Abstract
StarBRITE is a one-stop, web-based research portal designed to meet the day-to-day needs of the Vanderbilt University and Meharry Medical College research community during the planning and conduct of research studies. StarBRITE serves as the main online location for research support addressing issues such as identification and location of resources, identification of experts, guidance for regulatory applications and approvals, regulatory assistance, funding requests, research data planning and collection, and serves as a central repository for educational offerings. To date, there have been more than 590,038 StarBRITE hits by more than 6582 cumulative users. We present here StarBRITE design objectives, details about technical infrastructure and system components, status report and activity metrics for the first 2.75-years of operation, and a report of lessons learned during organizing, launching and refining the portal.
Keywords: Biomedical Informatics, Clinical Research, Translational Research, Scientific Portfolio Management, Researcher Portal, Research Services
1. INTRODUCTION
The NIH Roadmap created a new national strategy requiring process and efficiency changes with an end goal to ultimately speed the translation of scientific discovery into effective healthcare. [1, 2] In October 2005, the National Center for Research Resources (NCRR), on behalf of the NIH, launched a new Roadmap Initiative: the Clinical and Translational Science Award (CTSA) program challenging academic health centers (AHCs) to improve the pace and uptake of translational research.[3] Critical program goals are to minimize barriers associated with moving basic science discoveries into clinical studies (T1) and clinical study findings into clinical practice (T2). [4, 5]
Organizations such as the American Medical Association (AMA), the Institute of Medicine, the Association of Academic Medical Colleges (AAMC) and the Clinical Research Forum (CRF) have analyzed clinical and translational research operations within member institutions.[6–8] Studies of AHCs have looked closely at clinical research infrastructure to identify gaps and potential solutions for translational research barriers. In 2007, members of the CRF Information Technology (IT) Roundtable subcommittee conducted a 2-year follow-up survey regarding the use of informatics and information technology within US academic medical centers (AMC)[7]. The survey indicated little progress made in research-driven IT infrastructure during the 2-year span, but cited evidence that institutions were currently planning for centralized and upgraded informatics capacity to support the research enterprise. In addition to basic IT infrastructure and services, survey respondents cited needs for the following informatics services: web-based grant proposal and study development applications; study conduct and administrative support applications such as enrollment tracking and electronic case report forms; electronic consent applications; research repositories; professional networking and collaboration software; and methods for integrating data from basic research, clinical research studies and clinical information systems.[7, 8] While some institutions reported partial coverage of informatics applications and services supporting the research enterprise, none reported having a comprehensive solution.
An internal research faculty/staff needs assessment survey was conducted at Vanderbilt in 2006 as part of our CTSA planning process. This survey identified true gaps in available resources as well as perceived gaps, where operational services actually existed but were not widely known. The survey highlighted a strong need for a one-stop, web-based research portal designed to meet the day-to-day needs of the research community during the planning and conduct of research studies. During CTSA planning exercises, this research portal was expected to be integral for the long-term success of the research enterprise and was therefore granted institutional support and resources prior to receiving CTSA funding. As a result, StarBRITE (Biomedical Research Integration, Translation and Education) was created in 2007 to support investigator awareness and training as well as the planning, conduct and management of studies within the Vanderbilt Institute for Clinical and Translational Research (VICTR) program.
StarBRITE is Vanderbilt’s virtual home for clinical and translational research, serving as a day-to-day guide for diverse scientific planning and research conduct. Embedded StarBRITE services address issues such as identification and location of resources, identification of experts, guidance for regulatory applications and approvals, regulatory assistance, funding requests, research data planning and collection, and serves as a central repository for educational offerings. StarBRITE audit logs and embedded usage tracking adhere to a ‘measure as you go’ philosophy with all components instrumented to help VICTR program management evaluate the overall CTSA research portfolio as well as subcomponents served through the portal. We believe that StarBRITE’s unique packaging of ‘just-in-time’ information and informatics services has contributed significantly to reducing research barriers within our VICTR program and furthermore that a description of the StarBRITE system will be useful and applicable to other institutions. We present here StarBRITE design objectives, details about technical infrastructure and system components, status report and activity metrics for the first 2.75-years of operation, and a report of lessons learned during organizing, launching and refining the portal.
2. StarBRITE
2.1 Design Objectives
The primary program goal in building StarBRITE was to bind data, information, and knowledge to effective action in order to promote and speed the design and conduct of research. A secondary goal was to routinely collect and analyze metrics to evaluate the impact of interventions designed to improve the research process. In developing and assembling components for inclusion, we followed three guiding principles:
Focus on researcher needs and maintain customer service focus;
Leverage existing resources; and
Plan for continuous improvement and expansion.
2.2 Technical Infrastructure
StarBRITE was developed at Vanderbilt University using the model view controller design pattern.[9] Front-end coding utilizes the PHP 5 object-oriented programming language (http://www.php.net), ExtJS cross-browser JavaScript library (http://www.extjs.com) with database abstraction using Zend Framework (http://framework.zend.com/). The software runs on an Apache web server (http://httpd.apache.org/) located behind university firewalls and access rights are limited to faculty, students and staff of Vanderbilt University and Meharry Medical College. LDAP-based methods are used to authenticate Vanderbilt users with automated table-based lookup authentication for Meharry Medical College research teams. StarBRITE back-end data are nominally stored in an Oracle 10g database server (www.oracle.com), but data extraction and integration operations also include persistent data connections and transfer with multiple external data sources using secure tunneling methods. StarBRITE uses a straightforward content management system (CMS) architecture to facilitate rapid and autonomous creation and editing of content by research support experts. Important features embedded within the CMS feature set include access control groups, WYSIWYG real-time editing tools, version auditing control and simple rollback options. StarBRITE uses common error logging functions across all sections and also a common click tracking methodology enabling seamless capture of user + access location + timestamp metrics. Context-sensitive help screens are database driven and employ snippet editing methods and logging similar to all other CMS content. Non-CMS data application modules (see next section) are embedded within StarBRITE using a standard template wrapper to create a consistent and seamless one-stop environment.
2.3 StarBRITE Components and Organizational Content
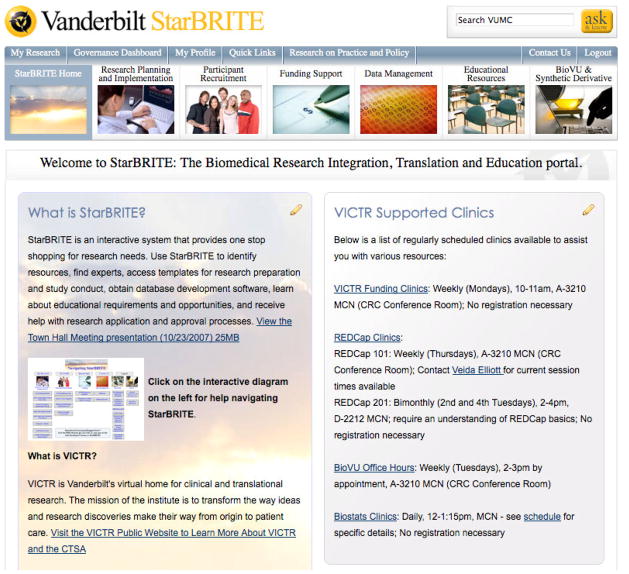
StarBRITE was designed to continually evolve to meet the needs of research teams and our CTSA program. The following content description represents a snapshot of system components and organization content after the first 2.75-years of operation. All informational content and embedded applications are presented within a consistent framework (Figure 1) organized into 10 major sections. Some major sections have existed in StarBRITE since inception, while others were added over time as we developed new classes of services to support the research enterprise. The guiding principal for StarBRITE’s organization framework is to put the right information into the hands of the right user at the right time. Following this principal, we defined major sections to correspond with classes of questions and services that a user might need at differing time points when creating hypotheses, planning for regulatory submission, acquiring funding and actively managing one or more clinical and translational research projects from inception to completion.
Figure 1.
StarBRITE Researcher Portal – Home Page
2.3.1 StarBRITE Home
This major section is StarBRITE’s default landing page and assists researchers in learning about new CTSA services, new funding opportunities and new policies expected to impact the global research enterprise. Examples of information panels include:
-
News items for research teams
Relevant information for research teams (e.g. 2009 NIH study section and grant scoring policies, NIH policy information for public access publishing)
-
CTSA services – promotion and awareness
New services or internal grant opportunities (e.g. BioVU DNA repository access policy and submission information)
-
CTSA news
Content concerning CTSA publications and program metrics.
2.3.2 Educational Resources
This major section is designed to assist research teams in understanding the milieu of educational opportunities available across Vanderbilt University and Meharry Medical Center where content is deemed particularly relevant for clinical and translational research. Offerings include:
-
Clinical and Translational Research Education Schedule
A centralized and combined listing of clinical and translational research education opportunities offered across all Vanderbilt departments. Topics and locations are typically presented two-weeks in advance and the on-line schedule is annotated with information concerning available credits for IRB and Responsible Conduct of Research training.
-
Responsible Conduct of Research Education (RCoRE)
Online registration and compliance tracking for students and trainees funded by the NIH who are required to receive training in responsible conduct of research.
-
Links to Training Programs and Available Training Resources
Examples include: Clinical and Translational Scientist Development - an integrated career development program for all physician-scientists, regardless of their scope of research, and for PhD-scientists engaged in translational or clinical research; Research Support Services Library – a catalog of books available for checkout to all research staff, faculty and students who work in research at Vanderbilt University; Clinical Research Immersion “Boot Camp” – online registration for a 7 hour session that provides clinical research staff with fundamental information for conducting clinical research at Vanderbilt; Required Training for Research Faculty and Staff – link to Collaborative IRB Training Initiative (CITI) training; Eskind Biomedical Library Resources – links to Vanderbilt University biomedical library and other electronic resources for researchers; Customized Education Sessions – researchers can request trainers to provide individual, departmental or large group educational sessions on topics related to the research enterprise.
2.3.3 Research Planning and Implementation
This major section is designed to assist research teams who are considering initiation of a new research project. Many of the sub-menu items deliver just-in-time information compiled by our Research Support Services team. Services also exist to assist research teams in gaining access to real or virtual experts necessary to formulate a study. Examples include:
-
Things to consider
Short descriptions and links to available resources (e.g. protocol, investigator’s brochure, confidential disclosure agreement, funding, staffing, required human subjects training, participant populations, help with study planning and design, feasibility budget, general resources);
-
Tools for grant writing
Organization of links, services and content designed to help research teams find relevant assistance when writing grant proposals (e.g. grant submission best practice guidelines, curated linkage to data and grant-related resources across all Office of Research programs, research resources grant-ready template language, listing of CTSA functions and infrastructure, power and sample size calculation tools).
-
Study Setup Assistance
Confidential, on-site consultative service sponsored by the Office of Research designed to assist teams initiating and conducting translational research projects;
-
Studio Requests
CTSA-funded 2-hour sessions bringing together relevant research experts in a particular methodology to focus on a specific stage of research (e.g. study design, grant preparation, manuscript preparation and finalization). [10]
-
Customized Action Plan
Short, structured computer-assisted interviews enabling research teams to answer questions about research plans and receive real-time guidance for efficiently navigating the regulatory review process and launching a study. [11]
2.3.4 Research on Practice and Policy
This major section is designed to provide research teams with access to VICTR core resources and services available for translating research into practice (‘T2’) Examples include:
-
Translating Clinical Discoveries into Practice Program
Stimulates and supports innovative health services, behavioral, epidemiologic and public health research that translates clinical discoveries into improved health outcomes.
-
Methodological Resource Cores
Encourages the development, implementation, analysis and interpretation of research that takes clinical discoveries into the practice setting and seeks to assess their impact on the health of communities. Methodological Cores include: Behavioral Measurement, Clinical Economics and Decision Analysis, Community-engaged Research, Database Analysis, Implementation Sciences/QI, Qualitative Research and Survey Research.
2.3.5 Funding Support
This major section is designed to assist research teams who need funding assistance for studies. Links to both external and internal institutional funding opportunities are presented to research teams here along with a comprehensive application process for CTSA pilot funding assistance. Available resources include:
-
VICTR Funding Requests and Scientific Portfolio Management
VICTR funding requests are managed through a single comprehensive submission tool designed to manage the entire VICTR research portfolio. The submission process includes a services ‘pick-list’ (> 400 resources) that serves to raise awareness of all CTSA funding possibilities and also to inform and drive the scientific and resource review process for individual studies. Projects are automatically categorized to one of three classes based on the total requested dollar amount of all services and resources (≤ $2,000, $2,000 to ≤ $10,000, and > $10,000). Once reviewed and approved by VICTR management, StarBRITE also provides instructions to research teams concerning redemption and tracking of all services.
-
Internal Pilot Funding Opportunities
Internal funding opportunities are often posted on the StarBRITE portal, with workflow for submission and tracking reused across programs. This resource is useful for departments and programs wishing to leverage existing infrastructure and greatly increases the efficiency of funding awareness, submission and evaluation.
-
External Funding Resources
Links to funding information deemed relevant for clinical and translational researchers (e.g. FIND Grants, Office of Research Funding, and the National Heart, Lung and Blood Institute);
2.3.6 Participant Recruitment
This major section is designed to assist research teams who are looking to recruit participants into active studies. Example services include:
-
Participant Recruitment Registry
ResearchMatch is a national disease-neutral research recruitment registry project designed to match potential participants with scientific teams conducting research at any participating CTSA institution.[12] ResearchMatch launched researcher services in March 2010 and replaced in StarBRITE a similar local Vanderbilt-specific recruitment registry operational since program launch.[13]
-
Clinical Study/Trial Public Registry
Tools enabling research teams to self-publish studies and trials to a public research awareness website.[14] Project publishing tools include auto-population of key study information fields from a central IRB data source and specific instructions concerning allowable content from IRB and Contracts regulatory groups.
-
Research Notifications Mass Email Distribution List
A service that allows researchers to email IRB-approved participant recruitment announcements to the Vanderbilt community. Members of the distribution list have the option to subscribe/unsubscribe with each email.
-
Record Counter
A ‘preparatory to research’ exploratory tool allowing researchers to query structured (e.g. ICD 9 codes, laboratory values, demographic fields) and unstructured (e.g. text keywords from clinic notes) data fields from a de-identified version of Vanderbilt’s electronic medical record called the Synthetic Derivative.[15] Queries may be performed for the purpose of determining the feasibility of conducting research studies at Vanderbilt, given historical patient conditions and volume. Aggregate datasets are returned in real-time and the Record Counter is available without IRB-consent.
2.3.7 Data Management
This major section is designed to assist research teams who need assistance with data planning, management and dissemination for research studies. Services include:
-
REDCap and REDCap-Survey
REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support traditional case report form data capture for research studies.[16] REDCap-Survey is a powerful tool designed for collecting data directly from study participants. Researchers create and design surveys in a secure web browser and engage respondents using a variety of notification methods. REDCap and REDCap-Survey are widely used in the Vanderbilt research enterprise and across a large consortium of institutional partners.[17]
2.3.8 BioVU & Synthetic Derivative
This major section is designed to provide research teams with access to de-identified data and biological samples necessary for the conduct of research. Services related to ‘secondary reuse of clinical data for the purpose of research’ are rapidly evolving. Current offerings include:
-
BioVU - De-Identified Biobank
A DNA biorepository of de-identified DNA extracted from discarded blood collected during routine clinical testing and linked to information in the Synthetic Derivative (SD), Vanderbilt’s de-identified electronic medical record.[15] The DNA biorepository project launched in February, 2007 and contains approximately 91,000 samples.
-
Synthetic Derivative – De-Identified Data Warehouse
A searchable database containing clinical information derived from Vanderbilt’s electronic medical record.[15] Investigators with IRB approval may use self-service user interface tools to query and view de-identified information for more than 1.95M patients. The resource is available to research teams with IRB-approval and may be used for hypothesis generation, retrospective research studies and large-scale genetic studies through linkage with de-identified DNA resources.
2.3.9 My Research
This major section is designed to provide rapid assimilation and display of project data across all regulatory domains governed by the Office of Research. Examples of data integration modules include:
-
Researcher Tools - Regulatory/Compliance Project Data
Data are collected and aggregated across regulatory group data systems (e.g. IRB, Grants, Contracts) into a cross-department research project data mart. From this data mart, StarBRITE provides researchers a snapshot of relevant information and stored documents in a single location.
-
Administrative Tools - Regulatory/Compliance Project Data
Regulatory group (IRB, Grants and Contracts, Department of Finance, VICTR) administrators and staff may view and cross-reference project-level data for all studies across the research enterprise.
2.3.10 Governance Dashboards
This major section is designed for use by VICTR administration in evaluating program components.
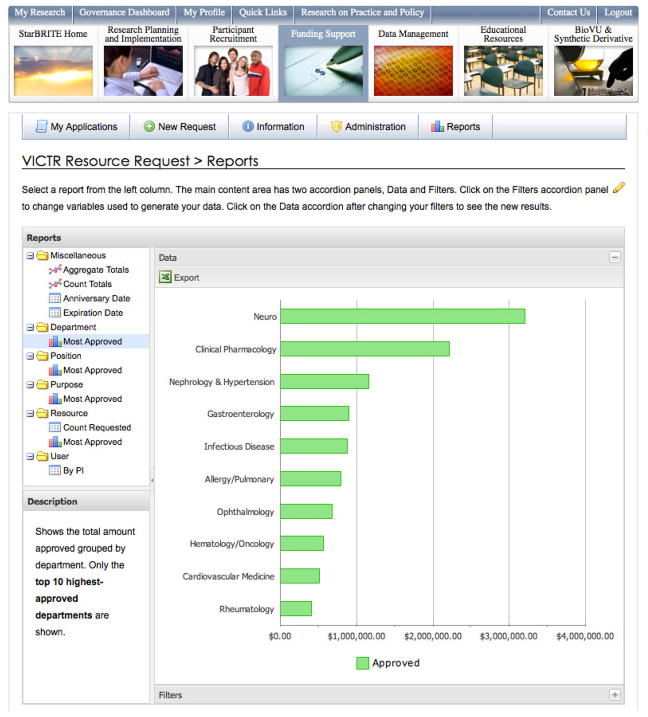
StarBRITE is instrumented to collect information about program utilization during normal use of the portal by research teams. Data are also routinely collected from external sources (e.g. VICTR patient care unit and laboratory systems) for presentation to institution leadership requiring information to evaluate programs. Drill-down data visualization dashboards (Figure 2) are available to evaluate the following programs: funding information for all VICTR supported projects, Customized Action Plans, Volunteer for Research Registry, REDCap, CRC Facility Utilization, VICTR Laboratory Utilization, Training Programs and StarBRITE utilization itself. Real-time graphics and summary statistics for program-related activity are routinely used to support VICTR program governance and advisory board meetings.
Figure 2.
Sample VICTR Dashboard - Resources Approved by Department (Top 10)3
2.3.11 User Questions and Comments
The majority of StarBRITE pages contain this minor section inviting end-users to ask a question or make a suggestion concerning the research portal. Additionally, a Contact Us section provides an opportunity for research teams to submit research-related questions for response within 1-business day. All information requested through StarBRITE is automatically logged and forwarded by electronic mail to the Vanderbilt Research Support Services group.
3. STATUS UPDATE
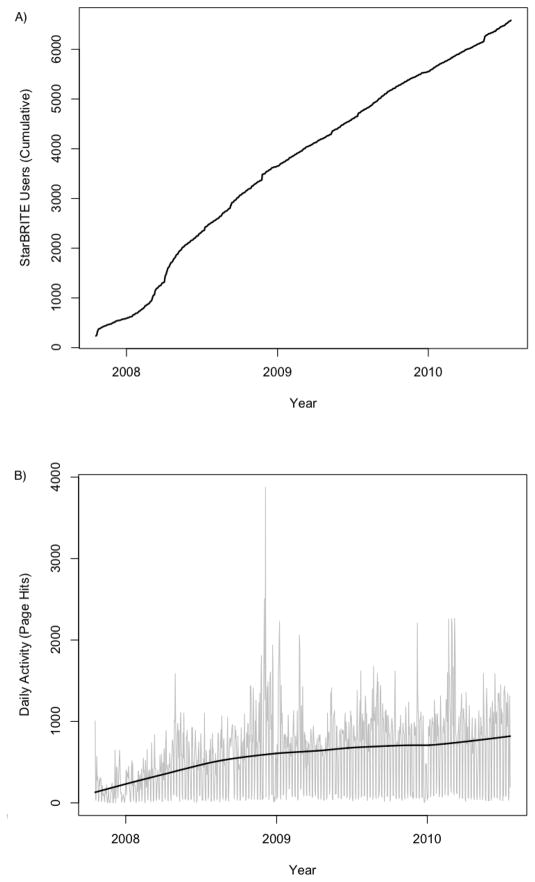
StarBRITE was formally introduced to the Vanderbilt University and Meharry Medical College research community on October 23, 2007 during a town hall meeting designed to introduce a new CTSA award. Since this time, the number of individuals entering and using the system has steadily grown. Vanderbilt University employs 20,000 full- and part-time individuals and has approximately 12,500 undergraduate- and graduate-students, with the majority of schools (College of Arts and Science, Blair School of Music, Divinity School, School of Engineering, Graduate School, Law School, School of Medicine, School of Nursing, Owen Graduate School of Management, Peabody College of Education and Human Development) and facilities housed within a single campus.[18] Meharry Medical College employs approximately 1,000 full- and part-time individuals and includes a medical school, dental school, graduate school and an allied health school.[19] We logged usage activity for nearly 6,582 unique faculty-, staff- and student-users during the time period between October 2007 and July 2010 (Figure 3A). We also tracked daily activity of the StarBRITE portal during the same time interval (Figure 3B). These data suggest sustained utilization by our research community and we believe the absolute number of users in the system, the rate of new users entering the system over time and also unsolicited positive feedback from our research community all present strong evidence for success of the program.
Figure 3.
A) Cumulative StarBRITE Users; B) StarBRITE Daily Activity
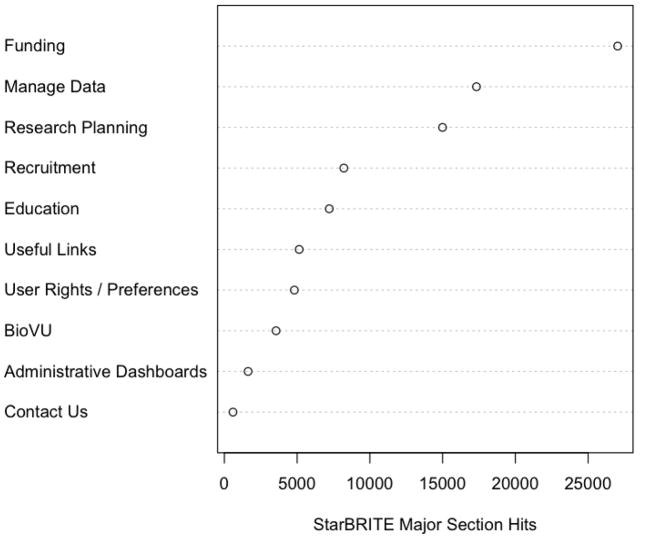
Understanding how researchers are leveraging the portal is important for system evaluation. Figure 4 provides high-level utilization information for each major StarBRITE section, where the x-axis represents the quantity of times an end-user landed on a specific major section page. These major section pages serve as launch sites for static sub-content pages and/or data-driven applications related to the major section content areas. End-user viewing counts of the major section pages serve as a strong surrogate measure of overall interest and need for classes of services within the StarBRITE portal. The dot plot shown in Figure 4 represents user activity across the entire 2.75-year project lifecycle, but it should be noted that StarBRITE is constantly evolving and offerings within each major section have changed over time. Examples include the BioVU major section (added during the 2009 calendar year) and the Research on Practice and Policies major section (added in Q3 2010 and not included in Figure 4 counts).
Figure 4.
StarBRITE Major Section Activity Metrics (October, 2007 – July, 2010)33
StarBRITE content pages are instrumented to automatically record utilization metrics on a daily basis. Static information pages may only need to be viewed once to deliver value (e.g. procedures for setting up monitor access to view the electronic medical record), but data driven applications (e.g. REDCap data services, recruitment services) might be used many times by a single user on a single day. Table 1 provides a status update for many of StarBRITE’s embedded data applications. [11, 13, 15, 16] Co-location of static content pages and data-driven applications within the StarBRITE major section framework has proven effective and drives awareness for all services.
Table 1.
StarBRITE Current Application Metrics
Customized Action Plan
|
4. LESSONS LEARNED
StarBRITE was created with the understanding that the system would need to continuously evolve to meet the needs of diverse research teams. During the process of continuously building and launching new services, we learned lessons that might be valuable for other institutions considering researcher portal services:
A team approach which pairing informatics (to guide methodology) with research experts (to guide needs-based content and services) is beneficial. We paired a new group from the Office of Research Informatics with an existing group from Research Services. The combined group has met weekly since approximately one year prior to the official project launch and continues to meet on a regular basis.
Include highly utilized services in the portal in the initial iteration, to ensure usage and generate awareness and familiarity. Used systems will naturally improve given feedback from those interacting with the software.
Consider a range of researcher use cases from experienced to junior investigators
Meet the immediate needs of the research teams by lowering the burden of initiating and conduct of scientific research.
Build as little as possible, leveraging services already in place across the institution. Examples of systems and data that can be leveraged include: e-IRB + grants/contracts applications, recruitment support applications, core services information, laboratory systems data and electronic data capture programs.
Create a simple framework and set of common page layout designs for content. Adherence to a relatively flat content framework with minimal deviation from design templates will ensure maximum familiarity and continuity for end-users and eliminate need for expensive design discussion/programming over the course of evolving the program.
Building a CMS methodology to feed common layout pages with appropriate edit rights for content experts also ensures that new static content pages can be easily created and maintained with little work required from programmers.
Do not rely solely on an electronic system to assist research teams. We included within StarBRITE the ability for research teams to ask questions or make suggestions at any time. The Research Support Services group provides quick responses by answering directly or routing questions to appropriate sources for resolution. This group also analyzes questions/suggestions and uses the feedback to suggest new content for StarBRITE.
Launch the research portal via broad-based educational sessions designed to highlight the benefits of system usage.
Track usage of all system components. Building automated reports for real-time presentation and export of system and program metrics is much less labor intensive than assisting with evaluation metrics on an ad hoc basis.
5. CONCLUSION
StarBRITE has become an essential component of research support at Vanderbilt University and Meharry Medical College. We anticipate the system will perpetually evolve to meet the needs of our research community, but it has already proven effective at facilitating improvement in clinical and translational research plus measuring that improvement as a byproduct of system usage.
Acknowledgments
Supported in part by Vanderbilt CTSA grant 1 UL1 RR024975 from the National Center for Research Resources, National Institutes of Health. We also gratefully acknowledge assistance from administrative and technical groups within the Vanderbilt Institutional Review Board (Denise Roe, Julie Ozier, Dena Johnson, Jeff Roe, Chris Boeing, Erick Kapamas) and Grants and Contracts groups (Libby Salberg, Janet Frye, Chris Renner, Peggy Pobst).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
VI. REFERENCES
- 1.Overview of the NIH Roadmap. Office of Portfolio Analysis and Strategic Initiatives, National Institutes of Health; 2006. [Google Scholar]
- 2.Zerhouni EA. Clinical research at a crossroads: the NIH roadmap. J Investig Med. 2006;54:171–3. doi: 10.2310/6650.2006.X0016. [DOI] [PubMed] [Google Scholar]
- 3.Zerhouni EA, Alving B. Clinical and translational science awards: a framework for a national research agenda. Transl Res. 2006;148:4–5. doi: 10.1016/j.lab.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Woolf SH. The meaning of translational research and why it matters. JAMA. 2008;299:211–3. doi: 10.1001/jama.2007.26. [DOI] [PubMed] [Google Scholar]
- 5.Sung NS, Crowley WF, Jr, Genel M, Salber P, Sandy L, Sherwood LM, Johnson SB, Catanese V, Tilson H, Getz K, Larson EL, Scheinberg D, Reece EA, Slavkin H, Dobs A, Grebb J, Martinez RA, Korn A, Rimoin D. Central challenges facing the national clinical research enterprise. JAMA. 2003;289:1278–87. doi: 10.1001/jama.289.10.1278. [DOI] [PubMed] [Google Scholar]
- 6.Breaking the Scientific Bottleneck. Clinical Research: A National Call to Action. Washington, DC: 1999. [Google Scholar]
- 7.DiLaura R, Turisco F, McGrew C, Reel S, Glaser J, Crowley WF., Jr Use of informatics and information technologies in the clinical research enterprise within US academic medical centers: progress and challenges from 2005 to 2007. J Investig Med. 2008;56:770–9. doi: 10.2310/JIM.0b013e3175d7b4. [DOI] [PubMed] [Google Scholar]
- 8.Turisco F, Keogh D, Stubbs C, Glaser J, Crowley WF., Jr Current status of integrating information technologies into the clinical research enterprise within US academic health centers: strategic value and opportunities for investment. J Investig Med. 2005;53:425–33. doi: 10.2310/6650.2005.53806. [DOI] [PubMed] [Google Scholar]
- 9.Gamma E. Design patterns: elements of reusable object-oriented software. Addison-Wesley; 1995. [Google Scholar]
- 10.Kain K. Promoting translational research at Vanderbilt University’s CTSA institute. Dis Model Mech. 2008;1:202–4. doi: 10.1242/dmm.001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pulley JM, Harris PA, Yarbrough T, Swafford J, Edwards TE, Bernard GR. An informatics-based tool to assist researchers in initiating research at an academic medical center: Vanderbilt Customized Action Plan (V-CAP) Acad Med. 2009 doi: 10.1097/ACM.0b013e3181c481bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NIH Announces First National Research Study Recruitment Registry. Available at http://www.nih.gov/news/health/nov2009/ncrr-10.htm.
- 13.Harris PA, Lane L, Biaggioni I. Clinical research subject recruitment: the Volunteer for Vanderbilt Research Program www.volunteer.mc.vanderbilt.edu. J Am Med Inform Assoc. 2005;12:608–13. doi: 10.1197/jamia.M1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snyder B. New Web Site Aims to Boost Clinical Trial Recruitment. Vanderbilt-Ingram Cancer Center; 2008. ( http://www.vicc.org/news/?p=86) [Google Scholar]
- 15.Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, Masys DR. Development of a large-scale de-identified DA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84:362–9. doi: 10.1038/clpt.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.REDCap Consortium. Available at http://www.project-redcap.org.
- 18.Re: VU: Quick Facts About Vanderbilt. Available at http://www.vanderbilt.edu/info/facts/
- 19.Meharry Medical College Employee Statistics. Available at http://www.mmc.edu/students/employeestat.html.