PubMed] [Google Scholar]
- 26.McDonald C, Bullmore E, Sham P, Chitnis X, Suckling J, MacCabe J, et al. Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder: computational morphometry study. Br J Psychiatry. 2005;186:369–377. doi: 10.1192/bjp.186.5.369. [DOI] [PubMed] [Google Scholar]
- 27.Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97:215–225. doi: 10.1016/j.schres.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- 29.Weinberger DR. Cell biology of the hippocampal formation in schizophrenia. Biol Psychiatry. 1999;45:395–402. doi: 10.1016/s0006-3223(98)00331-x. [DOI] [PubMed] [Google Scholar]
- 30.Durany N, Michel T, Zochling R, Boissl KW, Cruz-Sanchez FF, Riederer P, et al. Brain-derived neurotrophic factor and neurotrophin 3 in schizophrenic psychoses. Schizophr Res. 2001;52:79–86. doi: 10.1016/s0920-9964(00)00084-0. [DOI] [PubMed] [Google Scholar]
- 31.Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- 32.Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2008;33:73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- 33.Peng Q, Masuda N, Jiang M, Li Q, Zhao M, Ross CA, et al. The antidepressant sertraline improves the phenotype, promotes neurogenesis and increases BDNF levels in the R6/2 Huntington’s disease mouse model. Exp Neurol. 2008;210:154–163. doi: 10.1016/j.expneurol.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou L, Huang KX, Kecojevic A, Welsh AM, Koliatsos VE. Evidence that serotonin reuptake modulators increase the density of serotonin innervation in the forebrain. J Neurochem. 2006;96:396–406. doi: 10.1111/j.1471-4159.2005.03562.x. [DOI] [PubMed] [Google Scholar]
- 35.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calabrese F, Molteni R, Maj PF, Cattaneo A, Gennarelli M, Racagni G, et al. Chronic duloxetine treatment induces specific changes in the expression of BDNF transcripts and in the subcellular localization of the neurotrophin protein. Neuropsychopharmacology. 2007;32:2351–2359. doi: 10.1038/sj.npp.1301360. [DOI] [PubMed] [Google Scholar]
- 37.Mannari C, Origlia N, Scatena A, Del Debbio A, Catena M, Dell’agnello G, et al. BDNF level in the rat prefrontal cortex increases following chronic but not acute treatment with duloxetine, a dual acting inhibitor of noradrenaline and serotonin re-uptake. Cell Mol Neurobiol. 2008;28:457–468. doi: 10.1007/s10571-007-9254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riecher-Rossler A, Seeman MV. Oestrogens and schizophrenia--introduction. Arch Womens Ment Health. 2002;5:91–92. doi: 10.1007/s007370200026. [DOI] [PubMed] [Google Scholar]
- 39.Rogoz Z, Skuza G, Legutko B. Repeated treatment with mirtazepine induces brain-derived neurotrophic factor gene expression in rats. J Physiol Pharmacol. 2005;56:661–671. [PubMed] [Google Scholar]
- 40.Balu DT, Hoshaw BA, Malberg JE, Rosenzweig-Lipson S, Schechter LE, Lucki I. Differential regulation of central BDNF protein levels by antidepressant and non-antidepressant drug treatments. Brain Res. 2008;1211:37–43. doi: 10.1016/j.brainres.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khundakar AA, Zetterstrom TS. Biphasic change in BDNF gene expression following antidepressant drug treatment explained by differential transcript regulation. Brain Res. 2006;1106:12–20. doi: 10.1016/j.brainres.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 42.Dias BG, Banerjee SB, Duman RS, Vaidya VA. Differential regulation of brain derived neurotrophic factor transcripts by antidepressant treatments in the adult rat brain. Neuropharmacology. 2003;45:553–563. doi: 10.1016/s0028-3908(03)00198-9. [DOI] [PubMed] [Google Scholar]
- 43.Lipska BK, Deep-Soboslay A, Weickert CS, Hyde TM, Martin CE, Herman MM, et al. Critical factors in gene expression in postmortem human brain: Focus on studies in schizophrenia. Biol Psychiatry. 2006;60:650–658. doi: 10.1016/j.biopsych.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 44.Weickert CS, Sheedy D, Rothmond DA, Dedova D, Fung SJ, Garrick T, et al. Selection of reference gene expression in a schizophrenia brain cohort. Australian and New Zealand Journal of Psychiatry. 2009 doi: 10.3109/00048670903393662. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 46.Lehman AF, Steinwachs DM. Translating research into practice: the Schizophrenia Patient Outcomes Research Team (PORT) treatment recommendations. Schizophr Bull. 1998;24:1–10. doi: 10.1093/oxfordjournals.schbul.a033302. [DOI] [PubMed] [Google Scholar]
- 47.Centorrino F, Price BH, Tuttle M, Bahk WM, Hennen J, Albert MJ, et al. EEG abnormalities during treatment with typical and atypical antipsychotics. Am J Psychiatry. 2002;159:109–115. doi: 10.1176/appi.ajp.159.1.109. [DOI] [PubMed] [Google Scholar]
- 48.Thompson M, Weickert CS, Wyatt E, Webster M. Decreased BDNF, TrkB-TK+ and GAD67 mRNA Expression in thPatients with Schizophrenia and Mood Disorders. J Psychiatr Res. 2009 doi: 10.1503/jpn.100048. Manuscript Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kozlovsky N, Shanon-Weickert C, Tomaskovic-Crook E, Kleinman JE, Belmaker RH, Agam G. Reduced GSK-3beta mRNA levels in postmortem dorsolateral prefrontal cortex of schizophrenic patients. J Neural Transm. 2004;111:1583–1592. doi: 10.1007/s00702-004-0166-3. [DOI] [PubMed] [Google Scholar]
- 50.Wong J, Webster MJ, Cassano H, Weickert CS. Changes in alternative brain-derived neurotrophic factor transcript expression in the developing human prefrontal cortex. Eur J Neurosci. 2009;29:1311–1322. doi: 10.1111/j.1460-9568.2009.06669.x. [DOI] [PubMed] [Google Scholar]
- 51.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res. 2005;136:29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 53.Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 54.Dwivedi Y. Brain-derived neurotrophic factor: role in depression and suicide. Neuropsychiatr Dis Treat. 2009;5:433–449. doi: 10.2147/ndt.s5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nair A, Vadodaria KC, Banerjee SB, Benekareddy M, Dias BG, Duman RS, et al. Stressor-specific regulation of distinct brain-derived neurotrophic factor transcripts and cyclic AMP response element-binding protein expression in the postnatal and adult rat hippocampus. Neuropsychopharmacology. 2007;32:1504–1519. doi: 10.1038/sj.npp.1301276. [DOI] [PubMed] [Google Scholar]
- 56.An JJ, Gharami K, Liao GY, Woo NH, Lau AG, Vanevski F, et al. Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134:175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pillai A. Brain-derived neurotropic factor/TrkB signaling in the pathogenesis and novel pharmacotherapy of schizophrenia. Neurosignals. 2008;16:183–193. doi: 10.1159/000111562. [DOI] [PubMed] [Google Scholar]
- 58.Chlan-Fourney J, Ashe P, Nylen K, Juorio AV, Li XM. Differential regulation of hippocampal BDNF mRNA by typical and atypical antipsychotic administration. Brain Res. 2002;954:11–20. doi: 10.1016/s0006-8993(02)03215-8. [DOI] [PubMed] [Google Scholar]
- 59.Lipska BK, Khaing ZZ, Weickert CS, Weinberger DR. BDNF mRNA expression in rat hippocampus and prefrontal cortex: effects of neonatal ventral hippocampal damage and antipsychotic drugs. Eur J Neurosci. 2001;14:135–144. doi: 10.1046/j.1460-9568.2001.01633.x. [DOI] [PubMed] [Google Scholar]
- 60.Bai O, Chlan-Fourney J, Bowen R, Keegan D, Li XM. Expression of brain-derived neurotrophic factor mRNA in rat hippocampus after treatment with antipsychotic drugs. J Neurosci Res. 2003;71:127–131. doi: 10.1002/jnr.10440. [DOI] [PubMed] [Google Scholar]
- 61.Pillai A, Terry AV, Jr, Mahadik SP. Differential effects of long-term treatment with typical and atypical antipsychotics on NGF and BDNF levels in rat striatum and hippocampus. Schizophr Res. 2006;82:95–106. doi: 10.1016/j.schres.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 62.Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Russo-Neustadt A, Beard RC, Cotman CW. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology. 1999;21:679–682. doi: 10.1016/S0893-133X(99)00059-7. [DOI] [PubMed] [Google Scholar]
- 64.Coppell AL, Pei Q, Zetterstrom TS. Bi-phasic change in BDNF gene expression following antidepressant drug treatment. Neuropharmacology. 2003;44:903–910. doi: 10.1016/s0028-3908(03)00077-7. [DOI] [PubMed] [Google Scholar]
- 65.Van Hoomissen JD, Chambliss HO, Holmes PV, Dishman RK. Effects of chronic exercise and imipramine on mRNA for BDNF after olfactory bulbectomy in rat. Brain Res. 2003;974:228–235. doi: 10.1016/s0006-8993(03)02584-8. [DOI] [PubMed] [Google Scholar]
- 66.Xu H, Steven Richardson J, Li XM. Dose-related effects of chronic antidepressants on neuroprotective proteins BDNF, Bcl-2 and Cu/Zn-SOD in rat hippocampus. Neuropsychopharmacology. 2003;28:53–62. doi: 10.1038/sj.npp.1300009. [DOI] [PubMed] [Google Scholar]
- 67.Garza AA, Ha TG, Garcia C, Chen MJ, Russo-Neustadt AA. Exercise, antidepressant treatment, and BDNF mRNA expression in the aging brain. Pharmacol Biochem Behav. 2004;77:209–220. doi: 10.1016/j.pbb.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 68.De Foubert G, Carney SL, Robinson CS, Destexhe EJ, Tomlinson R, Hicks CA, et al. Fluoxetine-induced change in rat brain expression of brain-derived neurotrophic factor varies depending on length of treatment. Neuroscience. 2004;128:597–604. doi: 10.1016/j.neuroscience.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 69.Vinet J, Carra S, Blom JM, Brunello N, Barden N, Tascedda F. Chronic treatment with desipramine and fluoxetine modulate BDNF, CaMKKalpha and CaMKKbeta mRNA levels in the hippocampus of transgenic mice expressing antisense RNA against the glucocorticoid receptor. Neuropharmacology. 2004;47:1062–1069. doi: 10.1016/j.neuropharm.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 70.Dwivedi Y, Rizavi HS, Pandey GN. Antidepressants reverse corticosterone-mediated decrease in brain-derived neurotrophic factor expression: differential regulation of specific exons by antidepressants and corticosterone. Neuroscience. 2006;139:1017–1029. doi: 10.1016/j.neuroscience.2005.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.