Abstract
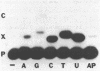
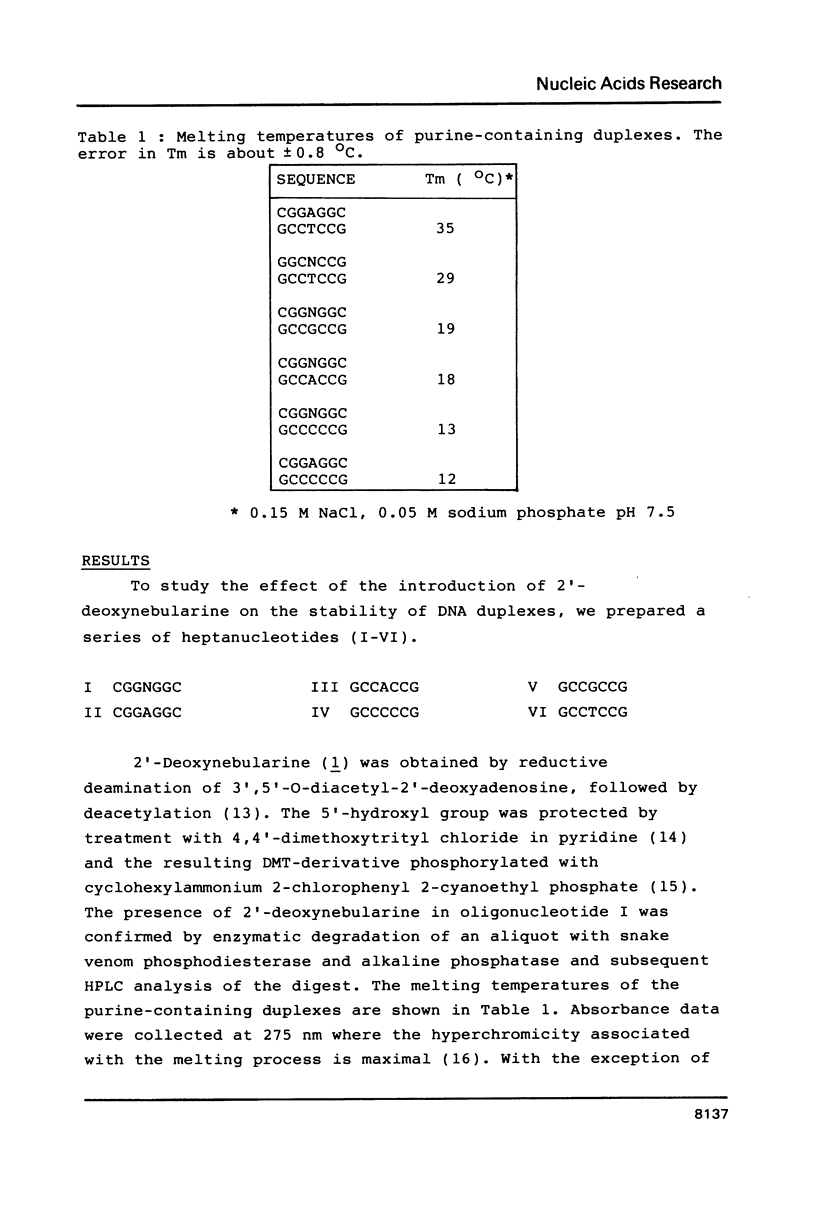
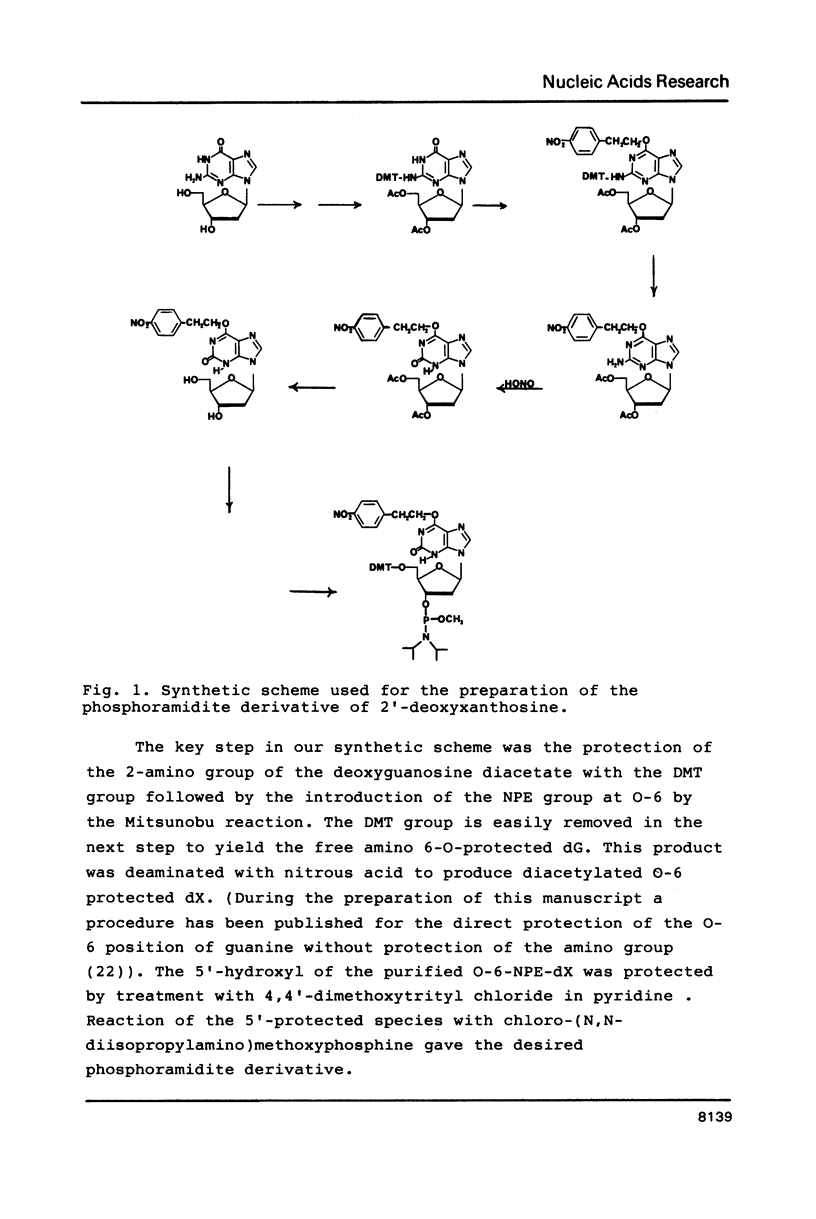
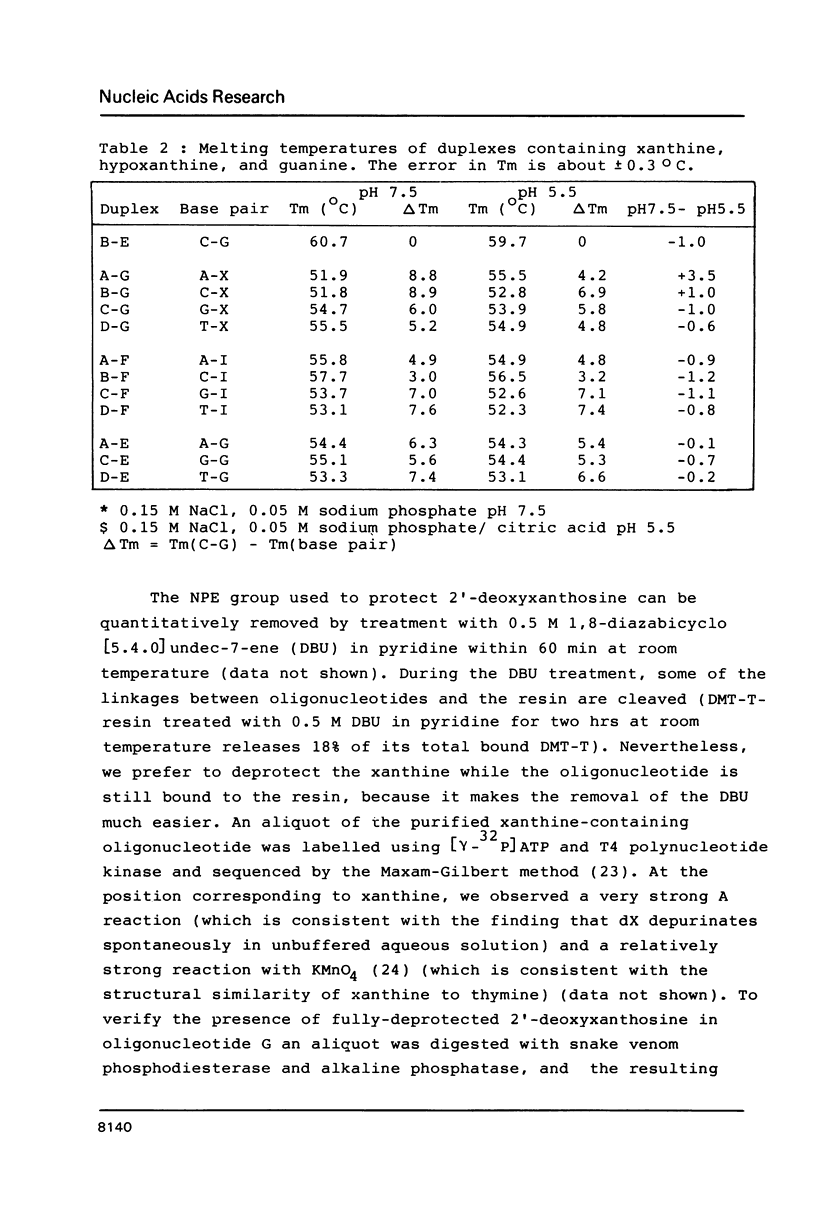
The preparation of synthetic oligonucleotides containing 2'-deoxynebularine (dN) and 2'-deoxyxanthosine (dX) is described. The thermal stabilities of duplexes containing dX, dN, and 2'-deoxyinosine (dI) base-paired with the four natural bases have been measured. Xanthine base pairs have stabilities at pH 5.5 that are similar to those of dI-containing duplexes at neutral pH. When xanthine is paired with adenine or cytosine an unusual stabilization of the duplex structure is observed at acid pH. Incorporation of base mispairs opposite template xanthine sites were measured using Drosophila DNA polymerase alpha. The relative nucleoside incorporation rates are in the order: T greater than C much greater than A approximately equal to G. These rates do not correlate with relative thermodynamic stabilities of base mispairs with xanthine obtained from Tm measurements: T greater than G greater than A approximately equal to C. We suggest that DNA polymerase misinsertion rates are greatest when the base mispair can be formed in accordance with Watson-Crick as opposed to other base pairing geometries even though other geometries, e.g. wobble, may result in a more stable final DNA product.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERT A. Quantitative studies of the avidity of naturally occurring substances for trace metals. III. Pteridines, riboflavin and purines. Biochem J. 1953 Jul;54(4):646–654. doi: 10.1042/bj0540646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboul-ela F., Koh D., Tinoco I., Jr, Martin F. H. Base-base mismatches. Thermodynamics of double helix formation for dCA3XA3G + dCT3YT3G (X, Y = A,C,G,T). Nucleic Acids Res. 1985 Jul 11;13(13):4811–4824. doi: 10.1093/nar/13.13.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks G. R., Boezi J. A., Lehman I. R. A high molecular weight DNA polymerase from Drosophila melanogaster embryos. Purification, structure, and partial characterization. J Biol Chem. 1979 Oct 10;254(19):9886–9892. [PubMed] [Google Scholar]
- Bessman M. J., Muzyczka N., Goodman M. F., Schnaar R. L. Studies on the biochemical basis of spontaneous mutation. II. The incorporation of a base and its analogue into DNA by wild-type, mutator and antimutator DNA polymerases. J Mol Biol. 1974 Sep 15;88(2):409–421. doi: 10.1016/0022-2836(74)90491-4. [DOI] [PubMed] [Google Scholar]
- Clayton L. K., Goodman M. F., Branscomb E. W., Galas D. J. Error induction and correction by mutant and wild type T4 DNA polymerases. Kinetic error discrimination mechanisms. J Biol Chem. 1979 Mar 25;254(6):1902–1912. [PubMed] [Google Scholar]
- Eritja R., Kaplan B. E., Mhaskar D., Sowers L. C., Petruska J., Goodman M. F. Synthesis and properties of defined DNA oligomers containing base mispairs involving 2-aminopurine. Nucleic Acids Res. 1986 Jul 25;14(14):5869–5884. doi: 10.1093/nar/14.14.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikus M., Shugar D. Properties of poly-xanthylic acid and its reactions with potentially complementary homopolynucleotides. Acta Biochim Pol. 1969;16(1):55–82. [PubMed] [Google Scholar]
- Inoue H., Imura A., Ohtsuka E. Synthesis and hybridization of dodecadeoxyribonucleotides containing a fluorescent pyridopyrimidine deoxynucleoside. Nucleic Acids Res. 1985 Oct 11;13(19):7119–7128. doi: 10.1093/nar/13.19.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaye M., de la Salle H., Schamber F., Balland A., Kohli V., Findeli A., Tolstoshev P., Lecocq J. P. Isolation of a human anti-haemophilic factor IX cDNA clone using a unique 52-base synthetic oligonucleotide probe deduced from the amino acid sequence of bovine factor IX. Nucleic Acids Res. 1983 Apr 25;11(8):2325–2335. doi: 10.1093/nar/11.8.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F. H., Castro M. M., Aboul-ela F., Tinoco I., Jr Base pairing involving deoxyinosine: implications for probe design. Nucleic Acids Res. 1985 Dec 20;13(24):8927–8938. doi: 10.1093/nar/13.24.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mehta J. R., Ludlum D. B. Synthesis and properties of O6-methyldeoxyguanylic acid and its copolymers with deoxycytidylic acid. Biochim Biophys Acta. 1978 Dec 21;521(2):770–778. doi: 10.1016/0005-2787(78)90316-7. [DOI] [PubMed] [Google Scholar]
- Mhaskar D. N., Goodman M. F. On the molecular basis of transition mutations. Frequency of forming 2-aminopurine-cytosine base mispairs in the G X C----A X T mutational pathway by T4 DNA polymerase in vitro. J Biol Chem. 1984 Oct 10;259(19):11713–11717. [PubMed] [Google Scholar]
- Michelson A. M., Monny C. Polynucleotide analogues. IX. Polyxanthylic acid. Biochim Biophys Acta. 1966 Dec 21;129(3):460–474. [PubMed] [Google Scholar]
- Millican T. A., Mock G. A., Chauncey M. A., Patel T. P., Eaton M. A., Gunning J., Cutbush S. D., Neidle S., Mann J. Synthesis and biophysical studies of short oligodeoxynucleotides with novel modifications: a possible approach to the problem of mixed base oligodeoxynucleotide synthesis. Nucleic Acids Res. 1984 Oct 11;12(19):7435–7453. doi: 10.1093/nar/12.19.7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka E., Matsuki S., Ikehara M., Takahashi Y., Matsubara K. An alternative approach to deoxyoligonucleotides as hybridization probes by insertion of deoxyinosine at ambiguous codon positions. J Biol Chem. 1985 Mar 10;260(5):2605–2608. [PubMed] [Google Scholar]
- Petruska J., Sowers L. C., Goodman M. F. Comparison of nucleotide interactions in water, proteins, and vacuum: model for DNA polymerase fidelity. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1559–1562. doi: 10.1073/pnas.83.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. M., Schmid C. W. Pyrimidine-specific chemical reactions useful for DNA sequencing. Nucleic Acids Res. 1980 Oct 24;8(20):4613–4619. doi: 10.1093/nar/8.20.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N. D., Biernat J., McManus J., Köster H. Polymer support oligonucleotide synthesis XVIII: use of beta-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984 Jun 11;12(11):4539–4557. doi: 10.1093/nar/12.11.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers L. C., Fazakerley G. V., Eritja R., Kaplan B. E., Goodman M. F. Base pairing and mutagenesis: observation of a protonated base pair between 2-aminopurine and cytosine in an oligonucleotide by proton NMR. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5434–5438. doi: 10.1073/pnas.83.15.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Kato K., Hayashizaki Y., Wakabayashi T., Ohtsuka E., Matsuki S., Ikehara M., Matsubara K. Molecular cloning of the human cholecystokinin gene by use of a synthetic probe containing deoxyinosine. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1931–1935. doi: 10.1073/pnas.82.7.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z. K., Ikuta S., Huang T., Dugaiczyk A., Itakura K. Solid-phase synthesis of polynucleotides. VIII: A simplified synthesis of oligodeoxyribonucleotides. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):383–391. doi: 10.1101/sqb.1983.047.01.045. [DOI] [PubMed] [Google Scholar]
- Tichy M., Fikus M. Investigations on the structures of xanthine-uracil and xanthine-adenine copolymers and their complexes with homopolynucleotides. Acta Biochim Pol. 1970;17(1):53–71. [PubMed] [Google Scholar]
- Watanabe S. M., Goodman M. F. On the molecular basis of transition mutations: frequencies of forming 2-aminopurine.cytosine and adenine.cytosine base mispairs in vitro. Proc Natl Acad Sci U S A. 1981 May;78(5):2864–2868. doi: 10.1073/pnas.78.5.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werntges H., Steger G., Riesner D., Fritz H. J. Mismatches in DNA double strands: thermodynamic parameters and their correlation to repair efficiencies. Nucleic Acids Res. 1986 May 12;14(9):3773–3790. doi: 10.1093/nar/14.9.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]