Abstract
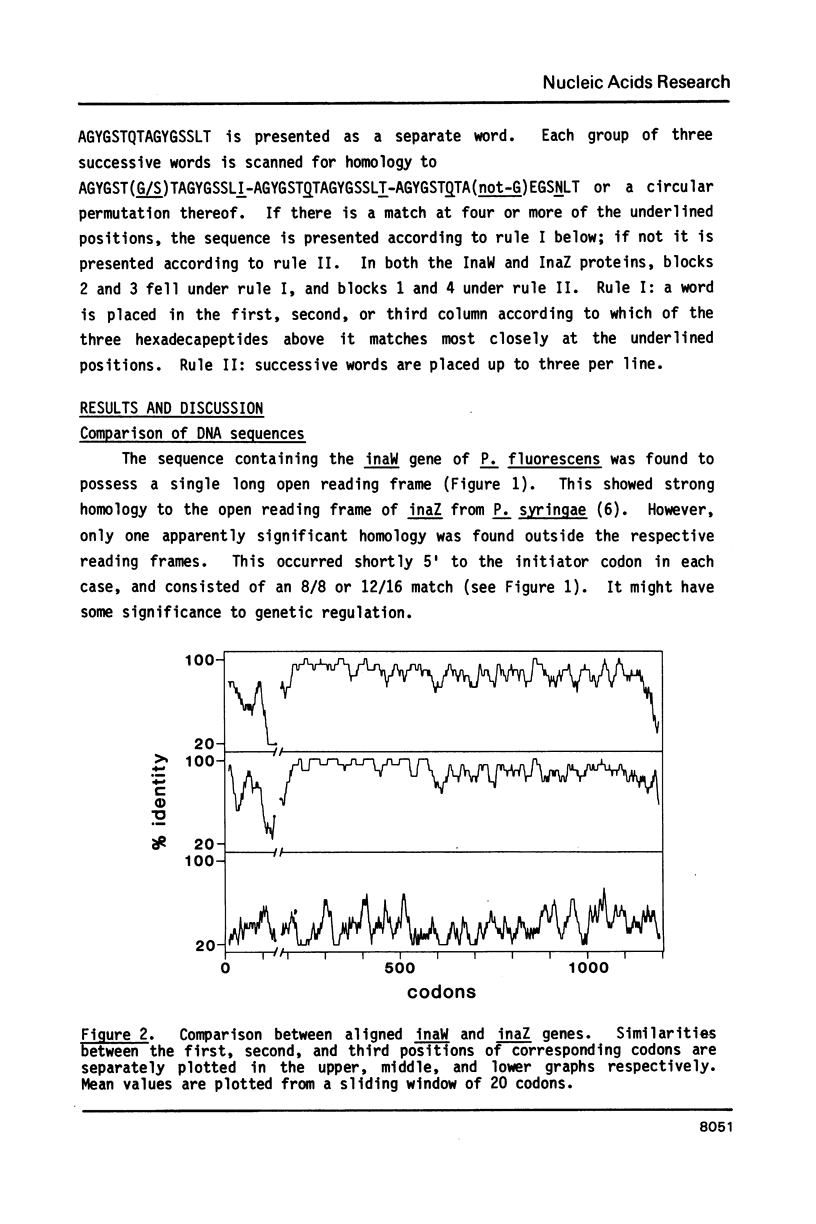
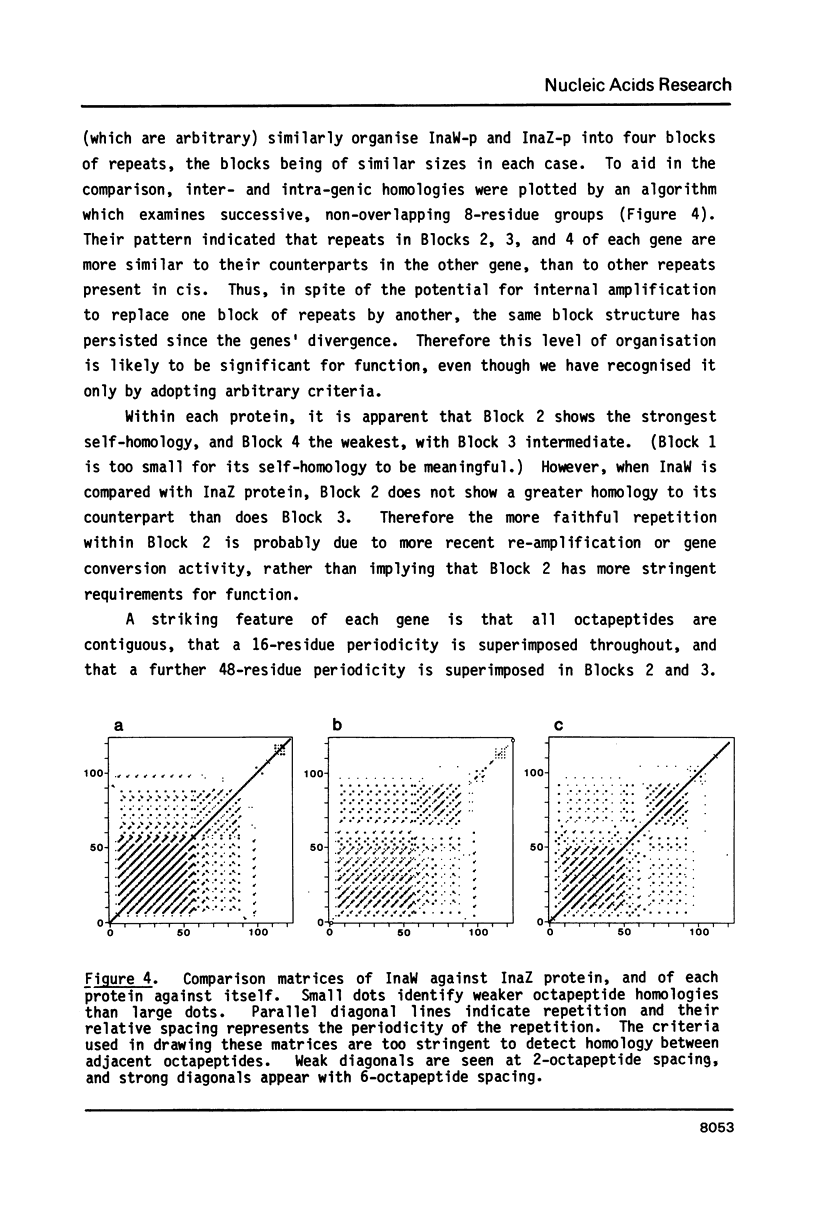
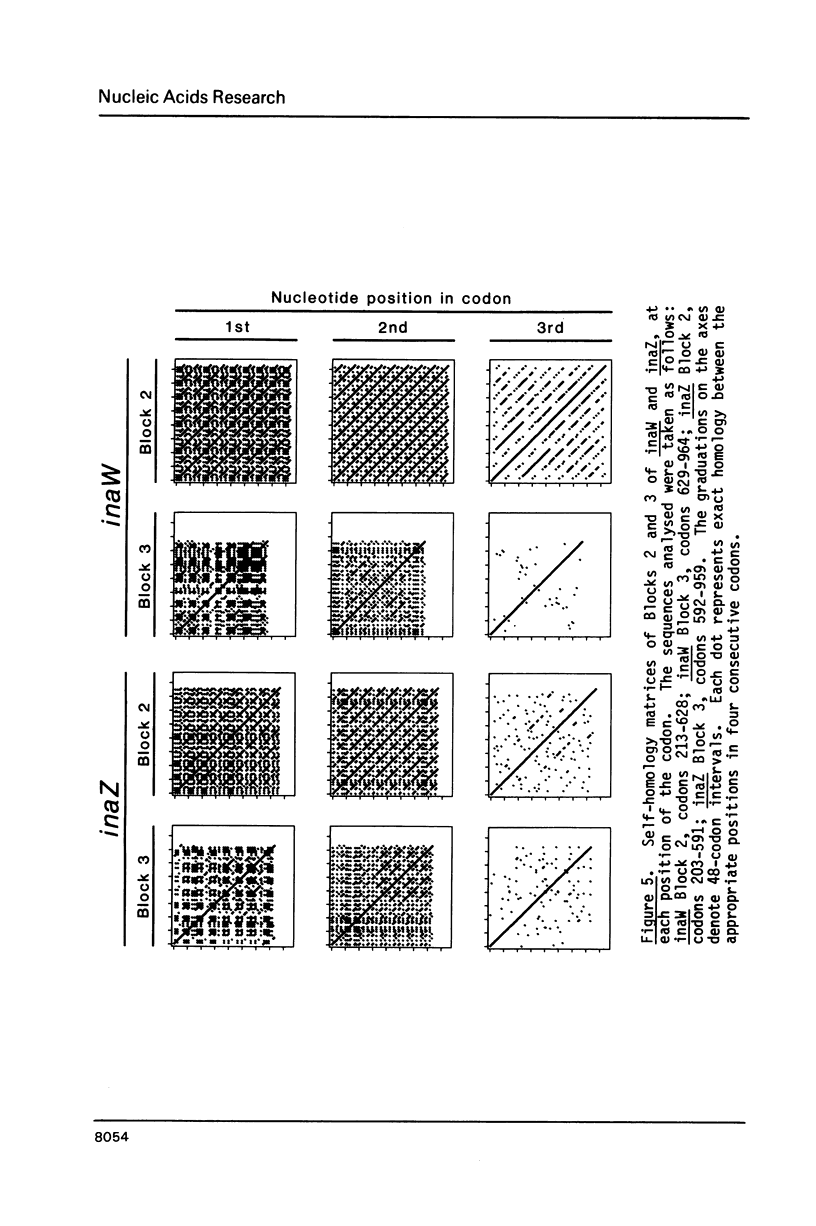
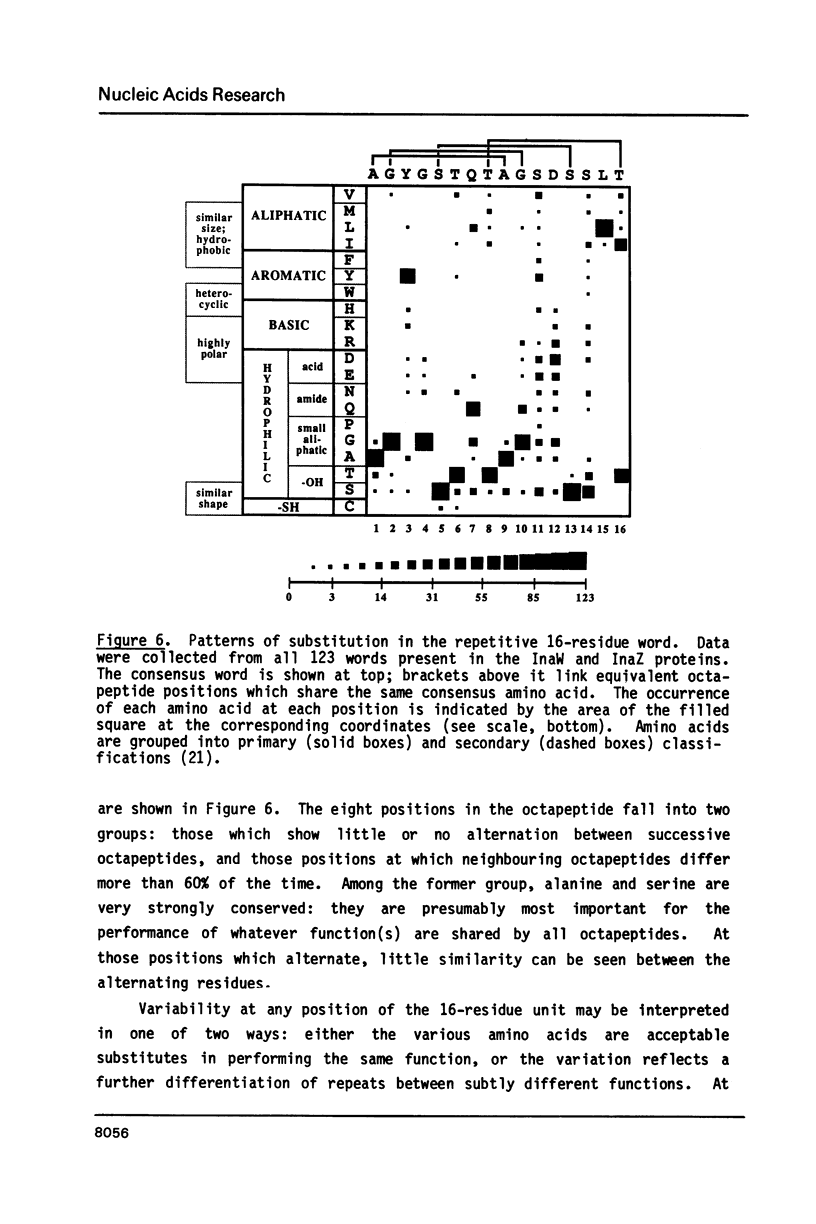
Sequence analysis shows that an ice nucleation gene (inaW) from Pseudomonas fluorescens is related to the inaZ gene of Pseudomonas syringae. The two genes have diverged by many amino acid substitutions, and have effectively randomized the third bases of homologous codons. By reference to their potential for change, it is shown that certain conserved features must have been maintained by selection pressure. In particular, their conservation of internal sequence repetition, with three orders of repeat periodicity in each gene, suggests that the pattern of repetition is significant to the gene products' function. We propose models for the structure of the gene products in which each order of periodicity would be required for the nucleation function.
Full text
PDF





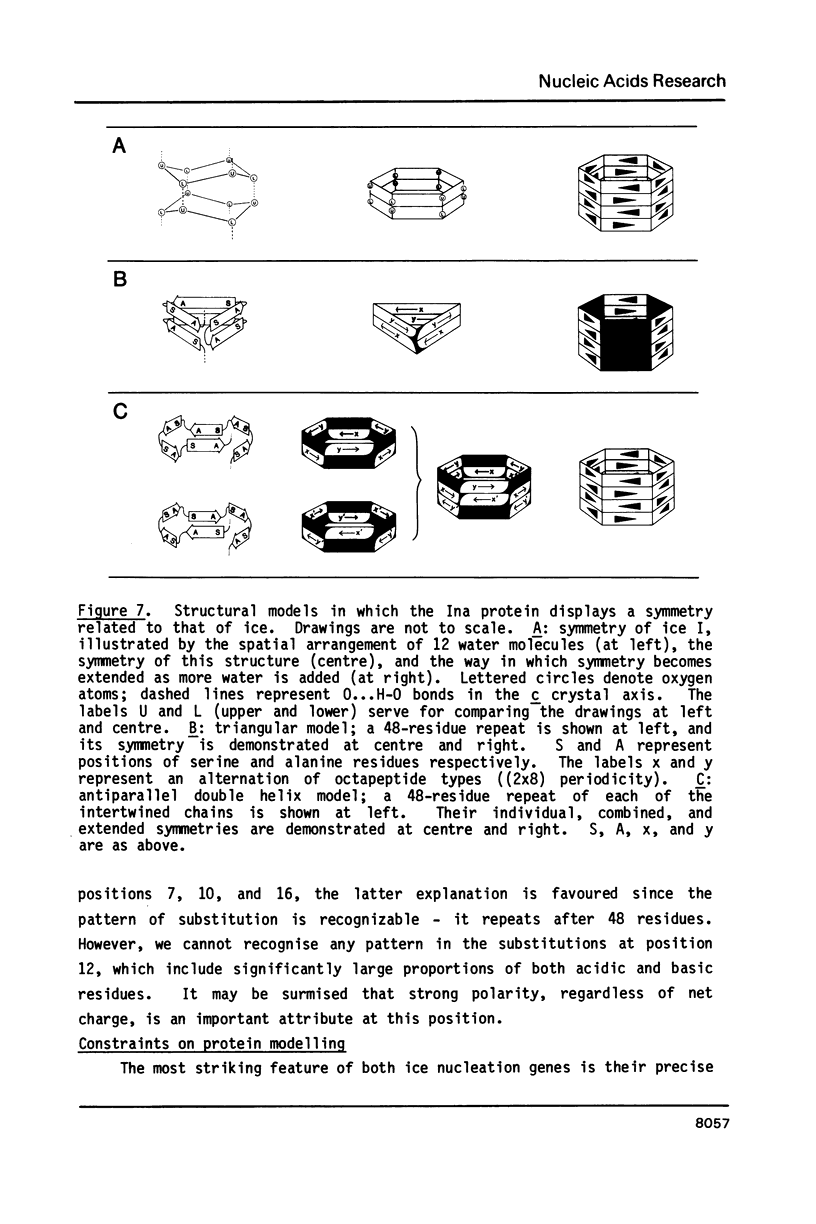



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnot D. E., Barnwell J. W., Tam J. P., Nussenzweig V., Nussenzweig R. S., Enea V. Circumsporozoite protein of Plasmodium vivax: gene cloning and characterization of the immunodominant epitope. Science. 1985 Nov 15;230(4727):815–818. doi: 10.1126/science.2414847. [DOI] [PubMed] [Google Scholar]
- Corotto L. V., Wolber P. K., Warren G. J. Ice nucleation activity of Pseudomonas fluorescens: mutagenesis, complementation analysis and identification of a gene product. EMBO J. 1986 Feb;5(2):231–236. doi: 10.1002/j.1460-2075.1986.tb04203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devries A. L., Lin Y. Structure of a peptide antifreeze and mechanism of adsorption to ice. Biochim Biophys Acta. 1977 Dec 20;495(2):388–392. doi: 10.1016/0005-2795(77)90395-6. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Ellis J., Svec P., Schlesinger D. H., Nussenzweig V. Identification and chemical synthesis of a tandemly repeated immunogenic region of Plasmodium knowlesi circumsporozoite protein. Nature. 1983 Sep 1;305(5929):29–33. doi: 10.1038/305029a0. [DOI] [PubMed] [Google Scholar]
- LUCAS F., SHAW J. T., SMITH S. G. The amino acid sequence in a fraction of the fibroin of Bombyx mori. Biochem J. 1957 Jul;66(3):468–479. doi: 10.1042/bj0660468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Gross J. K. Molecular cloning and characterization of winter flounder antifreeze cDNA. Proc Natl Acad Sci U S A. 1981 May;78(5):2825–2829. doi: 10.1073/pnas.78.5.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, Lenk R. P. Enhanced graphic matrix analysis of nucleic acid and protein sequences. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7665–7669. doi: 10.1073/pnas.78.12.7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki L. R., Galyan E. L., Chang-Chien M. M., Caldwell D. R. Ice nucleation induced by pseudomonas syringae. Appl Microbiol. 1974 Sep;28(3):456–459. doi: 10.1128/am.28.3.456-459.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Orser C., Staskawicz B. J., Panopoulos N. J., Dahlbeck D., Lindow S. E. Cloning and expression of bacterial ice nucleation genes in Escherichia coli. J Bacteriol. 1985 Oct;164(1):359–366. doi: 10.1128/jb.164.1.359-366.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlén M., Guss B., Nilsson B., Gatenbeck S., Philipson L., Lindberg M. Complete sequence of the staphylococcal gene encoding protein A. A gene evolved through multiple duplications. J Biol Chem. 1984 Feb 10;259(3):1695–1702. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Ycas M. De novo origin of periodic proteins. J Mol Evol. 1972 Dec 29;2(1):17–27. doi: 10.1007/BF01653939. [DOI] [PubMed] [Google Scholar]



