Abstract
Lethal factor is a protease, one component of Bacillus anthracis exotoxin, which cleaves many of the mitogen-activated protein kinase kinases (MEKs). Given the importance of MEK signaling in tumorigenesis, we assessed the effects of anthrax lethal toxin (LeTx) on tumor cells. LeTx was very effective in inhibiting mitogen-activated protein kinase activation in V12 H-ras-transformed NIH 3T3 cells. In vitro, treatment of transformed cells with LeTx caused them to revert to a nontransformed morphology, and inhibited their abilities to form colonies in soft agar and to invade Matrigel without markedly affecting cell proliferation. In vivo, LeTx inhibited growth of ras-transformed cells implanted in athymic nude mice (in some cases causing tumor regression) at concentrations that caused no apparent animal toxicity. Unexpectedly, LeTx also greatly decreased tumor neovascularization. These results demonstrate that LeTx potently inhibits ras-mediated tumor growth and is a potential antitumor therapeutic.
Mitogen-activated protein kinase (MAPK) kinases (MEKs) play pivotal roles in a variety of signal-transduction pathways, aspects of which are critical for cell cycle progression and differentiation (reviewed in ref. 1). Activated MAPK or elevated MAPK expression has been detected in a variety of human tumors, including breast carcinoma and glioblastoma, as well as primary tumor cells derived from kidney, colon, and lung tissues (2–5). MEK1 and MEK2 phosphorylate and activate extracellular signal-regulated/mitogen-activated protein kinases 1 and 2 (ERK/MAPK1/2) in response to activation by the ras pathway. Anthrax lethal factor (LF) is a protease that cleaves members of the MEK family including MEKs 1, 2 (6, 7) and 3 (8). LF-induced proteolysis of MEK1 blocks MAPK activation (6, 9). LF is produced by Bacillus anthracis, the Gram-positive bacterium responsible for the disease anthrax. B. anthracis produces an exotoxin consisting of three proteins; protective antigen (PA), LF, and edema factor (EF; for a recent review, see ref. 10). By itself, PA is nontoxic (11, 12). PA serves to translocate EF and LF from the exterior of the host cell to the cytoplasm via the endosomal pathway (13). EF is an adenylate cyclase that causes a dramatic elevation of cAMP concentrations within the host cell (13). Combinations of EF plus PA, or edema toxin, cause skin edema characteristic of anthrax, but are not toxic when injected intravenously into mice or rats (14, 15). By contrast, combinations of LF plus PA, or lethal toxin (LeTx), do not cause skin edema but are lethal when injected intravenously (14, 15). Based on previous work demonstrating a prominent role for the MEK-MAPK signaling pathway in cancer (2–5, 16), we evaluated the effect of LeTx on ras-mediated transformation in vitro and in vivo. Here we demonstrate that LeTx is a potent inhibitor of ras-mediated transformation in vitro. More importantly, we show that LeTx not only inhibits in vivo tumor growth, but also inhibits tumor vascularization at a concentration that has no apparent side effects.
Methods
Cell Lines and Treatments.
NIH 3T3 (490) mouse fibroblast cells expressing either empty vector (pDCR, mammalian expression vector) or transforming human H-ras (V12) protein (17) were grown in DMEM supplemented with 10% (vol/vol) FBS and 1% penicillin/streptomycin, and were maintained at 37°C in a humidified atmosphere of 5% CO2. PA, inactive LF(E687C), and LF were purified from cultures of B. anthracis, as described (18).
Cell Morphology, Immunoblotting, and Immunostaining.
Cells were cultured in 10-cm dishes or on slides under the conditions described above. When cells reached 30–50% confluency, 100 ng/ml PA plus 10 ng/ml inactive LF(E687C), PA plus LF (100 ng/ml PA plus 10 ng/ml LF), or PD98059, an inhibitor of MEK1 activation (50 μM from a 50 mM stock in DMSO), were added to the culture medium. Cells were cultured another 24 h, at which point they were lysed for immunoblotting (10 μg of protein per lane), or fixed for immunostaining as outlined (19, 20) with antibodies raised against human MEK1 (NT, 1:1,000; Upstate Biotechnology, Lake Placid, NY), phosphorylated MAPK (pTEpY, 1:4,000; Promega), MAPK1/2 (K-23/C-14, 1:4,000; Santa Cruz Biotechnology), cathepsin L (M-19, 1:100; Santa Cruz Biotechnology), β-tubulin (TUB2.1, 1:1,000; Sigma) or actin (AC-40, 1:250; Sigma), and Oregon Green-conjugated anti-mouse IgG antibody (1:250; Molecular Probes). Slides were examined with confocal laser scanning microscopy.
Cell Proliferation Analysis.
Cells were cultured in 96-well plates under the conditions described above. When cells reached 30–50% confluency, 100 ng/ml PA plus 10 ng/ml inactive LF(E687C) (21), PA plus LF [100 ng/ml PA plus 10 or plus 1 ng/ml LF (high and low, respectively)], or PD98059 (25 μM or 50 μM from a 50 mM stock in DMSO) were added to the culture medium. Cells were cultured another 48 h, at which point cell proliferation was assayed by using the CellTiter 96 Aqueous NonRadioactive Cell Proliferation Assay (Promega), according to the manufacturer's instructions. Data are presented relative to proliferation of control samples incubated in the presence of culture medium alone and as an average of at least three measurements (±SD).
In Vitro Tumorigenicity Assays.
Soft agar colony formation.
Trypsinized cells were washed with Ca2+/Mg2+-free PBS, resuspended at a concentration of 1 × 104 cells per ml in DMEM containing 10% calf serum and 0.5% (wt/vol) Noble agar (Difco), in the presence or absence of LeTx (100 ng/ml PA plus 10 ng/ml LF), as indicated below, and layered over a 0.5-ml solid plug of DMEM-containing 1% agar in 24-well plates. Cells were incubated at 37°C with 5% CO2 for 1 week, during which time the cells were monitored daily. The images shown in Fig. 2 were made at the end of this time. Each sample was assayed in triplicate in three separate experiments.
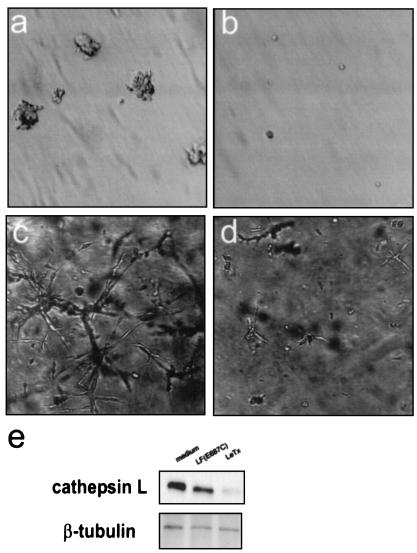
Figure 2.
Effects of LeTx on anchorage independent colony formation and extracellular matrix invasion. We evaluated the ability of V12 H-ras-transformed cells to form colonies in Noble agar (a and b) or invade Matrigel (c and d) in the presence (b and d) or absence (a and c) of LeTx. (e) Levels of cathepsin L were assayed by immunoblotting lysates of V12 H-ras-transformed NIH 3T3 cells which had been treated with medium alone, PA plus inactive LF(E687C), or LeTx. Blots were stripped and reprobed with antibodies raised against β-tubulin to control for lane loading.
Extracellular matrix invasion assay.
Three-dimensional Matrigel (Becton Dickinson) invasion assays were performed as described (22). Approximately 2.5 × 104 cells were mixed with growth factor-reduced Matrigel supplemented with medium alone, inactive LeTx [100 ng/ml PA plus 10 ng/ml LF(E687C)], or LeTx (100 ng/ml PA plus 10 ng/ml LF). The cell suspension was cultured for up to 1 week, during which time the cells were monitored daily. The images shown in Fig. 2 were made at day 4. Each sample was assayed in triplicate in three separate experiments.
In Vivo Tumorigenicity Assays.
V12 H-ras-transformed NIH 3T3 cells (105 cells in a volume of 100 μl) were injected s.c. into 10 athymic nude mice on the left and right sides of the dorsal area behind the last rib. When tumors reached a size of 5–7 mm (approximately 2 weeks), the mice were divided into two groups. Group A was sham-injected (insertion of the needle only) intratumorally on the left side and injected on the right side with 100 μl of buffered saline, whereas group B was injected on the left side with 100 μl of buffered saline and on the right side with 10 μg of PA plus 2 μg of LF in 100 μl of buffered saline. Injections continued once daily for a total period of 5 days. The sizes of the tumors were monitored after injection. When control tumors attained a diameter of 20 mm (approximately 3–4 weeks), the mice were euthanized, and the tumors were dissected, trimmed, and fixed in formalin for further analyses. Paraffin tissue sections (5 μm) were stained with mouse monoclonal antibodies against two endothelial cell markers, CD31 (BBa7, 1:100; Research Diagnostics, Flanders, NJ) and CD34 (M7 165, 1:25; Dako), were treated with FITC-conjugated secondary antibodies, and imaged with a Zeiss CLSM 410 confocal microscope. Images were made of representative sections that showed histologic features of a fibrosarcoma. The images were made with a water immersion 40× high numerical aperture lens and stored digitally to file.
Results
Cellular Consequences of LeTx Treatment.
To test the effects of LeTx on NIH 3T3 cells, we first confirmed that LeTx was active on both nontransformed and oncogenic V12 H-ras-transformed NIH 3T3 cells by performing immunoblot analyses on lysates of cells that had been incubated 24 h in the presence of inactive LeTx [PA plus LF(E687C), which contains a point mutation in its Zn2+-binding site], LeTx, or PD98059, a compound that preferentially inhibits MEK1 activation (refs. 23 and 24; Fig. 1 a and b). As described (6), treatment of both V12 H-ras-transformed and nontransformed NIH 3T3 cells with LeTx, but not inactive LeTx or PD98059, caused the loss of NH2-terminal epitopes of MEK1, indicating that LF had cleaved intracellular MEK1. We assayed the consequences of proteolysis on downstream MAPK activity by using antibodies specific for phosphorylated (active) MAPK. Whereas the levels of total MAPK remained constant under all conditions in both cell types, levels of phosphorylated MAPK decreased in response to LeTx, but not inactive LeTx. Treatment of cells with PD98059 caused a similar but less pronounced reduction in levels of phosphorylated MAPK in each cell type.
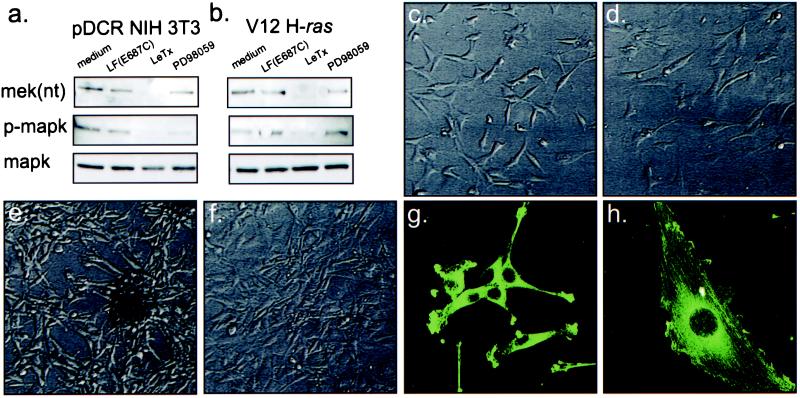
Figure 1.
The effects of LeTx on MAPK activation and cell morphology. Immunoblotting of lysates from nontransformed (pDCR NIH 3T3) (a) and V12 H-ras-transformed NIH 3T3 cells (b) show loss of NH2-terminal epitopes of MEK and phospho-epitopes of MAPK after treatment of cells with LeTx but not in cells treated with either medium alone, 100 ng/ml PA plus inactive 10 ng/ml LF(E687C), PA plus LF (100 ng/ml PA plus 10 ng/ml LF), or PD98059 (50 μM from a 50 mM stock in DMSO). Nontransformed cells (c) possessed an irregular, flattened morphology which was not substantially altered by 24 h exposure to LeTx (d). In contrast, after 24 h LeTx treatment, the well defined, elongated, spindle-like shape of V12 H-ras-transformed NIH 3T3 cells (e), reverted to a shape resembling a nontransformed cell (f). Immunostaining of V12 H-ras-transformed NIH 3T3 cells incubated in the absence (g) or presence (h) of LeTx for 24 h for actin (green) showed actin stress fibers formed after LeTx treatment.
It is well established that NIH 3T3 cells transformed by oncogenic V12 H-ras undergo a change in morphology from an irregular flattened shape into a spindle-like form with an associated loss in actin stress fibers (reviewed in ref. 25; Fig. 1 c and e). LeTx treatment was accompanied by a reversion of the cell shape of V12 H-ras-transformed NIH 3T3 cells from a spindle-like shape (Fig. 1 e and g) to an irregular flattened shape (Fig. 1f) with an enlarged nucleus and prominent actin stress fibers (Fig. 1h). Treatment of cells with PA plus inactive LF(E687C) had no effect on cell morphology (data not shown). Similarly, it has been reported that treatment of cells with PD98059 or U0126, a compound that acts in a fashion similar to PD98059 but inhibits both MEK1 and MEK2 (26), causes morphological reversion of transformed cells (24, 27).
MEK/MAPK signaling is known to play an important role in mitogenesis. To determine the effects of LeTx on cell growth, we cultured cells in 96-well plates in the presence or absence of PA plus LF or inactive LF(E687C) for 48 h (Table 1). For comparison, we also tested the effects of PD98059. Treatment with 50 μM of PD98059 for 48 h caused a 50% inhibition of growth of nontransformed NIH 3T3 cells, whereas only a 25% inhibition was found in cells transformed with V12 H-ras. In contrast, treatment with LeTx caused only a modest (20–35%) inhibition of nontransformed NIH 3T3 cell proliferation and did not significantly inhibit proliferation of V12 H-ras-transformed cells.
Table 1.
The effects of LeTx upon cell proliferation
| Cell type | Relative proliferation
|
|||||
|---|---|---|---|---|---|---|
| Untreated | PA + LF(E687C) | Low LeTx | High LeTx | 25 μM PD98059 | 50 μM PD98059 | |
| NIH 3T3 | 1 | 1.00 ± 0.04 | 0.81 ± 0.08 | 0.63 ± 0.07 | 0.70 ± 0.09 | 0.47 ± 0.03 |
| V12 H-ras | 1 | 0.94 ± 0.02 | 1.05 ± 0.02 | 0.87 ± 0.11 | 0.79 ± 0.07 | 0.77 ± 0.03 |
Cell proliferation was assayed in nontransformed (pDCR) or V12 H-ras-transformed NIH 3T3 cells treated with either medium alone, 100 ng/ml PA plus inactive 10 ng/ml LF(E687C), PA plus LF [100 ng/ml PA plus 10 or 1 ng/ml LF (high and low, respectively)], or PD98059 (25 μM or 50 μM from a 50 mM stock in DMSO) as described in Methods. Data are presented relative to proliferation of control samples incubated in the presence of culture medium alone and as an average of at least three measurements (±SD).
Effects of LeTx on Anchorage Independent Growth and Invasion.
Tumor growth and invasion is a complex multistep process that involves anchorage independent growth, motility, and proteolytic degradation of the extracellular matrix. Aspects of these processes may be simulated in vitro by measuring a cell's ability to (i) grow independently of substrate adhesion and form colonies in a soft agar suspension, and (ii) degrade an artificial extracellular matrix (basement membrane Matrigel). Nontransformed NIH 3T3 cells suspended in soft agar fail to proliferate, and they remain as single cells in suspension (28). By contrast, V12 H-ras-transformed cells continue to proliferate in the absence of substrate adhesion and form colonies of cells. However, LeTx completely prevented colony formation of V12 H-ras-transformed cells (Fig. 2 a and b). Moreover, LeTx prevented V12 H-ras-transformed cells from invading, or branching into, the extracellular matrix (Fig. 2 c and d). Invasiveness in Matrigel has been correlated with the expression of extracellular matrix-degradative enzymes such as the MAPK-dependent cysteine-protease, cathepsin L (29, 30). To determine whether LeTx inhibited cathepsin L expression, we treated lysates of cells with LeTx and subjected them to immunoblot analysis with antibodies to cathepsin L. Our results clearly demonstrated that LeTx blocked cathepsin L expression in V12 H-ras-transformed cells (Fig. 2e).
Effects of LeTx on in Vivo Tumorigenicity.
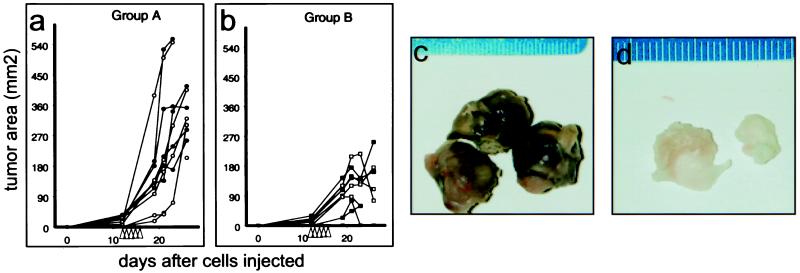
Because no in vitro assay can adequately mimic all aspects of tumorigenicity and predict potential therapeutic value, it was necessary to assess the effects of LeTx in vivo. We injected V12 H-ras-transformed cells s.c. into the left and right dorsal areas of 10 athymic nude mice. Preliminary experiments suggested that intratumoral injections of LeTx affected distant tumor growth via a systemic route. Therefore, when tumors had grown to a size of 5–7 mm in diameter, we divided the mice into two groups, A and B. In group A, we sham-injected tumors on the left side and injected those on the right side with buffered saline. In group B, we injected tumors on the left side with LeTx (10 μg of PA and 2 μg of LF) and injected those on the right side with buffered saline. We continued these injections once daily for a total of 5 days and monitored tumor size (length × width) thereafter. Whereas we found that tumors in mice of group A were considerably larger than those in group B (Fig. 3), we were unable to discern any differences between the sizes of tumors on the left and right hand sides of mice within the same group. Importantly, we observed that several tumors from mice in group B regressed in size over the course of the experiment. Moreover, although it was apparent that the average mass of tumors removed from mice in group B (1.35 ± 0.77 g) was significantly smaller than those removed from control mice in group A (3.67 ± 1.25 g; Student's t test, P = 0.002), we failed to find significant differences in the masses of tumors on the left and right hand sides of mice within the same group. These results also show that LeTx injected into one tumor of a group B mouse could systemically affect growth of the tumor implanted on the other side of the same animal. Remarkably, by using this dose regimen, all LeTx injected mice appeared healthy in all other respects throughout the course of the experiment.
Figure 3.
The effects of LeTx on V12 H-ras-transformed NIH 3T3 xenografts in athymic nude mice. Growth of tumors derived from V12 H-ras-transformed NIH 3T3 cells was measured after either sham injection or injection with Hanks' buffered saline solution (HBSS) (a), or after injection with either HBSS or HBSS containing PA and LF (b). Open symbols indicate the tumor on the left side; closed symbols indicate the tumor on the right. Tumor size is expressed as the product of their measured length and width. Arrowheads on the x axis indicate the times of injection. The appearance of tumors from group A (c) and group B (d) are shown adjacent to a ruler indicating tumor size in millimeters.
Histological examination of tumors excised from both groups of mice revealed classic fibrosarcoma tumors with high mitotic indices (Fig. 4 c and g). Whereas all tumors examined showed some degree of noninflammatory necrosis, tumors derived from the LeTx-injected group B mice showed a greater level of necrosis than tumors from control group A mice (Fig. 4 d and h). In addition, the external coloration of tumors removed from the two groups differed markedly; those from group A were a mottled red-purple, whereas those from group B were uniformly pale yellow (Fig. 3 c and d). This result strongly suggested that LeTx inhibits tumor angiogenesis in vivo. To verify this idea, we immunostained sections of these tumors with antibodies to CD31 (Fig. 4 a and e) and CD34 (Fig. 4 b and f), markers of vascularization (reviewed in ref. 31), and found that levels of each were dramatically reduced in tumors excised from LeTx injected mice.
Figure 4.
Histological analyses of tumors derived from LeTx-injected mice. Tumors excised from groups A (a–d) and B (e–h) mice were sectioned and immunostained with antibodies to the angiogenic markers CD31 (a and e) or CD34 (b and f) or stained by hemotoxylin and eosine and shown at low (×40) and high (×100) magnification.
Discussion
MEK inhibitors such as PD98059, U0126, and PD184352 inhibit tumor cell growth in vitro or in vivo (24, 26, 32). Each differs somewhat in their substrate specificities and affinities. PD98059 inhibits MEK1 and, to a lesser extent, MEK2, with IC50s of ≈4 and 50 μM, respectively (23). U0126 inhibits both MEK1 and MEK2, with IC50s of ≈0.07 and 0.06 μM, respectively (26). U0126 has also been reported to inhibit p70S6K phosphorylation through an MEK-independent mechanism (27). PD184352 inhibits MEK1 with an IC50 of ≈0.02 μM (32). Although LF has been demonstrated to inactivate only MEK1 (7, 10), it also cleaves and, therefore, probably inactivates MEK2 (7, 33) and MEK3 (9). Furthermore, based on sequence homology, it is likely that additional LF substrates will be found within the MEK family (11) or other regulatory transduction pathways. Indeed, we have found that LF also cleaves MEKs 4, 6, and 7 in vitro (N.S.D., unpublished data). Thus, the inhibitory activities of LF may not be assumed to be equivalent to those of other MEK inhibitors.
Treatment of V12 H-ras-transformed cells with LeTx was shown to cause a rapid and dramatic alteration of cell morphology and the appearance of actin stress fibers. This effect is likely to be mediated by inhibition of MEK1 and/or MEK2 activity, because similar effects are induced by PD98059 (24) and U0126 (27). Treatment of V12 H-ras-transformed cells with LeTx was also shown to inhibit soft agar colony formation as well as extracellular matrix invasion in vitro. Further, we found that LeTx inhibited expression of cathepsin L, an enzyme that is involved in the degradation of the extracellular matrix and whose expression requires high levels of MAPK activity in ras-transformed NIH 3T3 cells (29, 30). Again, because similar changes in soft agar colony formation have been reported to occur after treatment with U0126 (27), and the expression of dominant-negative MAPK1 and MAPK2 inhibits the ability of V12 H-ras-transformed cells to grow in soft agar and to invade Matrigel basement membrane (29), it is likely that these changes are largely mediated by LeTx inhibition of MEK1 and/or MEK2.
However, it was interesting that LeTx had only a modest effect on cell proliferation when compared with PD98059 (24) or PD184352 (32), particularly because LeTx more effectively inhibited MAPK in V12 H-ras-transformed cells than did PD98059 (see Fig. 1 a and b). This result indicates that the activities of LF and MEK inhibitors are not identical.
It is not immediately evident why LeTx does not inhibit the proliferation of V12 H-ras-transformed cells. However, this observation may explain, in part, why LeTx-injected mice appeared healthy in all respects throughout the experiment despite the fact that injected LeTx was able to affect uninjected tumors via a systemic route. Intravenous injection of a similar amount of LeTx into a mouse might have been expected to have a detrimental effect on the health of the mouse (the LD50 in mice is 12.5 μg of PA and 2.5 μg of LF; refs. 34 and 35). However, because the toxin was injected intratumorally rather than intravenously, the amount of toxin that became systemic may have been considerably less.
Each of the above in vitro assays measures isolated aspects of the transformed phenotype. Although useful, they cannot duplicate actual tumor growth in vivo. For example, the rate at which a primary tumor develops in vivo is not simply a reflection of the rate at which its cells divide, but rather is a function of a complex series of cellular activities including proliferation, vascularization, and invasion. Our results clearly demonstrate that LeTx reduces tumor growth in vivo. That this reduction was achieved in part by cell death is evident from the necrotic appearance of tumors excised from the LeTx-injected animals. The reason for this cell death is not clear, because in vitro studies failed to demonstrate a cytotoxic effect on these cells. It could be that sustained treatment of cells would eventually have led to a cytotoxic response in vitro. Alternatively, the necrosis may be related to the poor vascularization observed in tumors derived from LeTx-injected animals. It is not immediately apparent why LeTx should inhibit vascularization. Although a role for MEK/ERK activity in angiogenic growth factor signaling has been reported (36–41), Sebolt-Leopold et al. (32) failed to note any obvious effects of PD184352 on tumor vascularization. In addition, expression of dominant-negative MEK in murine angiosarcoma cells inhibits growth in soft agar, but has no effect on the tumorigenicity of xenografts in nude mice (42). However, the induction of fibroblast growth factor-binding protein, which binds and stabilizes the angiogenic stimulator fibroblast growth factor (43, 44), has recently been shown to depend on both MEK/MAPK and p38 signal transduction pathways (45). Thus, the combined inhibition of these pathways by LeTx may be necessary for the effective inhibition of tumor vascularization and concomitant growth. In conclusion, our results show that LeTx has a therapeutic potential that exceeds expectations based solely on its MEK1 inhibitory activity, and that its use in vivo may represent an effective strategy for inhibiting tumor growth.
Acknowledgments
We thank S. Boone and B. Buckner for technical assistance and Drs. A. Chopra and J.-F. Bodart for reviewing the manuscript. A portion of this work was sponsored by the National Cancer Institute under contract with Advanced BioScience Laboratories.
Abbreviations
- MAPK
mitogen-activated protein kinase
- MEK
MAPK kinases
- LF
anthrax lethal factor
- PA
protective antigen
- EF
edema factor
- LeTx
lethal toxin, a combination of LF plus PA
References
- 1.Lewis T S, Shapiro P S, Ahn N G. In: Advances in Cancer Research. Vande Woude G F, Klein G, editors. San Diego: Academic; 1998. pp. 49–139. [DOI] [PubMed] [Google Scholar]
- 2.Hoshino R, Chatani Y, Yamori T, Tsuruo T, Oka H, Yoshida O, Shimada Y, Ari-I S, Wada H, Fujimoto H, Kohno M. Oncogene. 1999;18:813–822. doi: 10.1038/sj.onc.1202367. [DOI] [PubMed] [Google Scholar]
- 3.Salh B, Bergman D, Marotta A, Pelech S L. Anticancer Res. 1999;19:741–748. [PubMed] [Google Scholar]
- 4.Sivaraman V S, Wang H, Nuovo G J, Malbon C C. J Clin Invest. 1997;99:1478–1483. doi: 10.1172/JCI119309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandell J W, Hussaini I M, Zecevic M, Weber M J, VandenBerg S R. Am J Pathol. 1998;153:1411–1423. doi: 10.1016/S0002-9440(10)65728-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duesbery N S, Webb C P, Leppla S H, Gordon V M, Klimpel KR, Copeland T D, Ahn N G, Oskarsson M K, Fukasawa K, Paull K D, Vande Woude G F. Science. 1998;280:734–737. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- 7.Vitale G, Pellizzari R, Recchi C, Napolitani G, Mock M, Montecucco C. Biochem Biophys Res Commun. 1998;248:706–711. doi: 10.1006/bbrc.1998.9040. [DOI] [PubMed] [Google Scholar]
- 8.Pellizzari R, Guidi-Rontani C, Vitale G, Mock M, Montecucco C. FEBS Lett. 1999;462:199–204. doi: 10.1016/s0014-5793(99)01502-1. [DOI] [PubMed] [Google Scholar]
- 9.Colanzi A, Deerinck T J, Ellisman M H, Malhotra V. J Cell Biol. 2000;149:331–339. doi: 10.1083/jcb.149.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duesbery N S, Vande Woude G F. Cell Mol Life Sci. 1999;55:1599–1609. doi: 10.1007/s000180050399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorne C B, Molnar D M, Strange R E. J Bacteriol. 1960;79:450–455. doi: 10.1128/jb.79.3.450-455.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanley J L, Sargeant K, Smith H. J Gen Microbiol. 1960;22:206–218. doi: 10.1099/00221287-22-1-206. [DOI] [PubMed] [Google Scholar]
- 13.Leppla S H. Proc Natl Acad Sci USA. 1982;79:3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanley J L, Smith H P. J Gen Microbiol. 1961;26:49–66. doi: 10.1099/00221287-26-1-49. [DOI] [PubMed] [Google Scholar]
- 15.Beall F A, Taylor M J, Thorne C B. J Bacteriol. 1962;83:1274–1280. doi: 10.1128/jb.83.6.1274-1280.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mansour S J, Matten W T, Hermann A S, Candia J M, Rong S, Fukasawa K, Vande Woude G F, Ahn N G. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 17.Webb C P, Van Aelst L, Wigler M H, Vande Woude G F. Proc Natl Acad Sci USA. 1998;95:8773–8778. doi: 10.1073/pnas.95.15.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leppla S H. In: Methods in Enzymology. Harshman S, editor. Orlando, FL: Academic; 1988. pp. 103–116. [Google Scholar]
- 19.Duesbery N S, Choi T, Brown K D, Wood K W, Resau J, Fukasawa K, Cleveland D W, Vande Woude G F. Proc Natl Acad Sci USA. 1997;94:9165–9170. doi: 10.1073/pnas.94.17.9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukasawa K, Vande Woude G F. Mol Cell Biol. 1997;17:506–518. doi: 10.1128/mcb.17.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klimpel K R, Arora N, Leppla S H. Mol Microbiol. 1994;13:1093–1100. doi: 10.1111/j.1365-2958.1994.tb00500.x. [DOI] [PubMed] [Google Scholar]
- 22.Jeffers M, Rong S, Vande Woude G F. Mol Cell Biol. 1996;16:1115–1125. doi: 10.1128/mcb.16.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 24.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chambers A F, Tuck A B. Crit Rev Oncog. 1993;4:95–114. [PubMed] [Google Scholar]
- 26.Favata M F, Horiuchi K Y, Manos E J, Daulerio A J, Stradley D A, Feeser W S, Van Dyk D E, Pitts W J, Earl R A, Hobbs F, et al. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 27.Fukazawa H, Uehara Y. Cancer Res. 2000;60:2104–2107. [PubMed] [Google Scholar]
- 28.Yang J J, Kang J S, Krauss R S. Mol Cell Biol. 1998;18:2586–2595. doi: 10.1128/mcb.18.5.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janulis M, Silberman S, Ambegaokar A, Gutkind J S, Schultz R M. J Biol Chem. 1999;274:801–813. doi: 10.1074/jbc.274.2.801. [DOI] [PubMed] [Google Scholar]
- 30.Silberman S, Janulis M, Schultz R M. J Biol Chem. 1997;272:5927–5935. doi: 10.1074/jbc.272.9.5927. [DOI] [PubMed] [Google Scholar]
- 31.Webb C, Vande Woude G F. J Neuroncology. 2000;50:71–87. doi: 10.1023/a:1006466605356. [DOI] [PubMed] [Google Scholar]
- 32.Sebolt-Leopold J S, Dudley D T, Herrera R, Van Becelaere K, Wiland A, Gowan R C, Tecle H, Barrett S D, Bridges A, Przybranowski S, et al. Nat Med. 1999;5:810–816. doi: 10.1038/10533. [DOI] [PubMed] [Google Scholar]
- 33.Vitale G, Pellizzari R, Recchi C, Napolitani G, Mock M, Montecucco C. J Appl Microbiol. 1999;87:288. doi: 10.1046/j.1365-2672.1999.00893.x. [DOI] [PubMed] [Google Scholar]
- 34.Ezzell J W, Ivins B E, Leppla S H. Infect Immun. 1984;45:761–767. doi: 10.1128/iai.45.3.761-767.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Welkos S L, Keener T J, Gibbs P H. Infect Immun. 1986;51:795–800. doi: 10.1128/iai.51.3.795-800.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta K, Kshirsagar S, Li W, Gui L, Ramakrishnan S, Gupta P, Law P Y, Hebbel R P. Exp Cell Res. 1999;247:495–504. doi: 10.1006/excr.1998.4359. [DOI] [PubMed] [Google Scholar]
- 37.Redlitz A, Daum G, Sage E H. J Vasc Res. 1999;36:28–34. doi: 10.1159/000025623. [DOI] [PubMed] [Google Scholar]
- 38.Yu Y, Sato J D. J Cell Physiol. 1999;178:235–246. doi: 10.1002/(SICI)1097-4652(199902)178:2<235::AID-JCP13>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 39.Rousseau S, Houle F, Landry J, Huot J. Oncogene. 1997;15:2169–2177. doi: 10.1038/sj.onc.1201380. [DOI] [PubMed] [Google Scholar]
- 40.Anand-Apte B, Zetter B R, Viswanathan A, Qiu R G, Chen J, Ruggieri R, Symons M. J Biol Chem. 1997;272:30688–30692. doi: 10.1074/jbc.272.49.30688. [DOI] [PubMed] [Google Scholar]
- 41.Eliceiri B P, Klemke R, Stromblad S, Cheresh D A. J Cell Biol. 1998;140:1255–1263. doi: 10.1083/jcb.140.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LaMontagne K R, Jr, Moses M A, Wiederschain D, Mahajan S, Holden J, Ghazizadeh H, Frank D A, Arbiser J L. Am J Pathol. 2000;157:1937–1945. doi: 10.1016/s0002-9440(10)64832-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu D Q, Kan M K, Sato G H, Okamoto T, Sato J D. J Biol Chem. 1991;266:16778–16785. [PubMed] [Google Scholar]
- 44.Czubayko F, Smith R V, Chung H C, Wellstein A. J Biol Chem. 1994;269:28243–28248. [PubMed] [Google Scholar]
- 45.Harris V K, Coticchia C M, Kagan B L, Ahmad S, Wellstein A, Riegel A T. J Biol Chem. 2000;275:10802–10811. doi: 10.1074/jbc.275.15.10802. [DOI] [PubMed] [Google Scholar]