Abstract
Insecticide-impregnated nets can kill triatomine bugs, but it remains unclear whether they can protect against Chagas disease transmission. In a field trial in Quequeña, Peru, sentinel guinea pigs placed in intervention enclosures covered by deltamethrin-treated nets showed significantly lower antibody responses to saliva of Triatoma infestans compared with animals placed in pre-existing control enclosures. Our results strongly suggest that insecticide-treated nets prevent triatomine bites and can thereby protect against infection with Trypanosoma cruzi. Anti-salivary immunoassays are powerful new tools to evaluate intervention strategies against Chagas disease.
Keywords: Triatoma infestans, Impregnated net, Sentinel guinea pig, Saliva, Antibody response
Intervention strategies aimed at preventing insect vectors of infectious diseases from feeding on their hosts, such as insecticide application and impregnated bed nets, are among the most effective public health measures of the last century (Hemingway et al., 2006). Nevertheless, evaluation of new or improved vector control measures has proven difficult. For diseases with lower incidence, such as Chagas disease, sample sizes necessary to evaluate novel vector control interventions can be prohibitively large.
Haematophagous arthropods have to overcome the defense mechanism of their hosts. These arthropods, such as triatomines, have evolved a wide range of salivary anti-haemostatic compounds, immunosuppressors and complement inhibitors that are injected into the host when feeding on blood (Ribeiro and Francischetti, 2003). This cocktail of salivary proteins can elicit a humoral immune response in hosts which has been used as an immunological marker of exposure to disease vectors such as mosquitoes, as well as a marker for transmission risk of infectious disease agents (e.g. Remoue et al., 2006). Immunoassays using salivary proteins show great promise to evaluate intervention measures against vector borne diseases (Drame et al., 2010).
Here we present empirical data from a trial of insecticide-impregnated nets on sentinel guinea pig corrals against transmission of Trypanosoma cruzi, the etiological agent of Chagas disease, by the insect vector Triatoma infestans. We used antibody responses of sentinel guinea pigs to salivary proteins of T. infestans to detect exposure to triatomines and compared these data with T. cruzi infection in sentinel guinea pigs.
We conducted the trial in Quequeña, Peru, a small town located approximately 25 km southeast of the city of Arequipa with an estimated population of 1,150 (Bayer et al., 2009). Guinea pigs are commonly raised for consumption and trade in Peru (Bayer et al., 2009).
In April of 2006, the peridomestic areas of all 192 inhabited homes of Quequeña were surveyed for the presence of T. infestans. An entomological survey was conducted with the aid of Tetramethrin (Sapolio “Mata Moscas” Tetramethrin 0.15%), an insecticide with a strong ‘flushing out’ effect on triatomine bugs (Levy et al., 2008). All captured female, male and second to fifth instar bugs were counted, staged, sexed and microscopically examined for the presence of T. cruzi as previously described (Levy et al., 2006). Ten households were identified in which T. infestans collected from guinea pig pens were found to carry T. cruzi. These households were invited to participate in the intervention study in June 2006. In each consenting household, two groups of four sentinel guinea pigs were placed in the peridomestic area. Sentinel animals were purchased from a distributor in Lima, Peru and had never been in contact with triatomines. The first group of four guinea pigs served as a control; these animals were placed in the original, existing guinea pig pen. The second group was placed in a newly constructed guinea pig enclosure (Levy et al., 2008). The enclosure measured 1.4 × 0.8 m, with metallic wire walls and a corrugated rubber roof. Long-lasting deltamethrin-impregnated net (PermaNet 2.0, 55 mg/m2 target deltamethrin concentration, 25 holes/cm2 mesh size; Vestergaard Frandsen, Lausanne, Switzerland) was draped over the enclosure. The netted enclosure was placed immediately adjacent to the original pen so that the distance from each pen to vector colonies was as closely matched as possible (Fig. 1). After 5 months all houses and peridomestic structures received insecticide treatment by the Arequipa Regional Ministry of Health Chagas disease control program. At the time of insecticide application, households were searched for one person-hour by trained triatomine collectors. In addition, all control and sentinel guinea pig enclosures were disassembled and exhaustively searched for triatomines; captured insects were examined for the presence of T. cruzi.
Fig. 1.
Photograph of a newly constructed guinea pig corral with an impregnated net and an original corral. Groups of sentinel guinea pigs were either placed in newly constructed guinea pig corrals which were covered with deltamethrin-impregnated nets, or pre-existing guinea pig pens (pictured behind the newly constructed pen).
Blood was drawn from sentinel guinea pigs through the saphenous or cephalic vein before exposure to triatomines and at 3 and 5 months after their placement in the field. Sera of pre-exposure guinea pigs served as negative controls in all immunoassays (trypomastigote excretion-secretion antigens (TESA)-ELISA, IFA, anti-T. infestans saliva ELISA). Sera from guinea pigs exposed repeatedly over a period of 5 months to triatomine bites derived from previous studies were used as positive controls in the anti-saliva ELISA (Schwarz et al., 2009a). Sera from animals infected once with 106 trypomastigotes of T. cruzi (Y strain, provided by Dra. E. Umezawa, Instituto de Medicina Tropical, Universidade de São Paulo, São Paulo, Brazil) served as positive controls in anti-T. cruzi immunoassays (TESA-ELISA, IFA). The Y strain of T. cruzi was originally isolated from an acute case of Chagas disease (Amato Neto, 2010). This strain was maintained in culture in the metacyclic stage until required. The use of animals was approved by the Institutional Animal Care and Use Committee (IACUC) of Johns Hopkins University, USA.
Because there is no optimal test for T. cruzi infection in guinea pigs, we used a number of assays. All animal sera were tested by ELISA using TESA as previously described (Umezawa et al., 2001). Antigen for the TESA-ELISA was obtained from the supernatant of infected monkey kidney LLC-MK2 cells. The protein concentration was measured using the Bradford quantification assay with BSA as a standard and the supernatants were stored at −70°C until use. Ninety-six well plates were coated with TESA at a final concentration of 2 μg/ml. The plates were incubated with guinea pig serum diluted 1:100 followed by a 1:10,000 dilution of horseradish peroxidase-conjugated goat anti-guinea-pig IgG (KPL Laboratories, USA) as secondary antibody. Each serum sample was analyzed in duplicate and each plate included a positive control and seven negative control sera. The O.D. was measured at 495 nm. Trypanosoma cruzi infection was considered as positive or negative in TESA-ELISA by using a cut-off value calculated from the final mean O.D.495nm (duplicate serum samples per specimen) of seven T. cruzi negative control sera plus 3 S.D. per ELISA assay.
Specimens tested positive for T. cruzi infection by TESA-ELISA were confirmed by standard IFA (Camargo and Rebonato, 1969). Epimastigotes of T. cruzi (Y strain) were cultured for 10 days until the culture reached its logarithmic phase of growth in liver infusion tryptose medium with 10% FBS. A dilution of 30–40 epimastigotes/well was used for IFA. Sera of sentinel guinea pigs and FITC-conjugated goat anti-guinea pig IgG (KPL) were diluted 1:16, 1:32, 1:64 and 1:128. Positive and negative control sera of anti-T. cruzi antibodies were tested in parallel. Sera reacting at the dilution of 1:32 and higher were considered positive.
Two parasitological tests were also performed on all sentinel animals: direct observation of blood in hematocrit tubes (La Fuente et al., 1984) and xenodiagnosis. Xenodiagnosis was applied after blood was draw from sentinel guinea pigs for serological tests (Vega Chirinos and Náquira Velarde, 2006). Briefly, 10 starved nymphs (third or fourth instars) were allowed to feed on guinea pigs for 20 min. Blood-fed triatomines were examined individually for the presence of trypomastigotes in the triatomine feces by microscopy after 30 days. Uninfected insects were re-examined again after 60 days.
Pooled crude saliva of 300 starved fifth instar and adult T. infestans was obtained from a colony maintained in Arequipa, Peru. Salivation of the triatomines was induced by stimulating the insects proboscis with forceps while breathing (providing CO2) towards the insect. Saliva was collected using capillary pipettes (Amino et al., 2001). The protein concentration of the saliva homogenate was determined using the Bradford method. The saliva was aliquoted and stored at −20°C and minus;70°C until use.
Anti-T. infestans saliva ELISAs were performed as previously described (Schwarz et al., 2009a). Only sentinel guinea pig sera from the first blood draw, after guinea pigs were kept in the field for 3 months, were used. Sera of guinea pigs collected at 5 months could not be used for the salivary ELISA because the animals had been exposed to vector saliva during the xenodiagnoses at 3 months. ELISA plates were coated with 0.5 μg total salivary protein of T. infestans per well. The plates were incubated with guinea pig serum diluted 1:100 and peroxidase-conjugated goat anti-guinea pig IgG diluted 1:5,000 as secondary antibody. Each serum sample was analyzed in duplicate and each plate included a positive and several negative control sera. The O.D. was recorded at 492 nm. The final O.D.492nm of the anti-saliva IgG ELISA assays was determined by calculating the mean O.D.492nm of the duplicate wells of each serum sample and subtracting the mean O.D.492nm of the negative control. Antibody responses of guinea pigs from the original and intervention corrals with netting were compared using a Mann-Whitney Rank Sum test (non-normal distribution of data) using the statistical programme SigmaStat 3.1 (Systat Software).
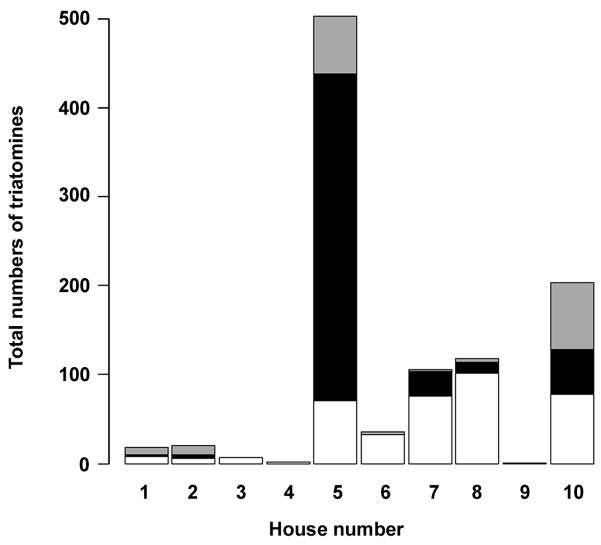
A total of 157 triatomines were collected in all guinea pig pens of the 10 households prior to placement of sentinel animals in these households. All triatomines were identified as T. infestans. Trypanosoma cruzi was observed in 44.3% of insects. Triatomine collections were repeated at the end of the netting trial, at which time all 10 households received insecticide treatment by the local authorities of the Chagas disease control program. The insecticide application greatly facilitated triatomine collection compared with the manual searches for bugs with the aid of a flushing out agent. Overall, 1,005 T. infestans were captured in and around the guinea pig corrals of the 10 study households (Fig. 2). No triatomines were found in the protected pens. Insect density was greatest in household no. 5, with 503 bugs. Surprisingly, although for all 10 households T. cruzi-infected T. infestans were detected in April 2006, no T. cruzi infection was present in triatomines collected in four participating dwellings (nos. 3, 4, 6 and 9) at the end of the netting trial.
Fig. 2.
Triatoma infestans infestation of guinea pig corrals in 10 households of Quequeñe, Peru. Households were sprayed with insecticides by the Arequipa Regional Ministry of Health in December 2006 and trained personal searched the entire households for triatomines for 1 h. All adult insects and second through the fifth instars were examined for the presence of Trypanosoma cruzi from all captured T. infestans (gray bars) in the guinea pig corrals. The black and white bars indicate the total numbers of T. cruzi-infected and non-infected bugs, respectively.
Our target sample size for TESA-ELISA and IFA was 160 guinea pig sera. One household withdrew from the study after the third month, leaving 152 sera for analyses. However, only 115 sera were available for analyses by TESA-ELISA and IFA due to insufficient amounts of sera from 37 blood draws. Only one of these 115 was confirmed seropositive. The infected guinea pig was kept in an original corral of household no. 5. One out of 96 sentinel guinea pigs tested by xenodiagnosis was found to be infected by T. cruzi. Not all 152 guinea pigs could be examined because some households refused the second round of xenodiagnosis on the animals. The T. cruzi-infected guinea pig was also kept in the original corral of household no. 5. Both positive animals were detected during the 3 month testing period. No additional animals were found to be positive for T. cruzi infection when tested at the end of the trial, after 5 months in the field. No T. cruzi infection in animals was detected by direct observation of blood in hematocrit tubes.
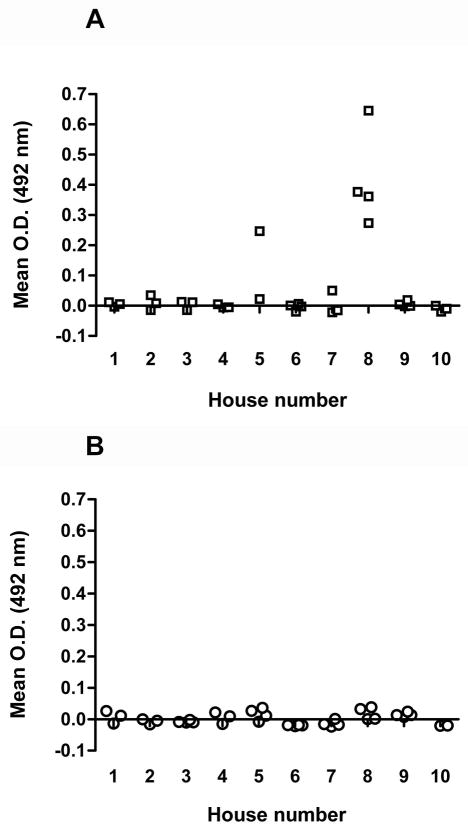
Exposure of sentinel guinea pigs to triatomines was characterized by antibody responses of animals to saliva of T. infestans. Out of 64 analyzed guinea pig sera, significant antibody responses were only detected for five guinea pigs (Mann-Whitney Rank Sum test, P < 0.01, Fig. 3A). All five were from the original corrals. All other animal sera reacted only very weakly in ELISA tests with triatomine saliva. The antibody responses of the other animals could not be distinguished from the responses of the negative control (Fig. 3). One of the five animals with strong antibody responses to triatomine salivary antigens was the T. cruzi-infected guinea pig from the original corral of household no. 5, the dwelling from which 503 insects were collected in December 2006 (Fig. 3A). Unfortunately, the antibody response of the second T. cruzi-infected animal could not be analysed because no serum was left for this ELISA.
Fig. 3.
Antibody responses of sentinel guinea pigs to salivary proteins of Triatoma infestans. Sentinel guinea pigs were either placed in original corrals (A, squares) or corrals with deltamethrin-impregnated nets (B, circles) of 10 households in the town of, Peru, in June 2006. Three months later the antibody response of guinea pigs to T. infestans saliva was measured by ELISA. The final O.D.492nm was determined by calculating the mean O.D.492nm of the duplicate wells of each guinea pig serum and subtracting the O.D.492nm of the mean O.D.492nm of the negative control sera per household.
Household application of pyrethroid insecticide is the standard for control of the Chagas disease vector T. infestans and has shown great success in the Southern Cone Initiative, a collaborative multi-national effort to eliminate transmission of T. cruzi by T. infestans (Schofield et al., 2006). The success of the Southern Cone Initiative, however, has not been universal. While transmission of T. cruzi by T. infestans has been disrupted in countries with greater resources, it continues in poorer areas where vector control activities could not, or have not, been sustained (Gürtler, 2009). Alternative means of vector control are very much needed in such areas.
Deltamethrin impregnated netting has been shown previously to slow the dispersal of T. infestans. In a study conducted in an urban community of Arequipa, Peru, nets were evaluated by counting vectors that infested bricks placed in new sentinel enclosures (Levy et al., 2008). Although a significant decrease in vector numbers was observed in enclosures protected by netting, the question remained whether bugs from colonies elsewhere in the household were capable of navigating through the nets and feeding on the animals. If so the nets might neither decrease the frequency of bites by vectors on hosts nor lower the risk of infection by T. cruzi. The results presented in this study show that animals protected by nets are in fact significantly less exposed to triatomine saliva suggesting, to our knowledge for the first time, that nets protect hosts from bites by T. infestans.
Exposure to vectors among sentinel guinea pigs in our study was very heterogeneous. It is not clear why so few animals in the control enclosures reacted strongly to T. infestans saliva. It is possible that vectors continued to feed on existing animals in the control enclosures, rather than feeding on the newly-placed sentinel guinea pigs. Thus, our estimate of the effect of netting may be inflated. It is also possible that the presence of the impregnated netting nearby affected the feeding of vectors in control enclosures, which would deflate our estimates. On the other hand, animals in one household in particular had very elevated antibody titers against salivary antigen from the vector. It is no coincidence that the guinea pig with confirmed T. cruzi infection was from household no. 5 with the highest number of triatomines. These animals can be categorized as highly exposed to triatomine bites according to the classification of antibody levels of guinea pigs from low and highly infested T. infestans habitats of previous studies (Schwarz et al., 2009a).
Haematophagous arthropods have evolved a wide range of salivary proteins, some of which are shared between species and across genera (Ribeiro and Francischetti, 2003). Cross-reacting IgG epitopes of salivary proteins of T. infestans and antigens in the saliva of other biting arthropods in ELISA assays may have affected our findings, and probably caused low-level baseline antibody responses in all animals. However, we carefully checked sentinel animals for ticks and fleas and found none—the absence of these other ectoparasites on the study animals may have reduced cross-reactivity and led to the success of the trial. The development of triatomine-specific salivary antigens as exposure markers of triatomine bites on guinea pigs would reduce cross-reactions (Schwarz et al., 2009b).
In conclusion, impregnated netting and other interventions are especially promising for the control of triatomine insects, as they can be made available directly to those people at risk of Chagas disease infection. Our immunological approach reported in this study will be of great use in facilitating the evaluation of vector control interventions against Chagas disease. It can serve as a surrogate measure for the degree of exposure to insect bites on sentinel animals and thereby for the risk of T. cruzi infection. Furthermore, this immunoassay could be expanded for use in humans and for other vectors of T. cruzi.
Acknowledgments
We thank the community of Quequeña for their participation. We especially thank Rocio Rodriguez, Amparo Toledo and the sprayers and field collectors who worked on the study. We also thank Gregory Martin, Jeffrey Stancil, David Bentzel, Ellen Dotson, Robert Wirtz, Lucy Rubio, Gena Lawrence, Karina Oppe and Fernando Malaga for assistance and support. The authors thank Torben Frandsen for fabrication and donation of the guinea pig PermaNets, and Jesus Valenzuela for his advice and support. We would also like to thank the Pan American Health Organization (PAHO), the Canadian International Development Agency (CIDA), Ministerio de Salud del Perú (MINSA), Dirección General de Salud de las Personas (DGSP), Estrategia Sanitaria Nacional de Prevención y Control de Enfermedades Metaxénicas y Otras Transmitidas por Vectores (ESNPCEMOTVS), Dirección General de Salud Ambiental (DIGESA), Gobierno Regional de Arequipa and the Gerencia Regional de Salud de Arequipa (GRSA). Funding for this study came from NIH 5K01 AI079162-03, NIH 3K01AI079162-02S1, 3K01AI079162-03S1 and NIH P50 AI074285-04. This study also received financial support from the UNICEF/UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases (TDR Grant) and the Grant Agency of the Czech Republic, grant no. P302/11/P798.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amato Neto V. Origin of the “Y strain” of Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo. 2010;52:171. doi: 10.1590/s0036-46652010000300012. [DOI] [PubMed] [Google Scholar]
- Amino R, Tanaka AS, Schenkman S. Triapsin, an unusual activatable serine protease from the saliva of the hematophagous vector of Chagas disease Triatoma infestans (Hemiptera: Reduviidae) Insect Biochem Mol Biol. 2001;31:465–472. doi: 10.1016/s0965-1748(00)00151-x. [DOI] [PubMed] [Google Scholar]
- Bayer AM, Hunter GC, Gilman RH, Cornejo Del Carpio JG, Naquira C, Bern C, Levy MZ. Chagas disease, migration and community settlement patterns in Arequipa, Peru. PLoS Negl Trop Dis. 2009;3:e567. doi: 10.1371/journal.pntd.0000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo ME, Rebonato C. Cross-reactivity in fluorescence tests for Trypanosoma and Leishmania antibodies. A simple inhibition procedure to ensure specific results. Am J Trop Med Hyg. 1969;18:500–505. doi: 10.4269/ajtmh.1969.18.500. [DOI] [PubMed] [Google Scholar]
- Drame PM, Poinsignon A, Besnard P, Le Mire J, Dos-Santos MA, Sow CS, Cornelie S, Foumane V, Toto JC, Sembene M, Boulanger D, Simondon F, Fortes F, Carnevale P, Remoue F. Human antibody response to Anopheles gambiae saliva: an immuno-epidemiological biomarker to evaluate the efficacy of insecticide-treated nets in malaria vector control. Am J Trop Med Hyg. 2010;83:115–121. doi: 10.4269/ajtmh.2010.09-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürtler RE. Sustainability of vector control strategies in the Gran Chaco Region: current challenges and possible approaches. Mem Inst Oswaldo Cruz. 2009;104(Suppl 1):52–59. doi: 10.1590/s0074-02762009000900009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway J, Beaty BJ, Rowland M, Scott TW, Sharp BL. The Innovative Vector Control Consortium: improved control of mosquito-borne diseases. Trends Parasitol. 2006;22:308–312. doi: 10.1016/j.pt.2006.05.003. [DOI] [PubMed] [Google Scholar]
- La Fuente C, Saucedo E, Urjel R. The use of microhaematocrit tubes for the rapid diagnosis of Chagas disease and malaria. Trans R Soc Trop Med Hyg. 1984;78:278–279. doi: 10.1016/0035-9203(84)90299-2. [DOI] [PubMed] [Google Scholar]
- Levy MZ, Bowman NM, Kawai V, Waller LA, Cornejo del Carpio JG, Cordova Benzaquen E, Gilman RH, Bern C. Periurban Trypanosoma cruzi-infected Triatoma infestans, Arequipa, Peru. Emerg Infect Dis. 2006;12:1345–1352. doi: 10.3201/eid1209.051662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy MZ, Quispe-Machaca VR, Ylla-Velasquez JL, Waller LA, Richards JM, Rath B, Borrini-Mayori K, del Carpio JG, Cordova-Benzaquen E, McKenzie FE, Wirtz RA, Maguire JH, Gilman RH, Bern C. Impregnated netting slows infestation by Triatoma infestans. Am J Trop Med Hyg. 2008;79:528–534. [PMC free article] [PubMed] [Google Scholar]
- Remoue F, Cisse B, Ba F, Sokhna C, Herve JP, Boulanger D, Simondon F. Evaluation of the antibody response to Anopheles salivary antigens as a potential marker of risk of malaria. Trans R Soc Trop Med Hyg. 2006;100:363–370. doi: 10.1016/j.trstmh.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Ribeiro JMC, Francischetti IMB. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu Rev Entomol. 2003;48:73–88. doi: 10.1146/annurev.ento.48.060402.102812. [DOI] [PubMed] [Google Scholar]
- Schofield CJ, Jannin J, Salvatella R. The future of Chagas disease control. Trends Parasitol. 2006;22:583–588. doi: 10.1016/j.pt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Sternberg JM, Johnston V, Medrano-Mercado N, Anderson JM, Hume JC, Valenzuela JG, Schaub GA, Billingsley PF. Antibody responses of domestic animals to salivary antigens of Triatoma infestans as biomarkers for low-level infestation of triatomines. Int J Parasitol. 2009a;39:1021–1029. doi: 10.1016/j.ijpara.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz A, Helling S, Collin N, Teixeira CR, Medrano-Mercado N, Hume JC, Assumpcao TC, Marcus K, Stephan C, Meyer HE, Ribeiro JM, Billingsley PF, Valenzuela JG, Sternberg JM, Schaub GA. Immunogenic salivary proteins of Triatoma infestans: development of a recombinant antigen for the detection of low-level infestation of triatomines. PLoS Negl Trop Dis. 2009b;3:e532. doi: 10.1371/journal.pntd.0000532. doi:510.1371/journal.pntd.0000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa ES, Nascimento MS, Stolf AM. Enzyme-linked immunosorbent assay with Trypanosoma cruzi excreted-secreted antigens (TESA-ELISA) for serodiagnosis of acute and chronic Chagas’ disease. Diagn Microbiol Infect Dis. 2001;39:169–176. doi: 10.1016/s0732-8893(01)00216-4. [DOI] [PubMed] [Google Scholar]
- Vega Chirinos S, Náquira Velarde C. Ministerio de Salud, INdS. Manual de procedimientos de laboratorio para el diagnóstico de la trypanosomiosis americana (enfermedad de Chagas) 2. Vol. 26. Serie de Normas Técnicas; Lima: 2006. Capíutlo VII Xenodiagnóstico; p. 106. [Google Scholar]