Abstract
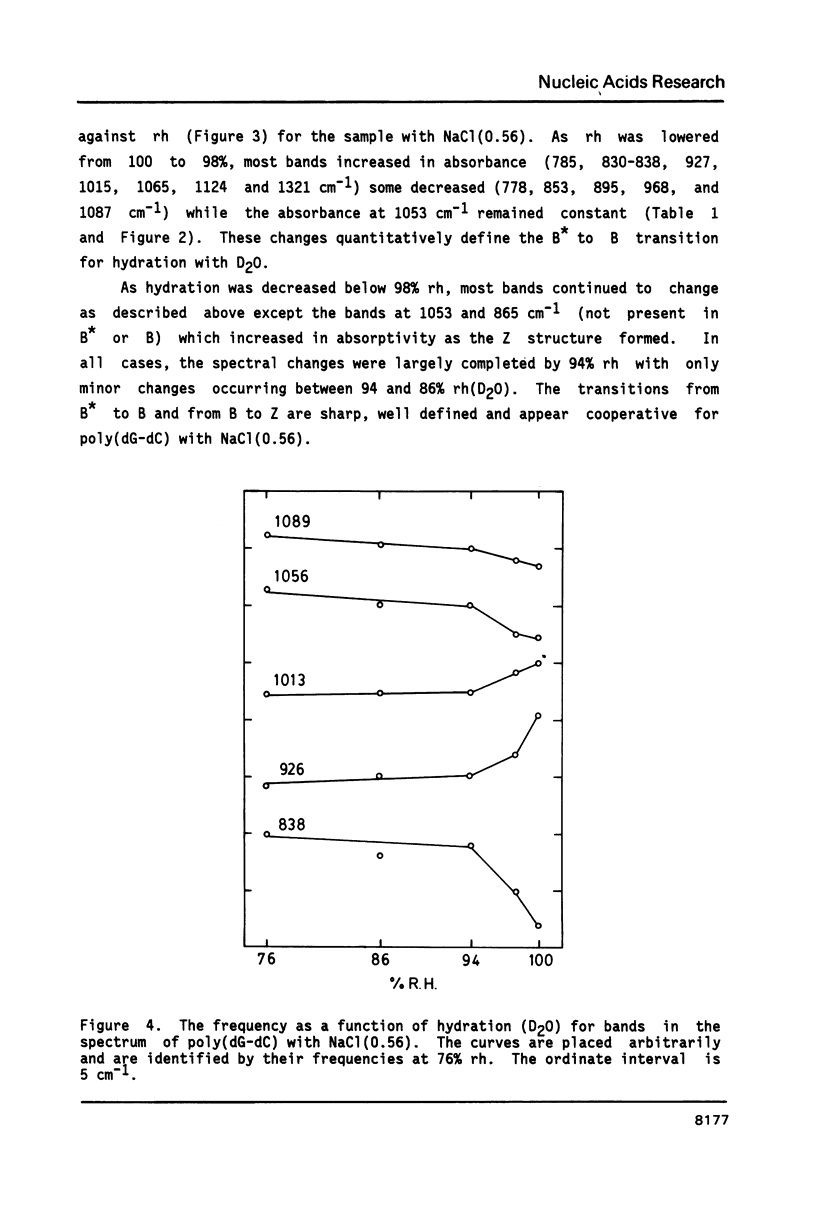
The poly(dG-dC) helical duplex forms a modified, B-family structure (B*) at very high hydration and a normal B structure at slightly lower hydration. The B* structure is slightly different in sugar-phosphate and base-stacking conformations than the B structure. Increasing the hydration or decreasing the NaCl content stabilizes B* with respect to B. Poly(dG-dC) forms the Z structure at low NaCl contents when the hydration is sufficiently reduced. At moderate NaCl content, the B to Z transition is sharp and cooperative for hydration with D2O. Hydration with H2O broadens the transition which occurs at lower hydration. This suggests that hydrogen bonding is stronger in the Z structure and helps stabilize Z over B. IR spectra may be used to quantitatively estimate the fractions of B and Z structures present in a sample. Some new indicator bands are described.
Full text
PDF


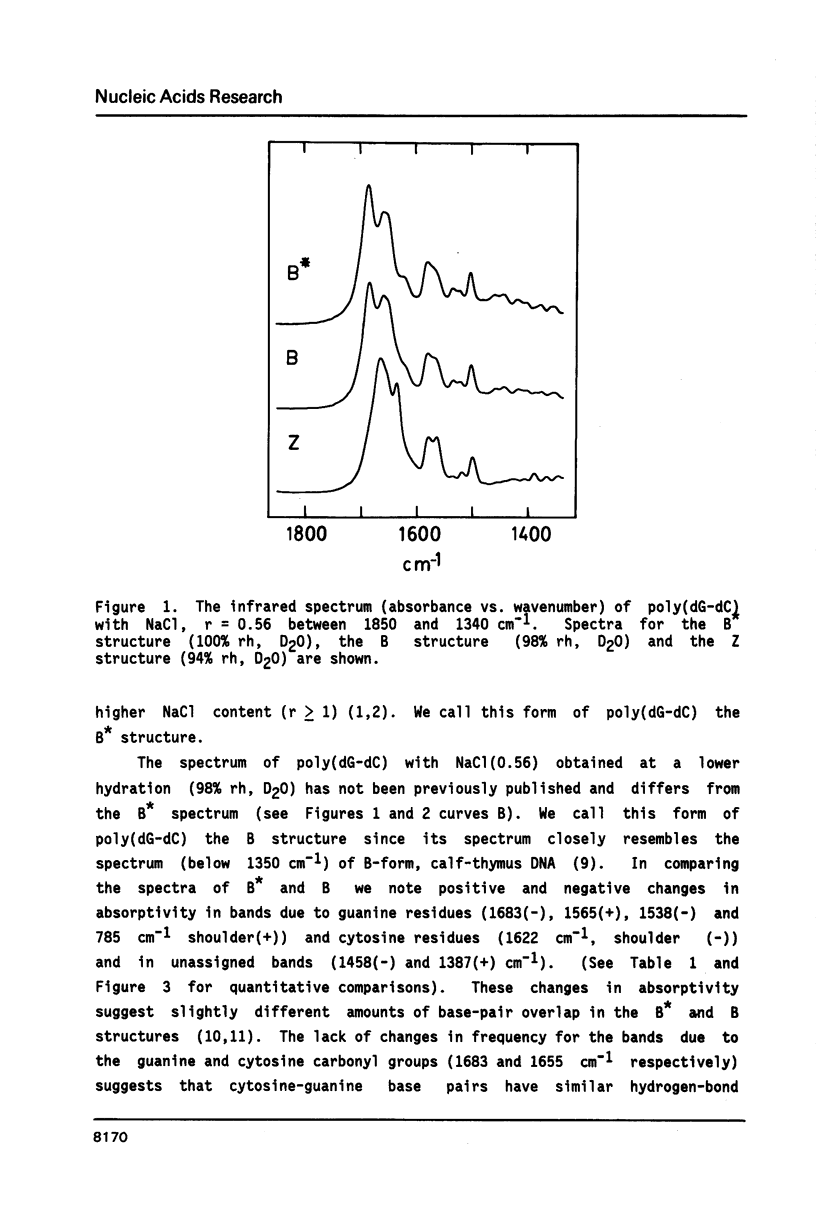
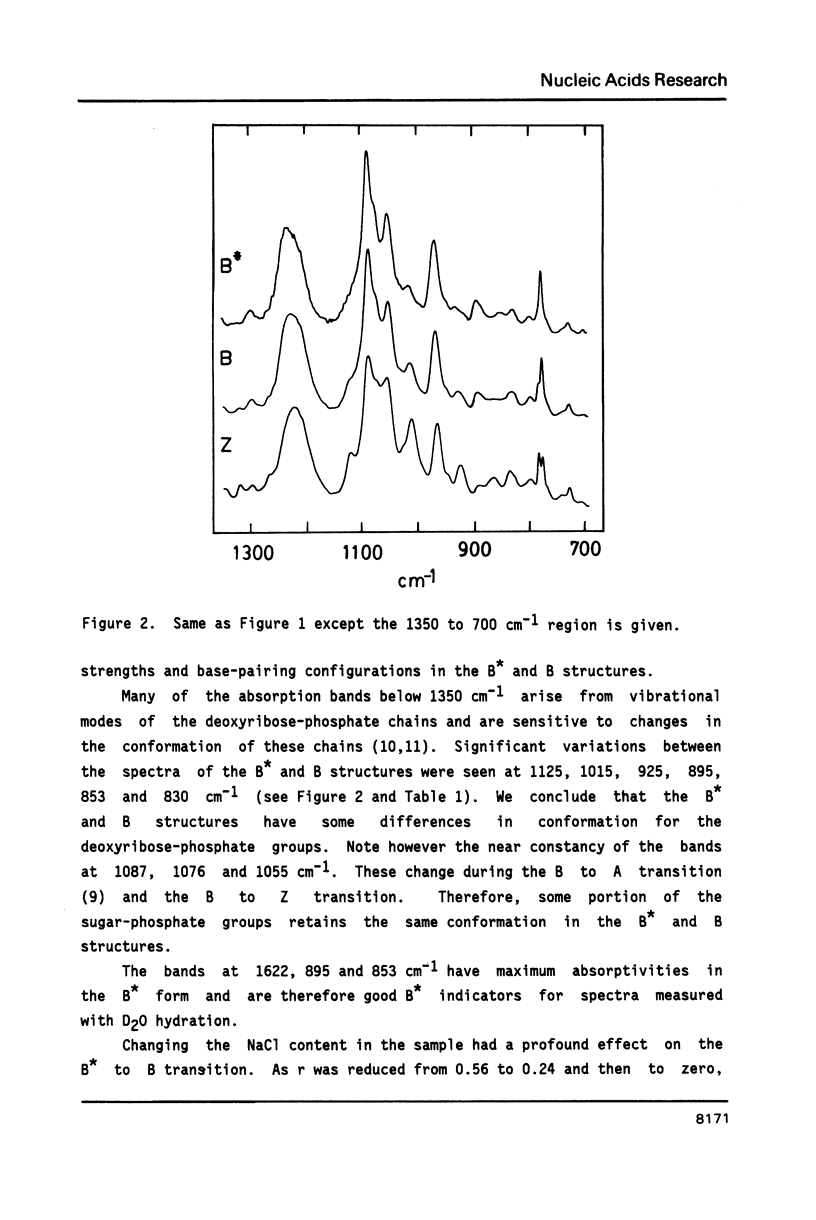
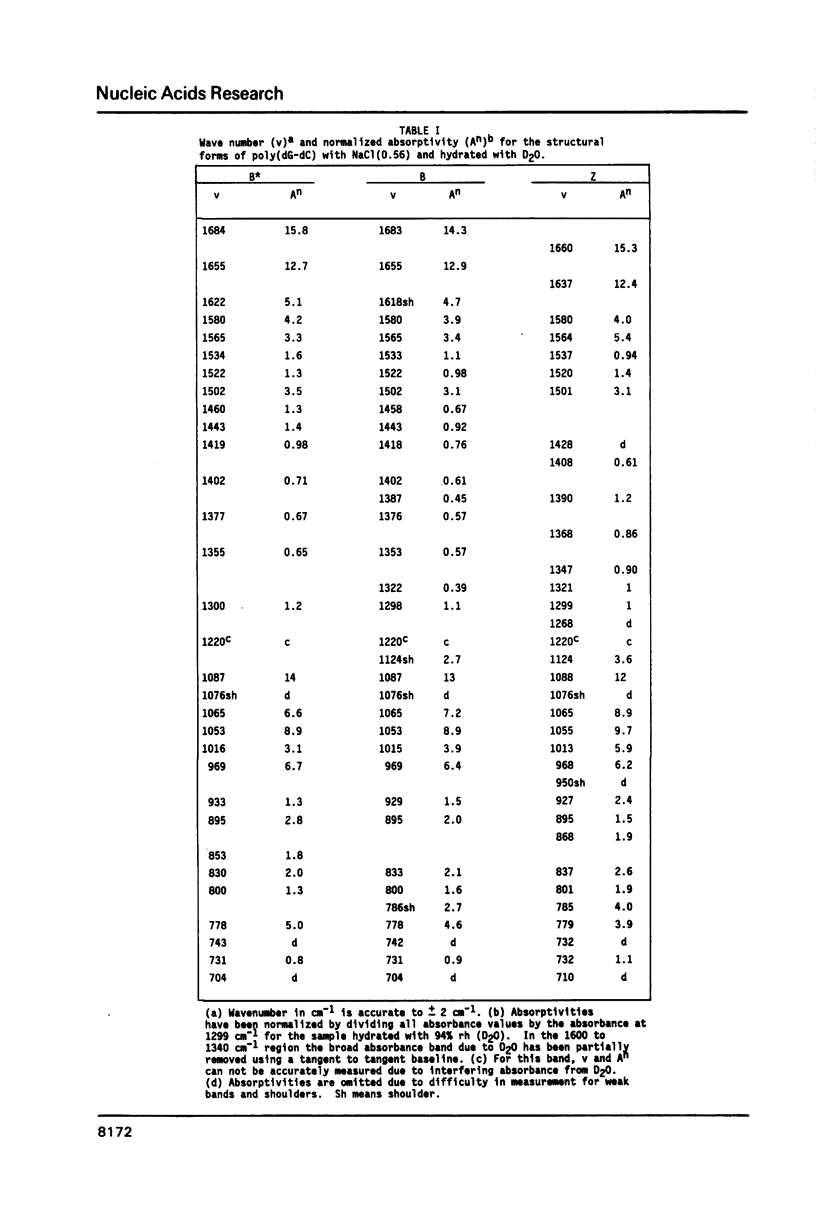
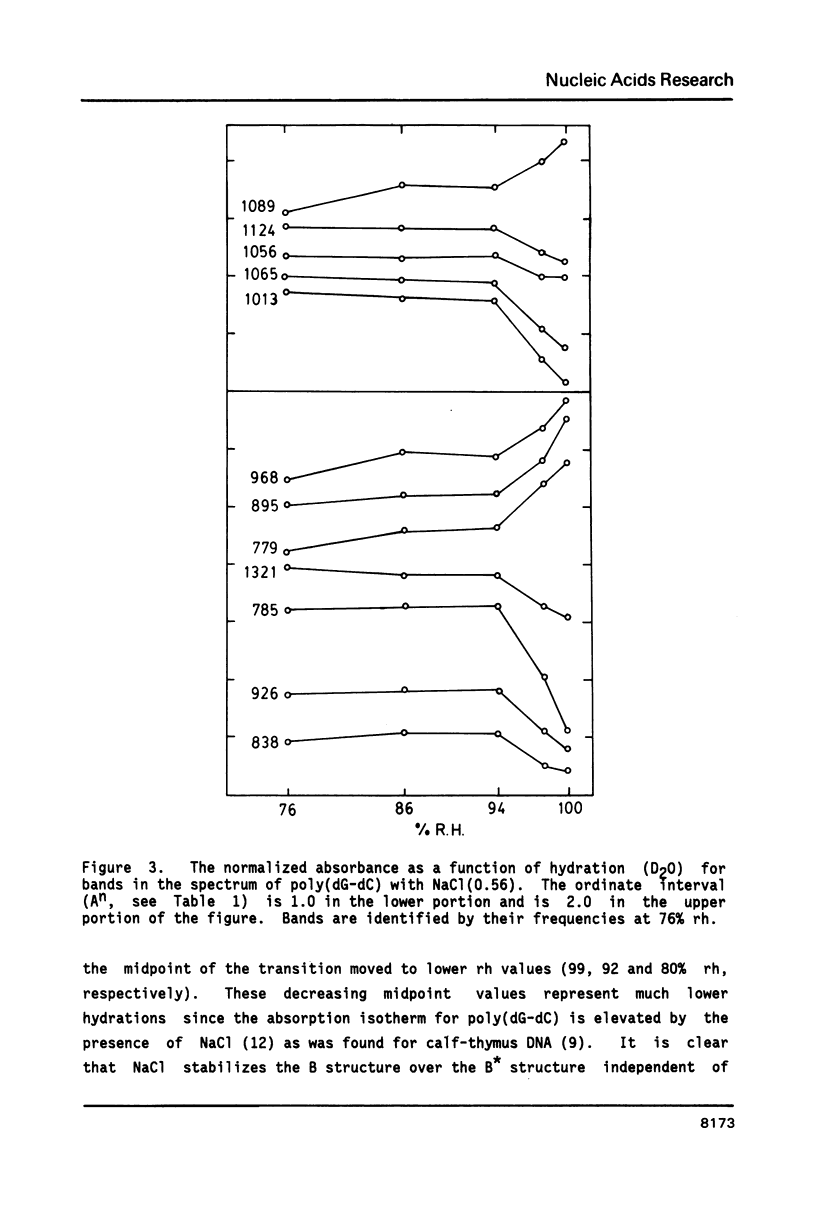








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Puigjaner L. C., Walker J. K., Hall I. H., Birdsall D. L., Ratliff R. L. Wrinkled DNA. Nucleic Acids Res. 1983 Mar 11;11(5):1457–1474. doi: 10.1093/nar/11.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behe M. J. Vacuum UV CD of the low-salt Z-forms of poly(rG-dC).poly(rG-dC), and poly(dG-m5dC).poly(dG-m5dC). Biopolymers. 1986 Mar;25(3):519–523. doi: 10.1002/bip.360250310. [DOI] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRico D. E., Jr, Keller P. B., Hartman K. A. The infrared spectrum and structure of the type I complex of silver and DNA. Nucleic Acids Res. 1985 Jan 11;13(1):251–260. doi: 10.1093/nar/13.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson R. E., Drew H. R., Conner B. N., Wing R. M., Fratini A. V., Kopka M. L. The anatomy of A-, B-, and Z-DNA. Science. 1982 Apr 30;216(4545):475–485. doi: 10.1126/science.7071593. [DOI] [PubMed] [Google Scholar]
- Dutta S., Parrack P. K., Sasisekharan V. Fibre diffraction of lithium DNA shows structural variability and deviation from a regular helical structure for the B-form. FEBS Lett. 1984 Oct 15;176(1):110–114. doi: 10.1016/0014-5793(84)80922-9. [DOI] [PubMed] [Google Scholar]
- Feuerstein B. G., Marton L. J., Keniry M. A., Wade D. L., Shafer R. H. New DNA polymorphism: evidence for a low salt, left-handed form of poly(dG-m5dC). Nucleic Acids Res. 1985 Jun 11;13(11):4133–4141. doi: 10.1093/nar/13.11.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard F. B., Frazier J., Miles H. T. Interbase vibrational coupling in G:C polynucleotide helices. Proc Natl Acad Sci U S A. 1969 Oct;64(2):451–458. doi: 10.1073/pnas.64.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Krimm S., Abe Y. Intermolecular interaction effects in the amide I vibrations of polypeptides. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2788–2792. doi: 10.1073/pnas.69.10.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie A. G., Arnott S., Chandrasekaran R., Ratliff R. L. Polymorphism of DNA double helices. J Mol Biol. 1980 Oct 15;143(1):49–72. doi: 10.1016/0022-2836(80)90124-2. [DOI] [PubMed] [Google Scholar]
- MILES H. T. Tautomeric forms in a polynucleotide helix and their bearing on the structure of DNA. Proc Natl Acad Sci U S A. 1961 Jun 15;47:791–802. doi: 10.1073/pnas.47.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilet J., Leng M. Comparison of poly(dG-dC).poly(dG-dC) conformations in oriented films and in solution. Proc Natl Acad Sci U S A. 1982 Jan;79(1):26–30. doi: 10.1073/pnas.79.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Roy K. B., Miles H. T. A thermally driven interconversion of B and Z-dna. Biochem Biophys Res Commun. 1983 Aug 30;115(1):100–105. doi: 10.1016/0006-291x(83)90974-9. [DOI] [PubMed] [Google Scholar]
- Taboury J. A., Bourtayre P., Liquier J., Taillandier E. Interaction of Z form poly(dG-dC).poly(dG-dC) with divalent metal ions: localization of the binding sites by I.R. spectroscopy. Nucleic Acids Res. 1984 May 25;12(10):4247–4258. doi: 10.1093/nar/12.10.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taillandier E., Taboury J., Liquier J., Sautière P., Couppez M. Structural transitions in DNAs and nucleohistones studied by I.R. spectroscopy. Biochimie. 1981 Nov-Dec;63(11-12):895–898. doi: 10.1016/s0300-9084(82)80282-4. [DOI] [PubMed] [Google Scholar]
- Wang A. J., Quigley G. J., Kolpak F. J., van der Marel G., van Boom J. H., Rich A. Left-handed double helical DNA: variations in the backbone conformation. Science. 1981 Jan 9;211(4478):171–176. doi: 10.1126/science.7444458. [DOI] [PubMed] [Google Scholar]
- Woisard A., Fazakerley G. V., Guschlbauer W. Z-DNA is formed by poly (dC-dG) and poly (dm5C-dG) at micro or nanomolar concentrations of some zinc(II) and copper(II) complexes. J Biomol Struct Dyn. 1985 Jun;2(6):1205–1220. doi: 10.1080/07391102.1985.10507633. [DOI] [PubMed] [Google Scholar]


