The optic cup ablation experiments of Hans Spemann (Spemann, 1901) introduced the concept of embryonic induction and established that presumptive retina provided signals required for lens development. Since then, lens induction has been a favored subject for developmental biologists wishing to understand the molecular mechanisms of inductive signaling. Much has been learned about the genetic regulation of lens induction and Fig. 1 summarizes some of the advances. The transcription factor Pax6 is centrally involved. It is both necessary (Ashery-Padan et al., 2000; Collinson et al., 2000) and sufficient (Altmann et al., 1997; Chow et al., 1999) for lens development and is induced by the fibroblast growth factor (FGF)(Faber et al., 2001; Gotoh et al., 2004) and bone morphogenetic protein 7 (BMP7) (Wawersik et al., 1999) signaling pathways that are required for early lens formation. Meis and Six family transcription factors have also been implicated in lens induction through their action at the Ectoderm Enhancer of the Pax6 gene (Zhang et al., 2002; Liu et al., 2006). The Sry family transcription factor Sox2 is involved in lens development (Kamachi et al., 2001; Kondoh et al., 2004) and has an essential, parallel function to Pax6 at pre-placodal stages (Smith et al., 2009). Sox2 is up-regulated by BMP4, the first signaling ligand to be implicated in lens induction (Furuta and Hogan, 1998). In a recent analysis, the Grainger group has provided evidence that the broadly expressed transcription factor Otx2 cooperates with the locally expressed Notch pathway transcriptional regulator suppressor of hairless (Su(H)) to up-regulate expression of FoxE3 and define lens placode ectoderm (Ogino et al., 2008). Since Su(H) is dependent on the Notch pathway ligand Delta2 that is expressed in the optic vesicle, this is an example of the type of lens induction signaling that would be anticipated from classical studies.
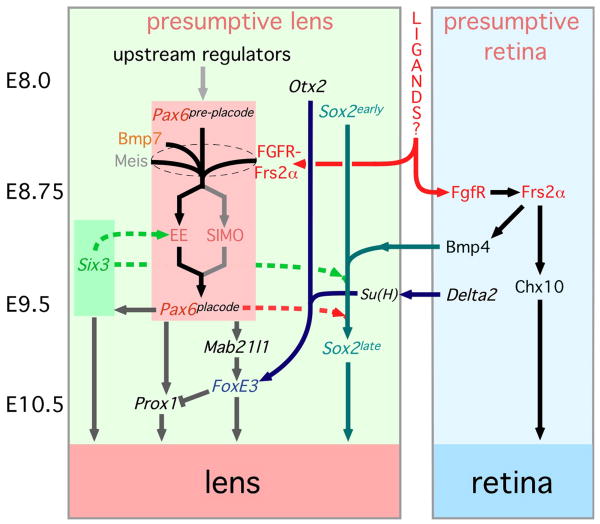
Figure 1. A model for the genetic regulation of lens induction.
The solid arrows represent pathway relationships that have been defined genetically by molecular epistasis analysis or that have been implied through pathway up- or down-regulation. For example, Prox1 expression is lost in mice with a placodal loss of Pax6 and so Prox1 is placed downstream. The dashed arrows represent direct physical interactions that have been defined by biochemical experiments. There are elements of speculation in this model. For example, Bmp4 expression is lost in the optic vesicle of Frs2α2F/2F mutant, but it is unclear whether this regulation occurs within the optic vesicle (the model shown) or is a consequence of Frs2α-dependent signaling to the presumptive retina from the presumptive lens. See the text for further explanation of these interactions. The word “ligands?” placed between the boxes representing the presumptive lens and presumptive retina is designed to indicate that so far, no conventional FGF ligands are known to be critical for this inductive interaction. On the left side of the figure, the approximate embryonic (E) stages at which these events occur are indicated.
In the case of the BMP and Notch signaling pathways, there is evidence for the involvement of particular ligand-receptor pairs in lens induction (Furuta and Hogan, 1998; Wawersik et al., 1999; Ogino et al., 2008). By contrast, despite the extensive evidence for FGF signaling involvement in lens and retinal induction, no essential ligands have thus far been documented. Expression of dominant-negative FGF receptors in the lens placode (Faber et al., 2001) and analysis of mutants for the FGF receptor adaptor FRS2α (Gotoh et al., 2004) both suggested that the FGF response in cells of the presumptive lens was required for development to proceed. Explant studies in the chick have also implicated the FGF pathway in reciprocal signaling (lens-to-retina)(Nguyen and Arnheiter, 2000) and this is consistent with reduced phospho-ERK immunoreactivity in the presumptive retina of FRS2α mutant mice (Gotoh et al., 2004). Later stages of lens development also require FGF signaling. Lens fiber cell differentiation can also be suppressed with dominant-negative, or Ig-fusion FGF receptors (Chow et al., 1995; Robinson et al., 1995a; Govindarajan and Overbeek, 2001). Furthermore, fiber cell differentiation can be enhanced by FGF ligands either in culture or in vivo (McAvoy and Chamberlain, 1989; Robinson et al., 1995b). Experiments performed in the lens system have also demonstrated redundancy in the FGF signaling system. In a genetic tour-de-force, the Robinson lab has conditionally deleted FGF receptors 1-3 from lens fiber cells and shown that only when all six alleles are deleted do fiber cells fail to differentiate (Zhao et al., 2008).
One possible explanation for the lack of evidence demonstrating an essential FGF ligand in lens induction is functional redundancy. There are certainly multiple FGF ligands expressed in the early eye. The surface ectoderm and presumptive lens express FGFs 1 and 2 (de Iongh and McAvoy, 1993), FGF8 (Kurose et al., 2005) and FGF15 (designated FGF19 in humans and in the chick)(Kurose et al., 2004; Kurose et al., 2005). Extensive expression studies by the Dorey group has shown that FGF ligands 1, 3, 13, 14 and 20 are all associated with the developing Xenopus tropicalis eye (Lea et al., 2009). This analysis also showed that FGFR3 is expressed in the lens, FGFR2 is found specifically in the outer epithelium of the eye and that FGFRs 1, 3 and 4 are also found in areas around the lens (Lea et al., 2009). Furthermore, different sub-domains of the developing mouse optic vesicle express FGF8, FGF9 and FGF15 (McWhirter et al., 1997; Vogel-Hopker et al., 2000; Kurose et al., 2004; Kurose et al., 2005).
Despite these expression patterns, there is still limited evidence for FGF ligand signaling in lens induction. For example, a double germ-line mutant for FGF1 and FGF2 gives no eye phenotype (Miller et al., 2000). Mutation of FGF9 similarly has no consequence for lens development (Zhao et al., 2001). Data supporting FGF19 functionality in the lens of the chick has shown that a soluble FGFR4 results in the induction of L-Maf (Kurose et al., 2005). Since L-Maf is known to induce the lens markers Prox1 and δ-crystallin, this suggests that inhibition of FGF19 (exclusively bound by FGFR4) is required for lens development. Nevertheless, these experiments still have yet to help identify a ligand that has a positive effect on lens induction. For example, the outcome of dominant-negative FGF receptor expression (Faber et al., 2001) and FRS2α mutation (Gotoh et al., 2004) both effect the expression of Pax6 and lens induction. Perhaps when the appropriate combination of FGF ligands is mutated, a lens induction phenotype will result.
Besides the obvious possibility of ligand redundancy, there are two other explanations we might consider to explain why, so far, no FGF family ligands have been identified as essential for lens induction. One is that there might be previously unrecognized ligands for the FGF receptors that function in this system. The recent discovery of Norrin, a non-Wnt family ligand that activates the canonical Wnt pathway through the Wnt receptor Fzd4 (Xu et al., 2004) and the discovery of IL34 as a new ligand for the CSF1 receptor cfms (Lin et al., 2008) are both reminders that unanticipated ligand-receptor interactions will emerge from time-to-time. A second explanation for the apparent lack of FGF ligands in the lens induction system is that this function might be partly fulfilled by N-cadherin since it has previously been recognized as an unconventional FGF receptor activating ligand (Trolice et al., 1997; Utton et al., 2001; Suyama et al., 2002).
The classical cadherins were originally identified as adhesion molecules (Hyafil et al., 1981). Consistent with this function, they were subsequently shown to be critical for morphogenesis during embryogenesis. Examples include a role in gastrulation movements in Xenopus laevis (Lee and Gumbiner, 1995), muscle cell movements in zebrafish (Cortes et al., 2003), epidermal morphogenesis in C. elegans (Pettitt et al., 2003) and tracheal tube fusion in Drosophila (Lee et al., 2003). However, cadherins also have a signaling function and like the integrin class of adhesion receptors (Hynes, 2002) are believed to mediate both outside-in and inside-out signaling (Gumbiner, 2005). Increased serine-threonine phosphorylation of cadherins or β-catenin can enhance adhesive strength (Lickert et al., 2000; Bek and Kemler, 2002) in an example of inside-out signaling. Cadherin outside-in signaling can be mediated, for example, by Rho family GTPases or Src (Yap and Kovacs, 2003; McLachlan et al., 2007). It has also been suggested that cadherins can act as alternative ligands for FGF receptors (Suyama et al., 2002).
During development of the eye there is a dynamic pattern of cadherin expression. Several cadherins are expressed when the optic vesicle and surface ectoderm come into close contact and exchange inductive signals that contribute to lens and retina formation (Leong et al., 2000; Xu et al., 2002; Pontoriero et al., 2009). Expression of N-cadherin in the presumptive lens and retinal epithelia has raised the possibility that N-cadherin might mediate signal exchange within the eye primordium. Recently it was shown that conditional deletion of N-cadherin in the presumptive lens ectoderm of the mouse resulted in relatively mild defects in lens morphogenesis (Pontoriero et al., 2009). By contrast, in mice with a germ-line mutation of N-cadherin, embryos show a complete failure of lens development (Fig. 2). Such embryos are difficult to produce due the early embryonic lethality of N-cadherin deficiency and must carry a cadherin rescue transgene with heart expression (αMHC-Ecad, (Luo et al., 2001)) if they are to progress to lens development stages. However, those embryos that survive show a very distinctive phenotype.
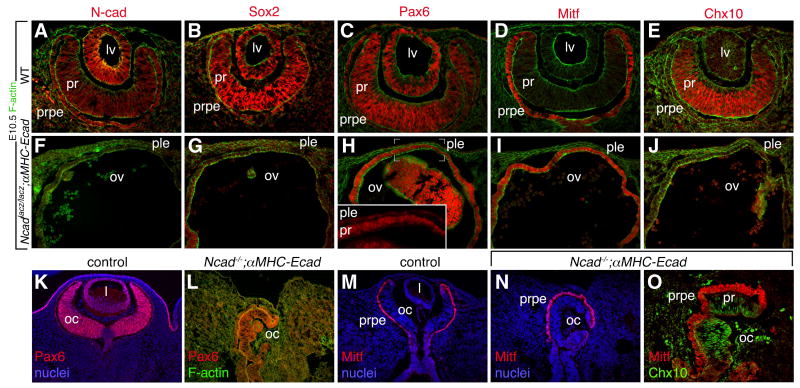
Figure 2. A failure of lens development in N-cadherin mutants.
Micrographs show the eye region of E10.5 (A-J) or E12.5 (K-O) embryos of the indicated genotypes labeled for the markers as color-coded above or on the panels. The absence of lens development is apparent at E10.5 examples (F-J) and E12.5 (L, N, O). lv – lens vesicle, pr – presumptive retina, prpe – presumptive retina pigmented epithelium, ple – presumptive lens ectoderm, ov – optic vesicle, oc- optic cup.
When examined at E10.5, Ncadlacz/lacz; αMHC-Ecad embryos showed a range of phenotypes. In the most extreme form, these embryos showed an arrest of eye development equivalent to approximately E9.0 with a complete absence of lens development and a general failure of eye morphogenesis (Fig. 2F-J). In the presumptive lens ectoderm, Pax6 expression was all but lost (Fig. 2H, inset) indicating that lens induction had failed. In Ncadlacz/lacz; αMHC-Ecad embryos the optic vesicle could be identified through its expression of Mitf and Pax6 (Fig. 2H, I). However, optic vesicle patterning was absent in that Chx10 was not induced in central presumptive retina and Mitf was expressed throughout (Fig. 2I, J). In the milder form of the Ncadlacz/lacz; αMHC-Ecad eye phenotype (Fig. 2K-O) there is still a complete absence of lens (Fig. 2L) but there is an optic cup, albeit mis-oriented, with appropriate Chx10 and Mitf distribution (Fig. 2K-O). This form of the mutant is particularly interesting because the presence of an optic cup implies that induction of retina has occurred and in turn, that the presumptive lens and retinal epithelia were sufficiently close to permit signaling. Both these eye phenotypes suggest that N-cadherin has an important role in lens development. Since embryos with an N-cadherin deletion in surface ectoderm have only a mild lens development defect (Pontoriero et al., 2009), the combined data suggest that optic vesicle expression of N-cadherin may be a domain critical for lens development.
Though these data are provocative and suggest that N-cadherin might function as an unconventional FGF receptor activating ligand, the difficulty of generating mutant embryos and the systemic phenotype that these embryos display means that at present, we must be cautious in this conclusion. Furthermore, it remains possible that while an N-cadherin-FGF receptor interaction might contribute to lens induction signaling, this may still be enhanced by conventional FGF ligands as has been described in other systems (Trolice et al., 1997). This might suggest that only with combinations of N-cadherin and FGF ligand mutants will there be consequences for lens induction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altmann CR, Chow RL, Lang RA, Hemmati-Brivanlou A. Lens induction by Pax-6 in Xenopus laevis. Dev Biol. 1997;185:119–23. doi: 10.1006/dbio.1997.8573. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–11. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bek S, Kemler R. Protein kinase CKII regulates the interaction of beta-catenin with alpha-catenin and its protein stability. J Cell Sci. 2002;115:4743–53. doi: 10.1242/jcs.00154. [DOI] [PubMed] [Google Scholar]
- Chow RL, Altmann CR, Lang RA, Hemmati-Brivanlou A. Pax6 induces ectopic eyes in a vertebrate. Development. 1999;126:4213–22. doi: 10.1242/dev.126.19.4213. [DOI] [PubMed] [Google Scholar]
- Chow RL, Roux GD, Roghani M, Palmer MA, Rifkin DB, Moscatelli DA, Lang RA. FGF suppresses apoptosis and induces differentiation of fibre cells in the mouse lens. Development. 1995;121:4383–93. doi: 10.1242/dev.121.12.4383. [DOI] [PubMed] [Google Scholar]
- Collinson JM, Hill RE, West JD. Different roles for Pax6 in the optic vesicle and facial epithelium mediate early morphogenesis of the murine eye. Development. 2000;127:945–56. doi: 10.1242/dev.127.5.945. [DOI] [PubMed] [Google Scholar]
- Cortes F, Daggett D, Bryson-Richardson RJ, Neyt C, Maule J, Gautier P, Hollway GE, Keenan D, Currie PD. Cadherin-mediated differential cell adhesion controls slow muscle cell migration in the developing zebrafish myotome. Dev Cell. 2003;5:865–76. doi: 10.1016/s1534-5807(03)00362-9. [DOI] [PubMed] [Google Scholar]
- de Iongh R, McAvoy JW. Spatio-temporal distribution of acidic and basic FGF indicates a role for FGF in rat lens morphogenesis. Dev Dyn. 1993;198:190–202. doi: 10.1002/aja.1001980305. [DOI] [PubMed] [Google Scholar]
- Faber SC, Dimanlig P, Makarenkova HP, Shirke S, Ko K, Lang RA. Fgf receptor signaling plays a role in lens induction. Development. 2001;128:4425–38. doi: 10.1242/dev.128.22.4425. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Hogan BLM. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh N, Ito M, Yamamoto S, Yoshino I, Song N, Wang Y, Lax I, Schlessinger J, Shibuya M, Lang RA. Tyrosine phosphorylation sites on FRS2alpha responsible for Shp2 recruitment are critical for induction of lens and retina. Proc Natl Acad Sci U S A. 2004;101:17144–9. doi: 10.1073/pnas.0407577101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan V, Overbeek PA. Secreted FGFR3, but not FGFR1, inhibits lens fiber differentiation. Development. 2001;128:1617–27. doi: 10.1242/dev.128.9.1617. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–34. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Hyafil F, Babinet C, Jacob F. Cell-cell interactions in early embryogenesis: a molecular approach to the role of calcium. Cell. 1981;26:447–54. doi: 10.1016/0092-8674(81)90214-2. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Tanouchi A, Sekido R, Kondoh H. Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes Dev. 2001;15:1272–86. doi: 10.1101/gad.887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh H, Uchikawa M, Kamachi Y. Interplay of Pax6 and SOX2 in lens development as a paradigm of genetic switch mechanisms for cell differentiation. Int J Dev Biol. 2004;48:819–27. doi: 10.1387/ijdb.041868hk. [DOI] [PubMed] [Google Scholar]
- Kurose H, Bito T, Adachi T, Shimizu M, Noji S, Ohuchi H. Expression of Fibroblast growth factor 19 (Fgf19) during chicken embryogenesis and eye development, compared with Fgf15 expression in the mouse. Gene Expr Patterns. 2004;4:687–93. doi: 10.1016/j.modgep.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Kurose H, Okamoto M, Shimizu M, Bito T, Marcelle C, Noji S, Ohuchi H. FGF19-FGFR4 signaling elaborates lens induction with the FGF8-L-Maf cascade in the chick embryo. Dev Growth Differ. 2005;47:213–23. doi: 10.1111/j.1440-169X.2005.00795.x. [DOI] [PubMed] [Google Scholar]
- Lea R, Papalopulu N, Amaya E, Dorey K. Temporal and spatial expression of FGF ligands and receptors during Xenopus development. Dev Dyn. 2009;238:1467–79. doi: 10.1002/dvdy.21913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Gumbiner BM. Disruption of gastrulation movements in Xenopus by a dominant-negative mutant for C-cadherin. Dev Biol. 1995;171:363–73. doi: 10.1006/dbio.1995.1288. [DOI] [PubMed] [Google Scholar]
- Lee M, Lee S, Zadeh AD, Kolodziej PA. Distinct sites in E-cadherin regulate different steps in Drosophila tracheal tube fusion. Development. 2003;130:5989–99. doi: 10.1242/dev.00806. [DOI] [PubMed] [Google Scholar]
- Leong L, Menko AS, Grunwald GB. Differential expression of N- and B-cadherin during lens development. Invest Ophthalmol Vis Sci. 2000;41:3503–10. [PubMed] [Google Scholar]
- Lickert H, Bauer A, Kemler R, Stappert J. Casein kinase II phosphorylation of E-cadherin increases E-cadherin/beta-catenin interaction and strengthens cell-cell adhesion. J Biol Chem. 2000;275:5090–5. doi: 10.1074/jbc.275.7.5090. [DOI] [PubMed] [Google Scholar]
- Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, Halenbeck R, Wu G, Zhou A, Behrens D, Hollenbaugh D, Linnemann T, Qin M, Wong J, Chu K, Doberstein SK, Williams LT. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807–11. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- Liu W, Lagutin OV, Mende M, Streit A, Oliver G. Six3 activation of Pax6 expression is essential for mammalian lens induction and specification. Embo J. 2006;25:5383–95. doi: 10.1038/sj.emboj.7601398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Ferreira-Cornwell M, Baldwin H, Kostetskii I, Lenox J, Lieberman M, Radice G. Rescuing the N-cadherin knockout by cardiac-specific expression of N- or E-cadherin. Development. 2001;128:459–69. doi: 10.1242/dev.128.4.459. [DOI] [PubMed] [Google Scholar]
- McAvoy JW, Chamberlain CG. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development. 1989;107:221–8. doi: 10.1242/dev.107.2.221. [DOI] [PubMed] [Google Scholar]
- McLachlan RW, Kraemer A, Helwani FM, Kovacs EM, Yap AS. E-cadherin adhesion activates c-Src signaling at cell-cell contacts. Mol Biol Cell. 2007;18:3214–23. doi: 10.1091/mbc.E06-12-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhirter JR, Goulding M, Weiner JA, Chun J, Murre C. A novel fibroblast growth factor gene expressed in the developing nervous system is a downstream target of the chimeric homeodomain oncoprotein E2A-Pbx1. Development. 1997;124:3221–32. doi: 10.1242/dev.124.17.3221. [DOI] [PubMed] [Google Scholar]
- Miller DL, Ortega S, Bashayan O, Basch R, Basilico C. Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2 null mice [published erratum appears in Mol Cell Biol 2000 May;20(10):3752] Mol Cell Biol. 2000;20:2260–8. doi: 10.1128/mcb.20.6.2260-2268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M, Arnheiter H. Signaling and transcriptional regulation in early mammalian eye development: a link between FGF and MITF. Development. 2000;127:3581–91. doi: 10.1242/dev.127.16.3581. [DOI] [PubMed] [Google Scholar]
- Ogino H, Fisher M, Grainger RM. Convergence of a head-field selector Otx2 and Notch signaling: a mechanism for lens specification. Development. 2008;135:249–58. doi: 10.1242/dev.009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettitt J, Cox EA, Broadbent ID, Flett A, Hardin J. The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherin-catenin function during epidermal morphogenesis. J Cell Biol. 2003;162:15–22. doi: 10.1083/jcb.200212136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontoriero GF, Smith AN, Miller LA, Radice GL, West-Mays JA, Lang RA. Co-operative roles for E-cadherin and N-cadherin during lens vesicle separation and lens epithelial cell survival. Dev Biol. 2009;326:403–17. doi: 10.1016/j.ydbio.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ML, MacMillan-Crow LA, Thompson JA, Overbeek PA. Expression of a truncated FGF receptor results in defective lens development in transgenic mice. Development. 1995a;121:3959–67. doi: 10.1242/dev.121.12.3959. [DOI] [PubMed] [Google Scholar]
- Robinson ML, Overbeek PA, Verran DJ, Grizzle WE, Stockard CR, Friesel R, Maciag T, Thompson JA. Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development. 1995b;121:505–14. doi: 10.1242/dev.121.2.505. [DOI] [PubMed] [Google Scholar]
- Smith AN, Miller LA, Radice G, Ashery-Padan R, Lang RA. Stage-dependent modes of Pax6-Sox2 epistasis regulate lens development and eye morphogenesis. Development. 2009 doi: 10.1242/dev.037341. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spemann H. Uber Correlationen in der Entwickelung des Auges. Verh Anat Ges. 1901;15:61–79. [Google Scholar]
- Suyama K, Shapiro I, Guttman M, Hazan RB. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell. 2002;2:301–14. doi: 10.1016/s1535-6108(02)00150-2. [DOI] [PubMed] [Google Scholar]
- Trolice MP, Pappalardo A, Peluso JJ. Basic fibroblast growth factor and N-cadherin maintain rat granulosa cell and ovarian surface epithelial cell viability by stimulating the tyrosine phosphorylation of the fibroblast growth factor receptors. Endocrinology. 1997;138:107–13. doi: 10.1210/endo.138.1.4836. [DOI] [PubMed] [Google Scholar]
- Utton MA, Eickholt B, Howell FV, Wallis J, Doherty P. Soluble N-cadherin stimulates fibroblast growth factor receptor dependent neurite outgrowth and N-cadherin and the fibroblast growth factor receptor co-cluster in cells. J Neurochem. 2001;76:1421–30. doi: 10.1046/j.1471-4159.2001.00140.x. [DOI] [PubMed] [Google Scholar]
- Vogel-Hopker A, Momose T, Rohrer H, Yasuda K, Ishihara L, Rapaport DH. Multiple functions of fibroblast growth factor-8 (FGF-8) in chick eye development. Mech Dev. 2000;94:25–36. doi: 10.1016/s0925-4773(00)00320-8. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R. BMP7 Acts in Murine Lens Placode Development. Dev Biol. 1999;207:176–188. doi: 10.1006/dbio.1998.9153. [DOI] [PubMed] [Google Scholar]
- Xu L, Overbeek PA, Reneker LW. Systematic analysis of E-, N- and P-cadherin expression in mouse eye development. Exp Eye Res. 2002;74:753–60. doi: 10.1006/exer.2002.1175. [DOI] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, Nathans J. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–95. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- Yap AS, Kovacs EM. Direct cadherin-activated cell signaling: a view from the plasma membrane. J Cell Biol. 2003;160:11–6. doi: 10.1083/jcb.200208156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Friedman A, Heaney S, Purcell P, Maas RL. Meis homeoproteins directly regulate Pax6 during vertebrate lens morphogenesis. Genes Dev. 2002;16:2097–107. doi: 10.1101/gad.1007602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yang T, Madakashira BP, Thiels CA, Bechtle CA, Garcia CM, Zhang H, Yu K, Ornitz DM, Beebe DC, Robinson ML. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol. 2008;318:276–88. doi: 10.1016/j.ydbio.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Hung FC, Colvin JS, White A, Dai W, Lovicu FJ, Ornitz DM, Overbeek PA. Patterning the optic neuroepithelium by FGF signaling and Ras activation. Development. 2001;128:5051–60. doi: 10.1242/dev.128.24.5051. [DOI] [PubMed] [Google Scholar]