Abstract
Corticotropin releasing factor (CRF) in the amygdala is involved in stress responses. Moreover, dopaminergic neurotransmission in the brain reward system including the amygdala plays a significant role in the pathology of cocaine addiction. Our study analyzed CRF-induced synaptic plasticity, its pharmacological sensitivity, and interactions with the dopamine (DA) system in the basolateral (BLA) to lateral capsula central amygdala (lcCeA) pathway after a two week withdrawal from repeated cocaine administration. A physiologically relevant CRF concentration (25 nM) induced long-term potentiation (LTP) that was enhanced after cocaine withdrawal. In saline-treated rats, CRF-induced LTP was mediated through N-methyl-D-aspartate (NMDA) receptors, L-type voltage gated calcium channels (L-VGCCs), and CRF1 receptors. However, in cocaine-withdrawn animals, activation of CRF1 and CRF2 receptors was found to enhance LTP. This enhanced CRF-induced LTP after cocaine withdrawal was mediated through endogenous activation of both D1-like and D2-like receptors. Furthermore, expression of the D1 receptor (D1R) but not the D2R, D3R, D4R or D5R was significantly increased after cocaine withdrawal. It was also found that CRF1 but not CRF2 protein expression was increased suggesting that elevated levels of these proteins contributed to the enhancement of CRF-induced LTP during cocaine withdrawal. In summary, CRF interactions with the DA system in the amygdala may represent a fundamental neurochemical and cellular mechanism linking stress to cocaine-induced neuronal plasticity.
Keywords: synaptic transmission, CRF receptors, field EPSP, GABAergic inhibition, cocaine withdrawal, basolateral amygdala to central amygdala
INTRODUCTION
Corticotropin releasing factor (CRF), a 41-amino acid peptide, known for its neuroendocrine and behavioral mechanisms underlying the stress response (Bale and Vale, 2004) plays a prominent role in the actions of drugs of abuse, particularly cocaine (Sarnyai et al., 1992; Sarnyai et al., 2001; Goeders, 2002). Specifically, a CRF antagonist administered intracerebroventricularly produces dose-dependent inhibition of cocaine-induced locomotor activity (Sarnyai et al., 1992). Likewise, CRF is involved in the maintenance of cocaine self-administration (Goeders and Guerin, 2000) and in stress- and cue-induced reinstatement of cocaine-seeking behavior (Erb et al., 1998; Erb et al., 2001) suggesting a role for endogenous CRF in cocaine-induced behavioral plasticity.
Evidence suggests that the amygdala represents an important locus for cocaine, stress and CRF interactions. It is also known that the central nucleus of the amygdala (CeA) is required for foot shock stress-induced reinstatement of cocaine seeking in rats trained to self-administer (McFarland et al., 2004). The CeA contains a large number of CRF-immunopositive cell bodies and terminals (Gray and Bingaman 1996) with a high density of CRF binding sites found in the basolateral amygdala (BLA) (De Souza et al., 1985; De Souza, 1987). Studies have shown that following short-term withdrawal from chronic cocaine, CRF mRNA levels (Zhou et al., 2003) and CRF release (Richter and Weiss, 1999) are dramatically increased in the amygdala, while CRF labeling decreases after short-term, but increases after long-term withdrawal (Zorrilla et al., 2001). This suggests that CRF associated signaling mechanisms may be significantly affected by cocaine withdrawal.
Actions of CRF in the amygdala are mediated through two major receptor types, CRF1 and CRF2 (Liu et al., 2004; Pollandt et al., 2006). CRF1 immunoreactivity is dense in the CeA (Chen et al., 2000). CRF-induced long-term potentiation (LTP) in the lateral amygdala (LA) to lateral capsula central amygdala (lcCeA) pathway in saline-treated animals is mediated primarily through activation of CRF2 (Pollandt et al., 2006). After cocaine withdrawal, an enhanced CRF-induced LTP is observed due to increase in CRF1 protein levels (Pollandt et al., 2006). This indicates that cocaine may affect specific CRF receptors in the CeA.
Dopamine (DA) and DA receptors (DRs) play a significant role in cocaine-induced neuroplasticity and modulation of neural activity in the amygdala. A D1-like receptor antagonist applied to the BLA blocks conditioned reinstatement of cocaine-seeking behavior (See et al., 2001). Additionally, DA itself can attenuate firing of BLA projection neurons and activation of BLA interneurons (Rosenkranz and Grace, 1999). DA is also known to gate synaptic plasticity in LA pathways by suppressing GABAergic inhibition (Bissiere et al., 2003). Other anatomical data provide evidence in the CeA for dopaminergic innervation of terminals with CRF-immunoreactive soma (Eliava et al., 2003). Thus, DA receptors in the BLA-lcCeA pathway may play a role in CRF-induced synaptic plasticity after cocaine withdrawal.
Some classic mediators of synaptic plasticity, such as N-methyl-d-aspartate (NMDA) receptors and voltage-gated calcium channels (VGCCs) are also involved in cocaine mechanisms and may influence CRF plasticity in the amygdala. NMDA receptor antagonists in the amygdala block locomotor sensitization of chronic cocaine (Kalivas and Alesdatter, 1993). Similarly, activation of L-type calcium channels mimic the induction (Lin et al., 2001) of cocaine sensitization while antagonists block the expression (Pierce et al., 1998). These data suggest that the above mediators may also be involved in cocaine-induced neuronal plasticity. However, interactions between these mediators and CRF-induced changes have not yet been investigated in the BLA-lcCeA pathway after cocaine withdrawal.
In the present study we tested the hypotheses that, 1) pharmacology of CRF-induced synaptic plasticity is altered after cocaine withdrawal, 2) DA receptors mediate CRF-induced plasticity and this interaction is altered after cocaine withdrawal and 3) the modulations in the DA system and CRF effects during cocaine withdrawal are caused by changes in protein expression. The results of these studies help define the interactions between CRF and the DA system, which may represent a fundamental mechanism linking stress to cocaine addiction.
MATERIALS AND METHODS
Subjects
All animal procedures were carried out in accordance to the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. Male Sprague-Dawley albino rats (Harlan, Houston, TX, USA) were used as subjects. Animals were randomly divided into cocaine and saline groups. Animals were acclimated for four days in the institutional animal housing facility controlled for temperature and humidity. Food and water were provided ad libitum. Animals were injected with cocaine (15 mg/kg) or saline (0.1 ml/kg) intraperitoneally (i.p.), once a day for seven consecutive days. After the treatment regimen, animals were subjected to either 24 hour or 14 day withdrawal period. Age of the animal ranged from 29-39 days for the 24 hour and 42-52 days for the 14 day withdrawal paradigms. Behavioral monitoring methods were carried out as previously described (Pollandt et al., 2006).
Locomotor Activity
To monitor cocaine effectiveness, we measured cocaine-induced conditioned locomotor activity in response to i.p. injections. Locomotor activity was measured as the total distance in centimeters (cm) traveled by the animal in an acrylic chamber (16” × 16” × 12”) for 15 minutes using a Versamax activity monitor system (Accuscan Instruments Inc., Columbus, OH, USA). The amygdala, while not known to be linked directly to locomotor sensitization, is involved in cocaine cues (See, 2002) occurring as a result of the handling and injection process. We used locomotor activity as a measure of neuronal adaptations resulting from the cocaine treatment and withdrawal paradigms.
Slice Preparation
Coronal brain slices were prepared either 24 hours or 14 days after the injection paradigm. No anesthetics were used prior to decapitation to avoid their influence on neuronal plasticity. Initially, serial coronal slices (500μm) were bathed in oxygenated, modified artificial cerebrospinal fluid (ACSF) solution (in mM), NaCl, (119); KCl, (3.0); NaH2PO4, (1.2); MgSO4, (1.2); CaCl2, (2.5); NaHCO3, (25); and glucose, (11.5) at room temperature (RT) for 1 hr. Slices were then submerged in a chamber (1.0 ml, 2.5 ml/min) and held at 30 ± 1°C for another hour before recording. A constant pH (7.4) was maintained by continuous superfusion (2ml/min) with oxygenated [95% oxygen/ 5% carbon dioxide (carbogen)] ACSF.
Electrophysiology
Field excitatory postsynaptic potentials (fEPSPs) were recorded with tungsten electrodes (2-5 MΩ) in coronal brain slices cut ~ 3.3 to 3.8mm from bregma (Paxinos and Watson, 1998) which contained the lateral capsula region of the CeA (lcCeA). These fEPSPs were evoked by stimulating fibers in the BLA using 150 μs pulses of varying intensity (3–15 V) applied at 0.05 Hz through concentric electrodes (50 kΩ). All experiments were performed in the presence of 10 μM picrotoxin (PTX) in ACSF except where noted. Initially, fEPSP magnitude was adjusted to 30% of maximum response and baseline values recorded for 10 minutes. For the experiments where CRF was superfused, drugs were added to the ACSF for 10 minutes prior to addition of CRF to ACSF and continued throughout CRF superfusion. Thereafter, fEPSPs evoked at a frequency of 0.05 Hz were recorded for an hour. High frequency stimulation (HFS) consisting of five trains of stimuli (100 Hz for 1 sec, 3 min intervals) applied to the BLA pathway was used to evoke long-term potentiation (LTP) in some experiments. Drug application preceded the HFS by 5 minutes and was continued for the duration of the stimulation. Drug induced changes in fEPSP slopes during drug application, during CRF or HFS treatments and for 10 minutes at the end of the experiment were calculated and normalized to baseline values.
Western Blotting
Amygdala tissue preparation
After withdrawal, rats were decapitated and the brain was sliced to obtain the amygdala as described above. The appropriate amygdala subregions were isolated and homogenized with lysis buffer (Mammalian Cellytic lysis buffer, SIGMA, St. Louis, MO) containing protease inhibitors (complete mini EDTA-free protease cocktail tablet, Pierce Biotechnologies, Rockford, IL) to obtain amygdala homogenate. The cell membrane fraction was obtained by homogenizing isolated amygdala subregions with lysis buffer containing (in mM), sucrose, (320); Tris-HCl, (25); EGTA, (2); EDTA, (2) and protease inhibitors and adjusted to pH 7.4. Individual samples were then centrifuged at 3,000 rpm for 15 min at 4°C and the supernatant collected and placed into ultra-clear centrifuge tubes (Beckman Coulter, Inc., Fullerton, CA). After spinning twice at 150,000 g for 20 min, the pellet fraction was dissolved in a minimum volume of lysis buffer with 1% Triton-X 100. Protein quantification was performed using the BCA protein assay (Pierce Biotechnologies, Rockford, IL, USA). Aliquots of 50 μg protein samples were stored at -80°C until further use. Each sample represented one animal.
Immunoblotting
A solution of 2X sample buffer with 1 mM DTT was added to the samples and then placed in water bath at 37°C for 30 min followed by a 5 min cooling on ice, unless otherwise indicated. Samples (50 μg per lane) were separated on a 10% acrylamide gel by SDS-PAGE and transferred to a nitrocellulose membrane overnight in a cold room. The membranes were then blocked for at least one hour at RT or overnight (O/N) at 4°C in LI-COR (LI-COR Biosciences, Lincoln, Nebraska) blocking buffer and then incubated overnight in primary antibodies (diluted in LI-COR blocking buffer). After removal of the antibody, the blot was washed with phosphate buffered saline (PBS) [composition (in mM), NaCl, (137); KCl, (2.7), Na2HPO4, (100), KH2PO4, (2)] containing 0.05% Tween 20 (PBST). All further steps were carried out in the dark to prevent the loss of sensitivity of the infrared dye secondary antibodies. The secondary antibodies (diluted in LI-COR blocking buffer) were applied for one hour at RT. Membranes were scanned directly by the Odyssey Infrared Fluorescent Imaging System (LI-COR Biosciences, Lincoln, NE, USA). As a loading control, blots were also probed with either N-cadherin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), or an actin antibody. Band densities were calculated using the integrated intensity values determined by the Odyssey software. Labeling was quantified by analyzing the ratio of the integrated intensity value of the protein specific antibody to the loading control in each lane to provide an integrated intensity ratio.
Antibodies
DA receptor rabbit polyclonal antibodies used were: D1R (AB20066 at 1:1000 dilution), D3R (AB42114 at 1:1000 dilution for whole amygdala, 1:400 for membrane fraction) and D4R (AB20424 at 1:500 dilution) from Abcam (Cambridge, MA). For certain blots, D1R antibody from Calbiochem (San Diego, CA) (324390 at 1:3000 dilution for membrane fraction) was used. Lastly, D1A (MAB5290 mouse monoclonal C-terminal at 1:250 dilution), D2R (AB5084P rabbit polyclonal at 1:800 dilution) and D5R (MAB5292 mouse monoclonal C-terminal at 1:250 dilution) from Millipore (Temecula, CA) were also used to identify DA receptors. The CRF receptor (rabbit polyclonal) antibodies used were CRF-R1/2 (H-215 at 1:200 dilution - against epitope 230-244 amino acids (aa) from the CRF1 receptor, Santa Cruz Biotechnologies, San Diego, CA) and CRHR2 (Novus Biologicals, Littleton, CO, at 1:400 dilution - against epitope 75-125 aa from the human CRF2 receptor). Other antibodies used were N-cadherin mouse monoclonal antibody to pan cadherin (CH-19) (AB6528-100, Abcam) at 1:5000 dilution, actin (MAB1501 - mouse monoclonal from Millipore) at 1:50,000 to 1:100,000 dilution and GAPDH (Clone 6C5 mouse monoclonal, Advanced Immunochemicals Inc., Long Beach, CA) at 1:100,000 for whole amygdala and 1:50,000 for membrane fraction. Goat anti-rabbit (926-32211-IRDye 800CW) and donkey anti-mouse (926-32222-IRDye 680), were diluted to 1:20,000 each with LI-COR blocking buffer for secondary antibodies.
Drugs
Cyclo(31–34)[D-Phe11,His12,C(a)MeLeu13,39,Nle17,Glu31,Lys34]Ac-Svg(8–40)trifluoroacetate salt (astressin2-B), picrotoxin (PTX), nimodipine (NIM), rat/human corticotropin releasing factor (CRF), D-2-amino-5-phosphonovaleric acid (APV), (R)-(+)-7-chloro-8-hydroxy -3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride [SCH23390 (SCH)] were all obtained from Tocris Cookson (Ellisville, MO). Raclopride (RAC), and 5-chloro-4-(N-(cyclopropyl)methyl-N-propylamino)-2-methyl-6-(2,4,6-trichlorophenyl) aminopyridine (NBI 27914 or NBI) were purchased from Sigma-Aldrich (St. Louis, MO) or Tocris Cookson (Ellisville, MO). Cocaine HCl was a gift from the National Institute of Drug Abuse. Cocaine was dissolved in 0.9% saline solution at 15 mg/kg.
Statistical analysis
Slices were cut from both hemispheres and one slice per hemisphere was used in each set of experimental treatments. On each experimental day, slices from saline-treated animals and matched cocaine-withdrawn animals were tested together. Analyses were performed using Clampfit 9.0 software (Molecular Devices, Sunnyvale, CA). Individual traces were filtered and six consecutive traces were averaged, the fEPSP slope was measured and plotted versus time. Averaged responses were measured 50-60 min after wash-out and normalized to baseline responses, for subsequent statistical analyses. For immunoblotting analysis, the means and standard error were calculated from a minimum of three samples.
To account for non-normal distribution of data, non-parametric tests were used for statistical analysis. Behavioral data was analyzed using either one-way ANOVA (Kruskal-Wallis test) or a repeated measures two-way ANOVA followed by a paired or unpaired t-test when significance was achieved. Western blot and electrophysiological data were analyzed using a Kruskal-Wallis test followed by a Mann-Whitney U or Wilcoxon matched pair test as appropriate for pair-wise comparison. Statistical significance was defined at P<0.05, with an increasing number of asterisks indicating greater significance.
RESULTS
Locomotor activity increased progressively during 7 days of cocaine administration and after 14 days withdrawal
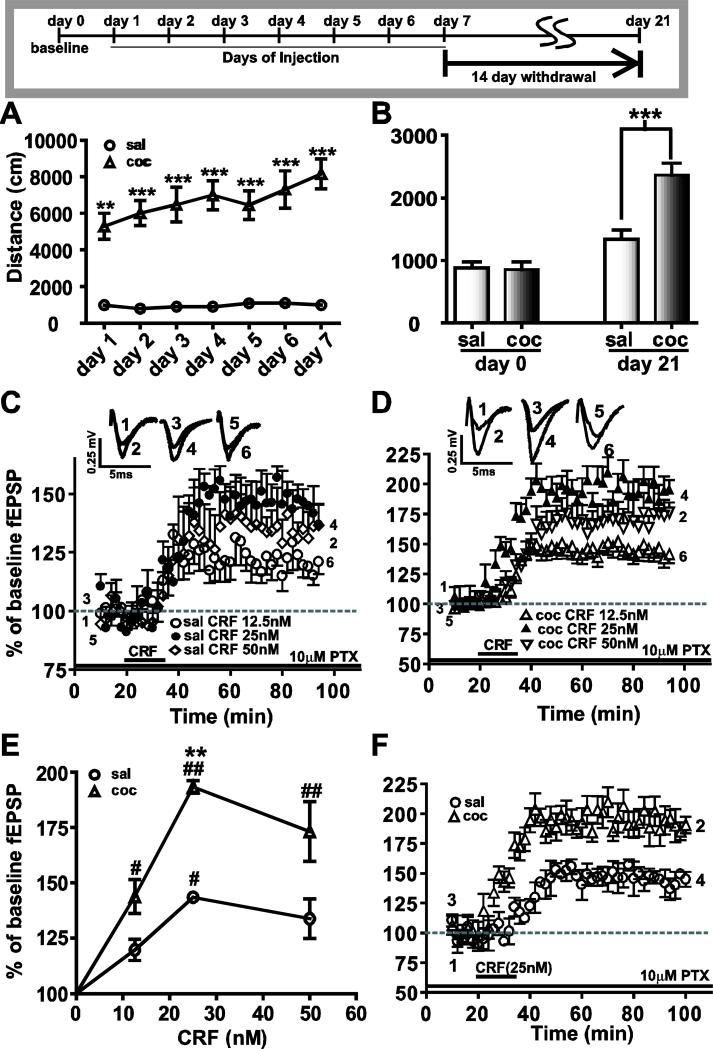
Locomotor activity was assessed 1) in a drug-free condition on day 0 (baseline or prior to the injections), 2) immediately following each drug injection during the 7 day repeated treatment regimen, and 3) on day 21 (after 14 day withdrawal). Locomotor activity is expressed as the total distance traveled (cm) during each 15 minute test period. Prior to any treatment, locomotor activity measurements showed no significant difference between the two groups (Fig.1B: Day 0, saline: 877.7 ± 98; cocaine: 859.8 ± 113.5, n=15; P>0.05). During the 7 days of repeated treatment regimen (Fig.1A), a repeated measures two-way ANOVA showed a significant main effect of drug (F(1,40)=70.04, ***P<0.0001), days (F(6,240)=3.42, **P<0.01) and drugXdays interaction (F(6,240)=3.42, **P<0.01). These results show that repeated cocaine administration progressively increased animal locomotion compared to saline. Additionally, when spontaneous locomotor activity was measured in animals after 14 days of withdrawal (Fig.1B), a two-way ANOVA comparing the two groups during baseline (day 0-drug free) and day 21 (drug free) indicated a significant effect of drug (F(1,28)=14.47, ***P<0.001), days (F(1,28)=47.48, ***P<0.0001) and drugXdays interaction (F(1,28)=12.56, **P<0.005). Thus, cocaine treatment induced changes in spontaneous locomotor activity that was evident 14 days after the last repeated injection (Fig.1B: saline: 1339.1 ± 143.5; cocaine: 2365.9 ± 187.5, n=15; **P<0.0005). The amygdala is important in cue-induced cocaine seeking behavior (See et al., 2003). Thus, cues associated with measuring locomotion per se may account for the increased locomotor activity.
Figure 1.
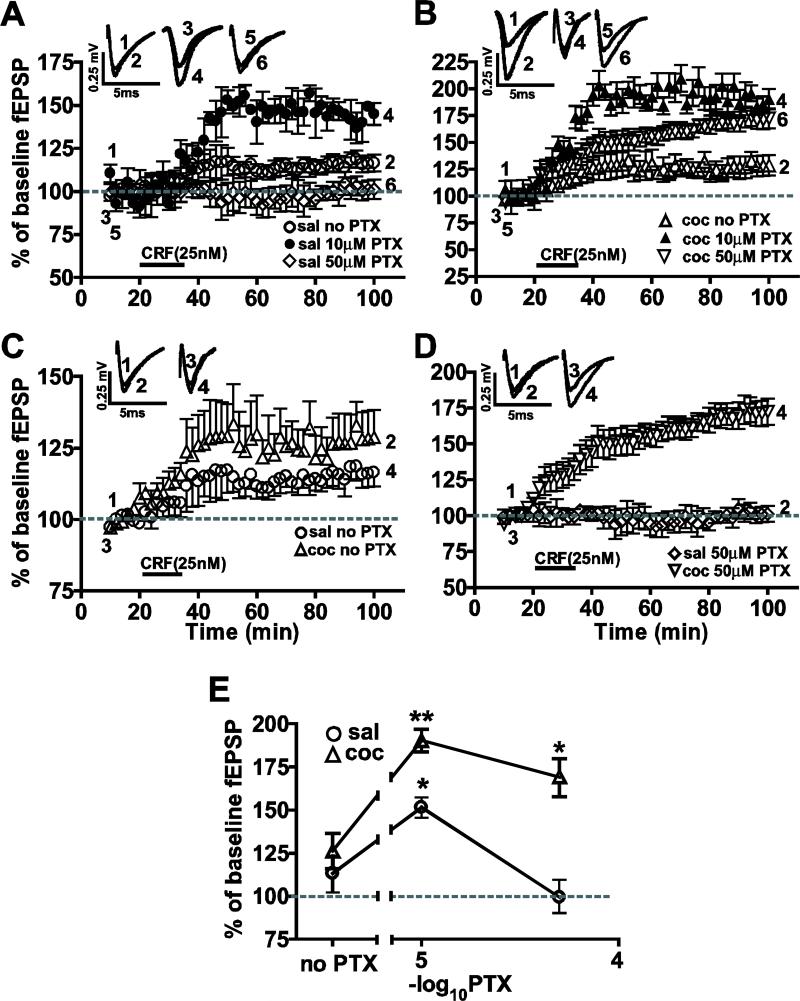
Cocaine treatment for 7 days enhanced CRF-induced LTP after 14 days withdrawal. The panel at the top depicts the time schedule of drug injections and test paradigm. (A) Locomotor activity (measured in distance traveled in centimeters during 15 minutes per day immediately after the drug injection along the Y-axis) in response to cocaine injection increases from day one to day seven of cocaine injections (triangular symbols), an effect which was absent in saline injected controls (circular symbols; **=P<0.01, ***=P<0.005). (B) When tested two weeks (day 21) after withdrawal from repeated cocaine injections (black bars) enhanced locomotor behavior was recorded suggesting continued neuronal adaptations to cocaine. In contrast, the saline-treated group showed no change (white bars). (C, D) Brain slices containing the BLA-lcCeA synaptic pathways obtained the saline-treated (C) and cocaine-withdrawn (D) groups at day 21 were tested with three different concentrations of CRF (12.5nM, 25nM and 50nM). CRF-induced LTP was plotted as percent of baseline fEPSP measured as a function of time in minutes. Traces of fEPSPs were recorded at the time points indicated by numbers placed on the graphs. (E) CRF at all concentrations resulted in enhanced LTP in cocaine-withdrawn group compared to saline-treated group. Concentration-response curves were plotted using the averaged fEPSPs from the last 10 minutes of LTP recording in slices from saline-treated (circles) and cocaine-withdrawn (triangles) animals. Based these data, 25nM was used CRF used in subsequent experiments. (F) CRF-induced LTP (25nM) in the cocaine-withdrawn group (triangles) was larger than LTP induced in the saline-treated group (circles) in the BLA-lcCeA synaptic pathway.
CRF-induced LTP in BLA-lcCeA pathway is enhanced at two weeks withdrawal from repeated cocaine administration
We analyzed the concentration-response relationship for CRF to identify the optimal concentration for electrophysiological studies in the BLA-lcCeA pathway. CRF application induced LTP, the magnitude of which significantly increased from the lowest to highest concentration in both saline-treated (Fig.1C: 12.5 nM=119.7 ± 1.4%, n=5; 25 nM=143.5 ± 2.0%, n=5; 50 nM=133.9 ± 1.2%, n=6; H=6.746, df=2, *P<0.05) and cocaine-withdrawn groups (Fig.1D: 12.5 nM=142.9 ± 1.4%, n=7; 25 nM=193.3 ± 2.8%, n=5; 50 nM=172.7 ± 2.4%, n=7; H=10.322, df=2, **P<0.001). Based on the concentration-response curve (Fig.1E), CRF 25 nM produced the maximum effect in the cocaine-withdrawn group and was used in all the subsequent studies in the BLA-lcCeA pathway. Two weeks after the 7 day repeated saline or cocaine treatment, CRF-induced LTP was significantly greater in the cocaine-withdrawn group compared to saline treatment group (Fig.1F, **P<0.005). The saline treatment group results were not different from acute slices prepared from control naïve animals (149.5 ± 14.6%, n=5; P>0.05, data not shown). In contrast, CRF-induced LTP could not be detected in slices from animals treated with saline or cocaine for 7 days and withdrawn for 24 hours (saline: 97.5 ± 4.9%, n=6; cocaine: 107.8 ± 3.7%, n=6; P>0.05, data not shown), an effect possibly due to stress (Alfarez et al., 2003) associated with injection-related handling.
CRF-induced LTP in rats is NMDA receptor-dependent
We previously demonstrated that LTP induced by HFS in the BLA-lcCeA pathway was NMDA receptor dependent (Fu and Shinnick-Gallagher, 2005). To test whether NMDA receptors are required for CRF-induced LTP in the saline-treated or cocaine-withdrawn groups, the NMDA antagonist, APV (50 μM) was applied 10 min before and during CRF superfusion. In all slices tested, APV completely blocked CRF-induced LTP (H=22.24, df=3, ***P<0.0001) both in saline-treated (Fig.2A) and cocaine-withdrawn (Fig.2B) groups (saline CRF: 149.2 ± 4.0%, n=8; saline CRF+APV: 110.6 ± 18.8%, n=5; cocaine CRF: 199.0 ± 7.5%, n=8; cocaine CRF+APV: 94.3 ± 18.9%, n=5; **P<0.005). These data suggest that the CRF-induced LTP observed after cocaine withdrawal is NMDA receptor-dependent. The antagonist (APV) alone and all other antagonists utilized in this study had no effect on baseline fEPSP recording before CRF or HFS application (see Table 1).
Figure. 2.
NMDA antagonist and VGCC blocker prevented induction of CRF-induced LTP in saline-treated and cocaine-withdrawn groups. Slices were obtained 14 days after the last saline or cocaine injection. Traces above show fEPSPs recorded at the time points indicated by the respective numbers on the graphs. APV (50 μM, A, B) and nimodipine (10 μM, C, D) blocked CRF-induced LTP in both animal groups.
Table 1.
Effect of each drug application on the baseline fEPSP magnitude.
| Drug Applied | Number of slices | Baseline (Mean ± SEM) | Drug before CRF (Mean ± SEM) | Wilcoxon sum of signed rank (W) | P-value | Significance @ P<0.05 |
|---|---|---|---|---|---|---|
| Sal CRF + APV | 5 | 99.1 ± 2.9 | 104.0 ± 10.1 | -1 | 1.0000 | ns |
| Coc CRF + APV | 5 | 99.6 ± 4.8 | 105.1 ± 5.4 | -1 | 1.0000 | ns |
| Sal CRF + NIM | 5 | 98.8 ± 2.1 | 87.9 ± 10.3 | 9 | 0.3125 | ns |
| Coc CRF + NIM | 5 | 100.2 ± 1.0 | 92.2 ± 7.1 | 7 | 0.4375 | ns |
| Sal CRF + NBI | 8 | 95.9 ± 1.4 | 99.7 ± 7.3 | -6 | 0.7422 | ns |
| Coc CRF + NBI | 8 | 98.6 ± 3.3 | 99.8 ± 4.4 | -4 | 0.8438 | ns |
| Sal CRF +Ast2B | 5 | 100.0 ± 1.0 | 103.3 ± 6.5 | -5 | 0.6250 | ns |
| Coc CRF + Ast2B | 5 | 100.0 ± 0.8 | 99.1 ± 5.6 | 1 | 1.0000 | ns |
| Sal CRF + SCH | 5 | 97.6 ± 3.3 | 106.3 ± 7.5 | -5 | 0.6250 | ns |
| Coc CRF + SCH | 5 | 96.5 ± 2.5 | 97.8 ± 5.8 | -1 | 1.0000 | ns |
| Sal CRF + RAC | 6 | 100.0 ± 0.9 | 107.2 ± 3.0 | -17 | 0.0938 | ns |
| Coc CRF +RAC | 7 | 100.0 ± 1.1 | 105.3 ± 4.6 | -12 | 0.3750 | ns |
| Sal HFS+SCH | 5 | 100.5 ± 2.2 | 98.0 ± 3.4 | 9 | 0.3125 | ns |
| Sal HFS+RAC | 5 | 100.5 ± 0.7 | 100.3 ± 1.0 | -5 | 0.6250 | ns |
| Coc HFS+SCH | 5 | 97.1 ± 2.8 | 103.4 ± 5.9 | -9 | 0.3125 | ns |
| Coc HFS+RAC | 8 | 100.8 ± 0.4 | 99.3 ± 0.7 | 18 | 0.2500 | ns |
Kruskal-Wallis test statistic=37.45; P=0.5408, ns=not significant
fEPSP magnitude was calculated during the time of the drug application which preceded the CRF or HFS treatment and followed the baseline measurements. It was then normalized to baseline values and averaged. The mean and standard error of mean was calculated for the averages from the number of slices indicated in the table. Kruskal-Wallis ANOVA followed by pairwise comparison using Wilcoxon revealed no significant differences between the baseline values and the drug application alone, suggesting that the drugs by themselves did not alter the baseline responses.
CRF-induced LTP is dependent on voltage gated calcium channels
In addition to NMDA receptors, we reported previously that electrically induced HFSLTP in the BLA-lcCeA pathway was dependent on L-VGCCs (Fu and Shinnick-Gallagher, 2005). To examine the contribution of L-VGCCs to CRF-induced LTP after saline treatment or cocaine withdrawal, nimodipine (NIM, 10 μM), an L-VGCC antagonist, was added before and during CRF superfusion (Fig.2C, D). The CRF-induced LTP was blocked (H=22.24, df=3, ***P<0.0001) in both saline-treated (saline CRF: 149.2 ± 4.0%, n=8; saline CRF+NIM: 111.2 ± 16.0%, n=5; **P<0.005) and cocaine-withdrawn groups (cocaine CRF: 199.0 ± 7.5%, n=8; cocaine CRF+NIM: 117.4 ± 16.8%, n=5; **P<0.005). Thus, NMDA receptors and L-VGCC channels contribute to CRF-induced plasticity in the BLA-lcCeA pathway in both saline-treated and cocaine-withdrawn groups.
Both CRF1 and CRF2 receptors contributed to the enhanced CRF-induced LTP in observed during cocaine withdrawal
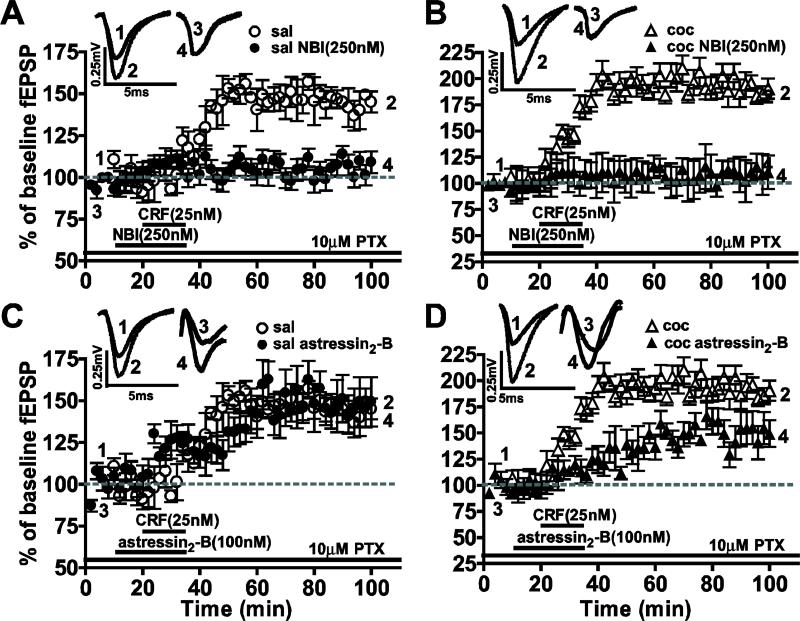
We examined the role of CRF1 and CRF2 receptors in mediating the CRF-induced LTP by applying selective antagonists (Fig. 3). The CRF1 antagonist, NBI 27914 (250 nM), superfused 10 min before and during CRF application, blocked CRF-induced LTP (H=26.19, df=3, ***P<0.0001) in slices from both saline-treated (saline CRF: 149.2 ± 4.0%, n=8; saline CRF+NBI: 104.1 ± 15.9%, n=8; ***P<0.0005) and cocaine-withdrawn groups (cocaine CRF: 199.0 ± 7.5%, n=8; cocaine CRF+NBI: 110.2 ± 13.0%, n=8; ***P<0.0005). Treatment with the selective CRF2 antagonist, astressin2-B (100 nM) resulted in significant LTP reduction in the cocaine-withdrawn group (cocaine CRF: 199.0 ± 7.5%, n=8; cocaine CRF+astressin2-B: 149.5 ± 2.4%, n=5; **P<0.005). No measurable effect was observed on CRF-induced LTP in slices from saline-treated animals (saline CRF: 149.2 ± 4.0%, n=8; saline CRF+astressin2-B: 149.0 ± 1.8%, n=5; P>0.05). These data suggest that while CRF1 receptors alone are required for CRF-induced LTP in both treatment groups, CRF2 receptors in the BLA to lcCeA pathway contribute to the enhanced CRF-induced LTP. However, activation of the CRF2 receptors alone are not sufficient to generate CRF-induced LTP in the cocaine-withdrawn group.
Figure 3.
CRF-induced LTP was blocked by CRF1 antagonists in saline-treated group whereas the enhanced LTP in the cocaine-withdrawn group involved CRF1 and CRF2 receptors. Traces above show fEPSPs recorded at the time points in the graphs below. The CRF1 antagonist inhibited induction of CRF-LTP in slices from both saline-treated (A) and cocaine-withdrawn (B) rats. The CRF2 antagonist had no effect in saline-treated group (C) yet significantly depressed the enhanced CRF-induced LTP (D) in the cocaine-withdrawn group.
Enhanced CRF-induced LTP is blocked in cocaine-withdrawn animals by D1- and D2-like antagonists
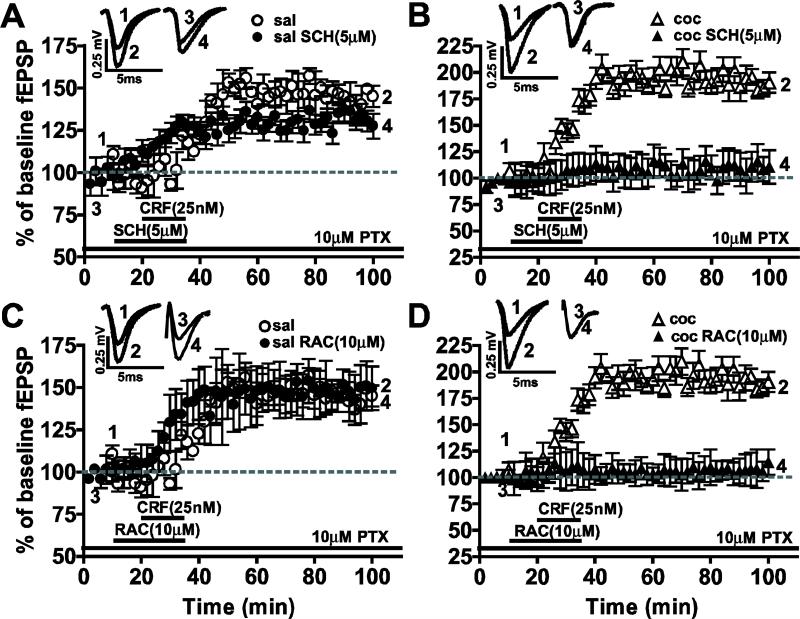
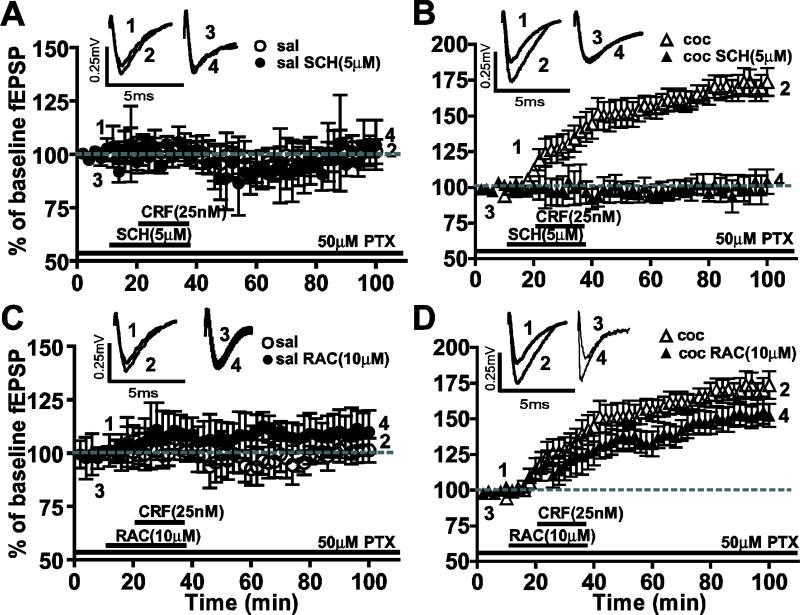
We tested whether DA receptors were involved in CRF-induced LTP (Fig. 4). The D1-like receptor antagonist SCH23390 (5 μM) produced no significant reduction in CRF-induced LTP in the saline-treated group (saline CRF: 149.2 ± 4.0%, n=8; saline CRF+SCH: 144.1 ± 4.05%, n=5; P>0.05). Conversely, SCH23390 blocked the enhancement of CRF-induced LTP observed in cocaine-withdrawn group (cocaine CRF: 199.0 ± 7.5%, n=8; cocaine CRF+SCH: 110.2 ± 11.63%, n=5; **P<0.005). When D2-like receptor antagonist raclopride (10μM RAC) was applied, CRF-induced LTP was unaffected in the saline-treated group (saline CRF: 149.2 ± 4.0%, n=8; saline CRF+RAC: 149.5 ± 2.881%, n=10; P>0.05) whereas RAC blocked the enhancement of CRF-induced LTP in the cocaine-withdrawn group (cocaine CRF: 199.0 ± 7.5%, n=8; cocaine CRF+RAC: 108.7 ± 4.8%, n=7; ***P<0.0005). These results suggest that the mechanisms underlying CRF-induced LTP are different in saline-treated versus cocaine-withdrawn groups.
Figure 4.
CRF-induced LTP in the saline-treated group was not significantly affected by DA antagonists whereas the enhanced CRF-induced LTP in the cocaine-withdrawn group was blocked by D1- and D2-like receptor antagonists. Traces above show fEPSPs recorded at the respective time points in corresponding graphs below. (A, B) The CRF-induced LTP in saline-treated group (A) was not significantly diminished, while the enhanced CRF-induced LTP in slices from cocaine-withdrawn animals (B) was completely blocked by the D1-like receptor antagonist, SCH23390. (C, D) CRF-induced LTP in saline-treated group (C) was not significantly affected by D2-like receptor antagonist, raclopride, but enhanced CRF-induced LTP in the cocaine-withdrawn group (D) was significantly reduced.
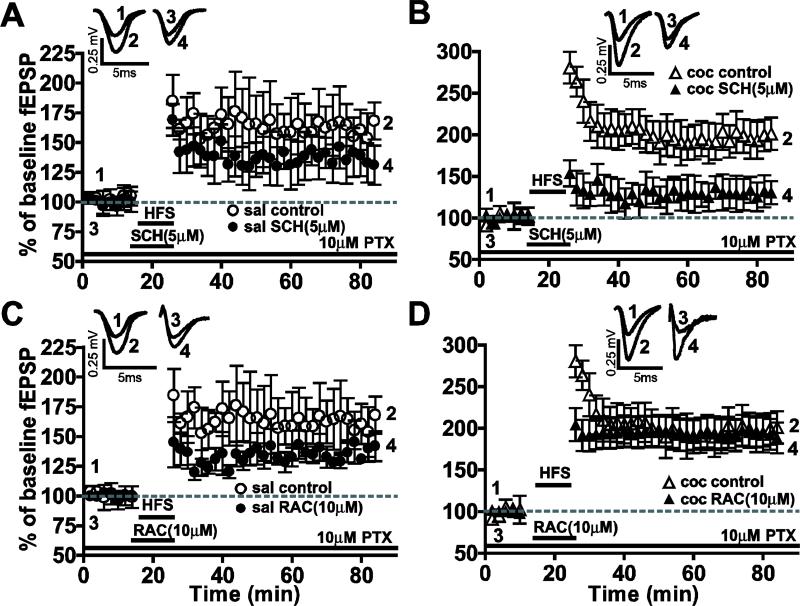
D1- but not D2-like receptor antagonists block the enhanced HFS–induced LTP recorded after cocaine withdrawal
To compare whether endogenous DA receptors also mediate electrically-induced LTP we analyzed the effects of DA receptor antagonists on LTP induced by HFS in the BLA-lcCeA pathway (Fig. 5). When the D1-like antagonist SCH23390 (5 μM) was applied 15 min before LTP induction, the HFS-LTP in slices from saline-treated group (saline: 160.4 ± 9.4, n=12; saline SCH: 135.9 ± 7.3%, n=5; P>0.05) was not significantly affected. However, HFS-induced LTP in the cocaine-withdrawn group (cocaine: 197.3 ± 11.0, n=12; cocaine SCH: 130.0 ± 3.305%, n=5; ***P<0005) was blocked by SCH23390. Conversely, when the D2-like antagonist RAC (10 μM) was applied, neither the saline-treated (saline: 160.4 ± 9.4, n=12; saline RAC: 138.9 ± 9.7%, n=5; P>0.05) nor the cocaine-withdrawn groups (cocaine: 197.3 ± 11.0, n=12; cocaine RAC: 197.5 ± 20.45%, n=8; P>0.05) were significantly affected. While DA receptor antagonists had no effect on CRF-induced LTP in the saline-treated group, induction of HFS- and CRF-induced LTP enhanced in the cocaine-withdrawn group may involve differences in DA receptor mechanisms.
Figure 5.
Tetanically induced LTP enhanced during cocaine withdrawal was blocked by D1-like not D2-like antagonists. Traces above show fEPSPs recorded at time points indicated in graphs below. The HFS-LTP was not significantly affected by D1-like antagonists in saline-treated group (A), while it was completely blocked in the cocaine-withdrawn group (B). Neither the HFS-LTP in saline-treated group (C) nor the enhanced HFS-LTP during cocaine withdrawal (D) was significantly affected by the D2-like antagonist.
CRF-induced LTP is controlled by GABAergic feed-forward inhibition
DA terminals make synaptic contact with GABA interneurons (similar to those represented as local interneurons in Fig. 12) in the BLA (Loughlin and Fallon, 1983; Lindvall et al., 1984; Snyder et al., 2000) and with CeA neurons containing CRF and other peptides (Asan 1998; Eliava et al., 2003). Moreover, CRF enhances GABAergic transmission in the CeA (Nie et al., 2004). These data suggest a convergence of CRF, DA and GABAergic synaptic mechanisms in the CeA. For this reason, we examined the possible role of GABAergic inhibition in the CRF-induced LTP in saline-treated and cocaine-withdrawn groups (Fig. 6) by applying different concentrations of picrotoxin (PTX), an antagonist of GABAA receptors. A two-way ANOVA showed a significant effect of the cocaine (drug) treatment (F(1,33)=97.16, ***P<0.0001), PTX concentration (F(2,33)=59.45, ***P<0.0001) and a significant drugXconcentration interaction (F(2,33)=14.48, ***P<0.0001). These results indicate that PTX had markedly different effects between the cocaine-withdrawn and saline-treated groups. In slices from the saline-treated group, the largest magnitude of CRF-induced LTP was recorded using 10 μM PTX (149.2 ± 4.0%, n=8; Fig. 6A) with a significant decrease (H=15.09, df=2, ***P<0.0005) measured in the absence of PTX (113.3 ± 4.17%, n=6; ***P<0.0005) and 50 μM PTX (99.46 ± 5.7%, n=7; ***P<0.0005). In the cocaine-withdrawn group, CRF-induced LTP was reduced (H=12.75, df=2, ***P<0.005) in the absence of any PTX (126.3 ± 8.35%, n=5; Fig. 6B) compared to 10 μM (199.4 ± 7.5%, n=8; **P<0.01) and 50 μM PTX (169.2 ± 7.8%, n=6; ***P<0.005).
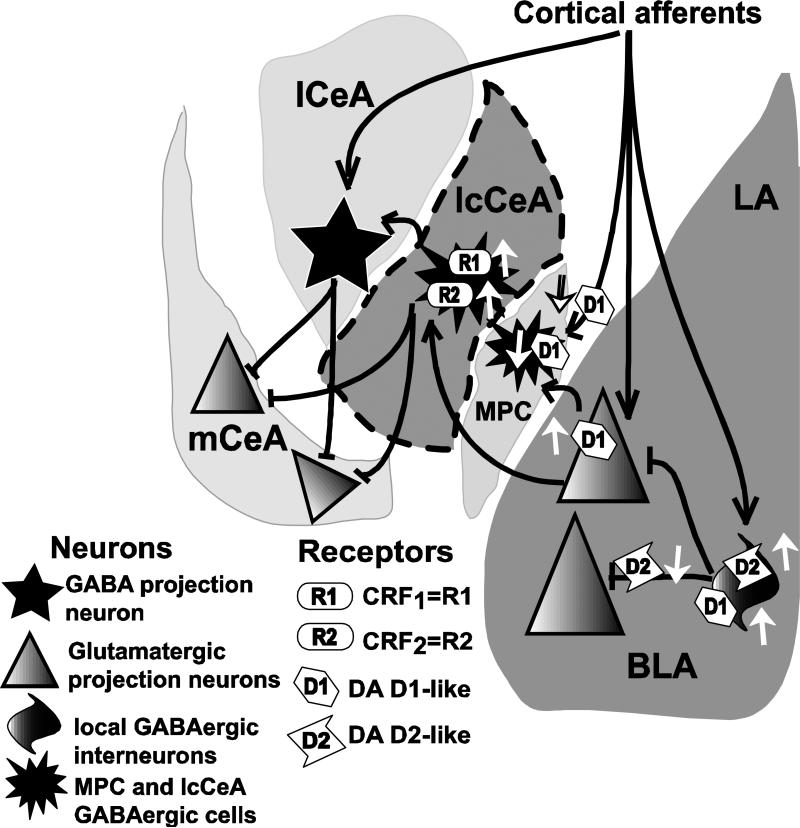
Figure 12.
Schematic diagram illustrating previously published DA actions and the effects of CRF application on fEPSP responses in the lcCeA recorded in the present study (see text). LA=lateral amygdala; BLA=basolateral amygdala; D1, D2=DA receptors; R1, R2=CRF receptors; MPC=medial paracapsula neurons; mCeA=medial central amygdala; lCeA=lateral CeA; lcCeA=lateral capsula CeA. Area within the dotted lines represents the region and the synaptic connections characterized in the present study. Black lines with arrowheads represent excitatory synapses while dark lines with flatheads represent inhibitory inputs. White arrows pointing upwards indicate increased activity of the receptor while those pointing downwards indicate reduction in activity. DA receptors are located on local (feed-forward) GABAergic interneurons (D1/D2, facilitatory), presynaptic receptors (D2, inhibitory), and pyramidal glutamatergic projection neurons (D1 facilitatory), in the BLA and MPC (D1, postsynaptic, inhibitory and D1, presynaptic, inhibitory). CRF1 and CRF2 receptor effects in this study, recorded from inhibitory GABAergic lcCeA neurons are stimulatory. The upregulation of CRF1 and CRF2 receptors in the lcCeA can then act on the GABAergic and non-GABAergic neurons in the lCeA and the mCeA (depicted in separate respective nuclei only for illustrative purposes) leading to downstream stimulation of target neurons involved in reward and emotion. In this model, when GABAergic inhibition is decreased, D2R effects are absent suggesting their primary influence in the enhanced CRF-induced LTP is to presynaptically block inhibitory BLA interneurons (see Bissiere et al., 2003). Cortical afferent input, as observed in other studies, is depicted here to show that there are additional levels of regulation present within the pathway.
Figure 6.
The enhanced CRF-induced LTP recorded in the cocaine-withdrawn group was dependent on the extent of GABAergic inhibition. (A) Concentration-response relationship for PTX in the saline-treated group showed the greatest potentiation in CRF-induced LTP at 10 μM (filled circles). (B) The enhanced CRF-induced LTP in the cocaine-withdrawn group occurred at 10 (filled triangles) and 50 μM PTX (inverted triangles). (C) Comparing CRF-induced LTP with GABAergic intact (no PTX) in slices showed no difference in CRF-induced LTP in the two treatment groups suggesting removal of inhibition was necessary for the enhanced potentiation after cocaine withdrawal. (D) At 50 μM PTX, an enhanced CRF-induced LTP was recorded in the cocaine withdrawal group (inverted triangle). (E) LTP differences between cocaine-withdrawn and saline-treated groups were measured at 10 μM and 50 μM PTX but not with GABAergic inhibition intact (no PTX).
When GABAergic inhibition was left intact (no PTX added, Fig. 6C), enhanced CRF-induced LTP following cocaine withdrawal was not apparent (saline: 113.3 ± 4.17%, n=6; cocaine: 126.3 ± 8.35%, n=5; P>0.05). Removal of GABAergic inhibition with increasing concentrations of GABAA antagonist (10 μM PTX, Fig. 1B; 50 μM PTX, Fig. 6D) unmasked significant differences between the saline and cocaine groups (H=21.73, df=3, ***P<0.0001).
When GABAergic inhibition was removed (50 μM PTX) D1- but not D2-like receptor antagonists blocked the enhanced CRF-induced LTP in the cocaine-withdrawn group (Fig. 7). There was no LTP in the presence of 50 μM PTX in the saline-treated group. The magnitudes of CRF-induced LTP in the presence and absence of the D1 antagonist were not significantly different in 50 μM PTX treated slices in the saline-treated group (saline CRF: 99.46 ± 5.7%, n=7; saline CRF+SCH: 102.0 ± 7.28%, n=3; P>0.05). In contrast, equivalent D1 antagonist blockade was recorded at 10 μM PTX (Fig. 4D) and 50 μM PTX in the cocaine-withdrawn group (cocaine CRF: 169.2 ± 7.8%, n=6; cocaine CRF+SCH: 123.6 ± 5.217%, n=4; **P<0.01). Conversely, the D2 antagonist failed to evoke a significant reduction in CRF-induced LTP in either treatment group in the presence of 50 μM PTX (saline CRF: 99.46 ± 5.7%, n=7; saline CRF+RAC: 109.7 ± 3.19%, n=4; P>0.05, cocaine CRF: 169.2 ± 7.8%, n=6; cocaine CRF+RAC: 152.8 ± 7.88, n=6; P>0.05). Decreasing the feed-forward inhibition removed the dependence of the CRF-induced LTP on the D2-like receptors in the cocaine-withdrawn group. These data suggest that inhibitory D2Rs may be located on GABAergic neurons. Nevertheless, D1R mediated effects in the enhanced CRF-induced LTP are still expressed with significant GABAergic inhibition removed.
Figure 7.
Effect of DA antagonists was influenced by removal of GABAergic inhibition. In the presence of 50 μM PTX, the D1-like antagonist, SCH23390, completely blocked enhanced CRF-induced LTP in the cocaine-withdrawn group (B) but not CRF-induced LTP recorded in saline-treated group (A) in the presence of 50 μM PTX. The D2-like antagonist, raclopride, did not block CRF-induced LTP in saline-treated group (C) or in the cocaine-withdrawn group (D) when recorded in 50 μM PTX as compared to 10 μM PTX (Fig. 4).
CRF1 receptor protein levels are increased in the amygdala after 14 day cocaine withdrawal
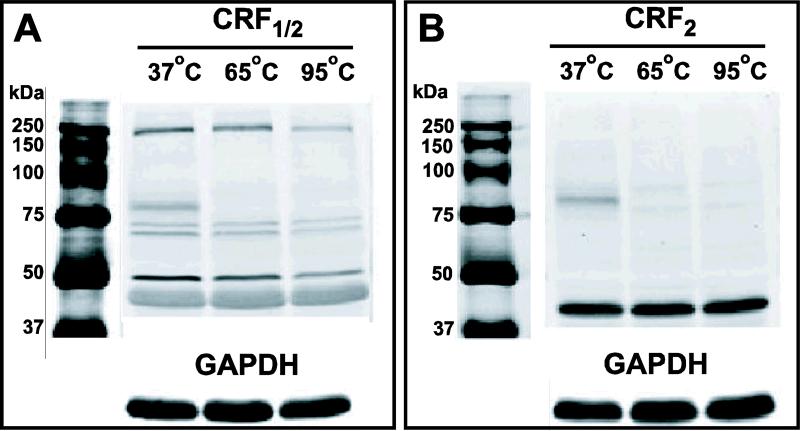
DA receptors are known to exist as dimers (Lee et al., 2004). Since increased temperatures can dissociate DA D2R dimers (Ng et al., 1996), we initially examined the effect of different temperatures on CRF and DA receptor protein expression. Rat heart tissue, which has abundant CRF2 expression (Perrin et al., 1995), was used for characterization. Robust expression of CRF receptor protein was detected at 37°C (Fig. 8). Band intensities decreased with increasing temperature. At lower temperatures, multiple bands at higher than expected molecular weights were also detected suggesting the presence of multimers. Based on these data, we prepared protein samples from amygdala tissue at a denaturation temperature of 37°C in all subsequent experiments to measure protein expression levels of CRF and DA receptors.
Figure 8.
Expression of CRF receptor protein in heart tissue decreased with increased temperature. Protein extracts from the heart tissue of one naïve rat was incubated with antibody at 37°C, 65°C or 95°C. Labeling of CRF1/2 (A) and CRF2 (B) protein showed temperature dependence. Note the presence of tissue-specific CRF2 labeling and higher oligomers of CRF1 at lower temperatures.
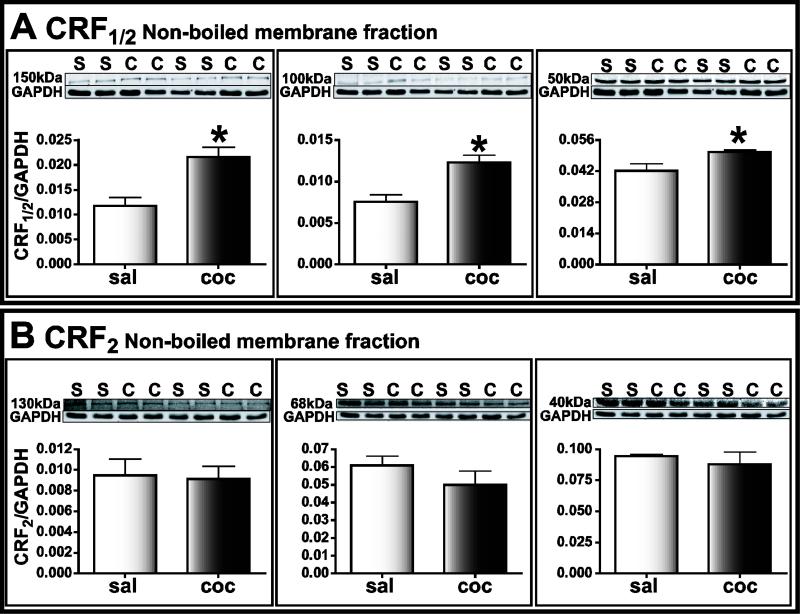
When the membrane fractions of amygdala tissue were compared at lower temperatures, CRF2 labeling detected at multiple molecular weights (130 kDa, 68 kDa, and 40 kDa, n=4) showed no significant difference in saline-treated (0.0095 ± 0.0016, 0.061 ± 0.0054, 0.095 ± 0.0014 respectively) or cocaine-withdrawn group (0.0091 ± 0.0012, 0.049 ± 0.0078, 0.088 ± 0.010 respectively, P>0.05; Fig. 9B). This result is consistent with our previous findings using higher temperatures (Pollandt et al., 2006). In contrast, the intensity of CRF1/2 bands (150 kDa, 100 kDa and 50 kDa) were significantly increased in the cocaine-withdrawn group (H=21.59, df=5, ***P<0.001, Fig. 9A; 150 kDa: saline=0.012 ± 0.002, cocaine=0.022 ± 0.002; 100 kDa: saline=0.0075 ± 0.0008, cocaine=0.012 ± 0.0009; 50 kDa: saline=0.043 ± 0.0032; cocaine=0.051 ± 0.0009, n=4; *P<0.05). CRF1/2 antibody is reported to recognize both CRF1 and CRF2 receptors (Karteris et al., 2005). However, we observed CRF1/2 bands at different molecular weights compared to those using CRF2 antibody This suggests that CRF1/2 antibody predominantly recognized CRF1 in our experimental conditions. Additionally, CRF1 receptor levels were not enhanced 24 hours after the last cocaine injection in either whole amygdala homogenate or membrane fractions (n=4, P>0.05, data not shown).
Figure 9.
CRF1 but not CRF2 receptor protein was increased in amygdala homogenates after cocaine-withdrawal. Each lane represents protein extracted from one animal. Upper panels: black and white images of fluorescent bands detected on immunoblots of samples from cocaine-withdrawn (C) and saline-treated (S) group. Lower panels: integrated intensity of bands expressed as a ratio of CRF receptor to GAPDH labeling. (A) CRF1/2 protein detected at 150 kDa, 100 kDa and 50 kDa was significantly different in intensity in the cocaine-withdrawn (dark bars) group compared to the saline-treated (light bars) group. (B). CRF2 labeled bands at 130 kDa, 68 kDa and 40 kDa in the cocaine-withdrawn group were not significantly different when compared to the same bands in saline-treated group.
Levels of D1R protein are increased in the amygdala after cocaine withdrawal
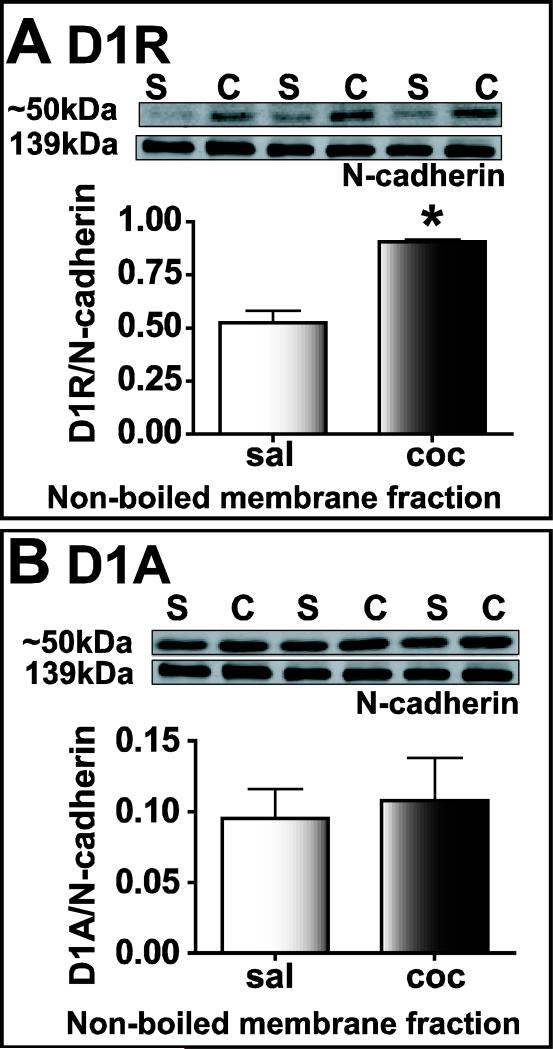
To determine if changes in DA receptor protein contributed to the enhanced CRF-induced LTP observed after cocaine withdrawal, we analyzed D1R protein expression in the membrane fractions of amygdala from saline-treated and cocaine-withdrawn rats (Fig. 10). D1R antibody raised against the N-terminus recognized a band at ~50kDa and this band was significantly increased in samples from cocaine-withdrawn animals (saline: 0.521 ± 0.06, n=3; cocaine: 0.90 ± 0.01, n=3; *P<0.05, Fig. 10A). In contrast, antibody raised against the C-terminal region of the D1 receptor labeled multiple bands (50 kDa, 75 kDa, and 100 kDa) and the protein levels were not different between the two treatment groups (n=4, P>0.05, Fig. 10B). The differences in labeling using the two antibodies was consistent and could be possibly related to the location of the epitopes on the receptor targeted by the antibody. In this regard, the cytoplasmic tail is involved in dimerization to some G-protein coupled receptors (GPCRs) (Bai, 2004; Breitwieser, 2004). Thus, it is possible that steric hindrance prevented binding of the C-terminal directed antibody to D1 receptor homo- or heterodimers (Jiang et al., 2006). D1R levels were not enhanced 24 hours after the last cocaine injection in either whole amygdala homogenate or membrane fractions (n=4, P>0.05, data not shown).
Figure 10.
N-terminal (A) but not C-terminal (B) antibodies detected an increase in D1 receptor protein in the non-boiled membrane fraction of amygdala homogenates. Each lane represents protein extracted from one animal. Upper panels: black and white images of fluorescent bands detected on immunoblots of samples from cocaine-withdrawn (C) and saline-treated (S) group. Lower panels: integrated intensity of bands expressed as a ratio of D1 receptor protein to N-cadherin.
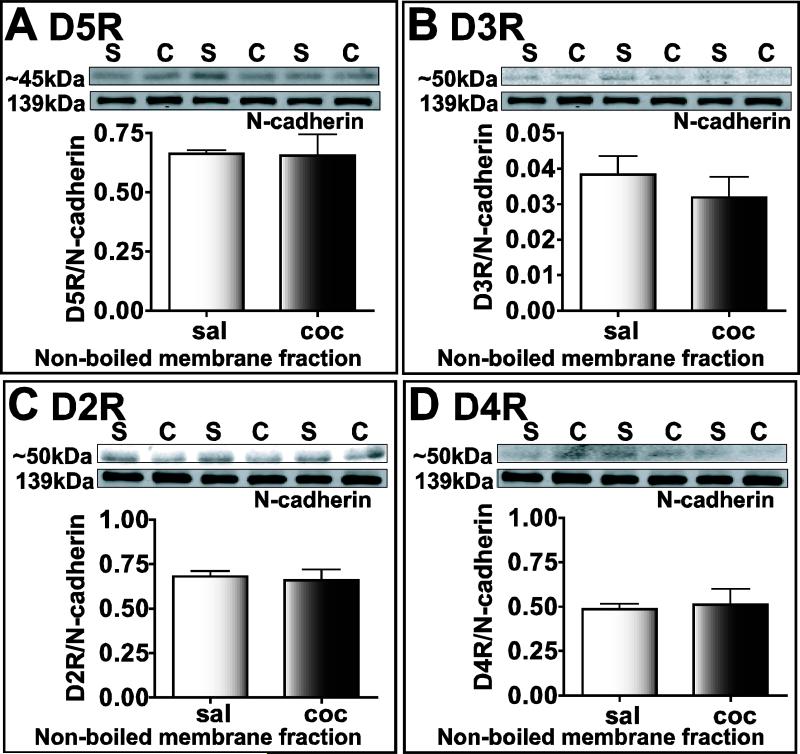
There was no significant difference in the expression of D5R receptor (~45 kDa, Fig. 11A), D3R (50kDa, Fig. 11B), D2R (50kDa, Fig. 11C), and D4R (50kDa, Fig. 11D) in the membrane fraction of saline-treated and cocaine-withdrawn animals (n=3, P>0.05). In a previous study, the D3R band was observed at 49 kDa (Matsukawa et al., 2007) suggesting that the higher bands that we observe under lower temperature conditions may either be homomultimers of D3R or heteromultimers with other DA receptors and/or possibly CRF receptors.
Figure 11.
D2R, D3R, D4R and D5R receptor protein levels do not increase in the cocaine-withdrawn group in the non-boiled membrane fraction of amygdala homogenates. Each lane represents protein extracted from one animal. Bands for cocaine-withdrawn and saline-treated groups, labeled as “C” and “S” respectively are shown on top, while graphs of integrated intensity ratio for D5R (A), D3R (B), D2R (C), and D4R (D) labeled bands to N-cadherin levels are plotted below.
DISCUSSION
The following novel findings were obtained in the present study: CRF-induced LTP in the BLA to lcCeA pathway was 1) enhanced after 14 day withdrawal from repeated cocaine administration and 2) mediated through L-type voltage gated calcium channels (in addition to the previously reported involvement of NMDA receptors). 3) due to activation of CRF1 receptors in the saline-treated group. In addition, enhanced CRF-induced LTP in the cocaine-withdrawn group involves 1) activation of both CRF1 and CRF2 receptors, 2) endogenously activated D1-like and D2-like receptors and 3) removal of GABAergic inhibition. The expression of D1R and CRF1 receptor protein were also found to be significantly increased in the cocaine-withdrawn group.
CRF-induced LTP in the BLA-lcCeA pathway is enhanced after 14 days, but not after 24 hours withdrawal to repeated cocaine administration
CRF-containing cell bodies are found primarily in the lateral CeA (lCeA), but with stress, CRF spills over into the lcCeA (Fenoglio et al., 2004). This suggests that the higher concentration of CRF (25 nM) used in our study [and also in the range detected in the amygdala during ethanol withdrawal (Merlo Pich et al., 1995; Richter et al., 1995; Richter and Weiss, 1999)] was within physiologically relevant levels.
The enhanced CRF-induced LTP in the BLA-lcCeA pathway was dependent on withdrawal time yet no CRF-induced LTP was recorded 24 hours after the last injection in either treatment group. Similarly, CRF1 and D1 receptor levels were not significantly increased after 24 hours, while increased levels were observed in the 14 day cocaine-withdrawn group. Expression levels of CRF mRNA in the CeA increase with age (Avishai-Eliner et al., 2001) but the levels of CRF1 receptors peak at 9 days and then decrease to reach adult levels by day 12 in the rat (Baram et al., 1997). These changes occur well before the age groups used in the present study (29–39 days for 24 h and 42–52 days for two week withdrawal groups). Thus, the age of the animals cannot account for the LTP differences observed. On the other hand, LTP recorded in the CA1 region and dentate gyrus is reduced in rats sacrificed 24 hours after a 21 day exposure to unpredictable stress (Alfarez et al., 2002). It is likely that repeated stress caused by the daily injections of saline or cocaine may have prevented LTP in the amygdala after 24 hours.
The enhanced CRF-induced LTP is dependent on CRF1 and CRF2 after cocaine withdrawal but with only CRF1 protein expression altered
CRF1 but not CRF2 protein expression was increased in the amygdala after cocaine withdrawal (Pollandt et al., 2006) even when using lower temperature conditions to increase oligomer detection (present study). Different molecular weights for CRF1 and CRF2 have been reported depending on the tissue, antibody source and epitope (Chatzaki et al., 2004; Karteris et al., 2005; Gounko et al., 2006; Gao et al., 2008; Markovic et al., 2008). Under the low temperature conditions used in this study, it is possible to detect dimers or multimers of homomeric or heteromeric receptors (Ng et al., 1996). CRF1 is known to form homodimers (Kraetke et al., 2005) and heterodimers (Young et al., 2007). Thus, it is possible that CRF2 receptors in the amygdala may associate with CRF1 to form heterodimers during cocaine withdrawal. This may account for its role in enhanced CRF-induced LTP despite no increase in CRF2 receptor levels.
Alternatively, changes in relative contributions of signaling molecules associated with CRF2 receptor pathway in the amygdala could result in the observed LTP effects. These changes may be similar to the self-regulatory mechanism of protein kinase A (PKA) induced CRF1 phosphorylation which attenuate kinase activity in intracellular pathways without affecting receptor levels (Papadopoulou et al., 2004).
Enhanced HFS- and CRF-induced LTP in the BLA-lcCeA pathway observed after cocaine withdrawal may result from different receptor associations, synapses and/or signaling mechanisms
CRF1 is largely responsible for changes in DA release induced by acute cocaine in the nucleus accumbens (NAcc), ventral tegmental area (VTA) (Lu et al., 2003) and also controls stressor-induced release of DA in VTA of cocaine treated animals (Wang et al., 2005). Similarly in the present study, CRF1 activation may cause DA release or interact with the increased endogenous DA to generate the enhanced CRF-induced LTP after cocaine withdrawal.
Receptor heterodimerization may play a role in unusual pharmacological responses where either a D1- or D2-like antagonist completely blocks cocaine sensitization-dependent synaptic plasticity (Goto and Grace, 2005). This phenomenon has been observed in the hippocampus (Goto and Grace, 2005), NAcc and prefrontal cortex (Rios et al., 2001). Heterodimerization between D1-like and D2-like receptors (O'Dowd et al., 2005; Rashid et al., 2007), specifically D5R and D2R dimers (Centonze et al., 2003) have been reported. In some cases, activation or inhibition of one receptor of a heterodimer can act as a dominant negative for the other (Bai, 2004). Thus, a complete blockade of the enhanced CRF-induced LTP observed by individual application of the D1- and D2-like antagonists in the present study could be explained by formation of D1R/D2R heterodimers which are then responsive to the endogenous DA levels generated by CRF1 activation.
Alternatively, CRF/DA receptor heterodimer formation in cocaine-withdrawn animals could contribute to the enhanced CRF-induced LTP. Formation of DA/CRF receptor heterodimers in the medial prefrontal cortex (mPFC) is proposed to explain the DA and CRF dependent enhanced synaptic transmission after cocaine withdrawal. In this study, exogenously applied DA is inhibitory while CRF is ineffective at glutamatergic synapses in saline-treated animals (Orozco-Cabal et al., 2008).
Enhanced D1-like mediated HFS-LTP observed in the cocaine-withdrawn group could be attributed to increased D1R levels alone, similar to the activation of D1 receptors known to mediate a phase of HFS-LTP in the spinal cord (Yang et al., 2004) and hippocampus (Frey et al., 1993; Huang and Kandel, 1994; Huang and Kandel, 1995).
The enhanced CRF-induced LTP after cocaine withdrawal may depend on GABAergic tone
The requirement of PTX to overcome GABAergic inhibition and unmask the enhanced CRF-induced LTP attests to the strong inhibitory tone within the slice preparation. Interestingly, a lesser blockade of the inhibition using 10 μM PTX resulted in a more robust CRF-induced LTP than at 50 μM PTX. This suggests that, similar to guinea pig intercalated neurons (Royer et al., 1999), the relative influence of sequential GABAergic inhibitory synapses [local interneurons, inhibitory medial paracapsular neurons (MPC) cells] to excitatory glutamatergic inputs in the BLA-lcCeA pathway may be subject to scaling of synaptic inhibition (Fig.12).
Stimulatory D1Rs on projection neurons and local interneurons in the BLA (Rosenkranz and Grace, 1999) suggest that output is dependent on the scaling of GABAergic inhibition. D1R activation hyperpolarizes MPC (also termed intercalated cells: ITC) interposed between BLA and lcCeA nuclei resulting in disinhibition of these amygdalar regions (Marowsky et al., 2005). Thus, a consequence of D1R activation is a shift in inhibitory and excitatory balance towards excitation in the BLA and CeA (Pape, 2005).
D2 receptors control synaptic plasticity in the LA by suppressing feed forward GABA inhibition (Bissiere et al., 2003). If a similar disinhibitory effect is present in the BLA, an excitatory action in the BLA-lcCeA pathway would result (Fig. 12). D2Rs also weakly stimulate local BLA interneurons (Kroner et al., 2005). D2R effects were absent after blocking GABAergic inhibition in the present study suggesting their primary influence on the enhanced CRF-induced LTP after cocaine withdrawal was blocking inhibitory interneurons. Thus, CRF-induced release of DA activates D1Rs that stimulate excitatory projections from the BLA, block inhibitory interneurons, and strongly suppress feed-forward inhibition in the BLA resulting in an enhanced LTP after cocaine withdrawal.
Functional relationships between CRF, DA and GABA provide a basis for DA modulation of the enhanced CRF-induced LTP observed after cocaine withdrawal
CRF1 induces LTP in the BLA-lcCeA (present study) while CRF2 induces LTP in the LA-lcCeA pathway (Pollandt et al., 2006). Postsynaptic CRF1 inhibits excitatory postsynaptic currents while CRF2 facilitates synaptic transmission both pre- and postsynaptically in the BLA-lCeA pathway (Liu et al., 2004). Other studies show that CRF can enhance GABAergic potentials when evoked through local stimulation within the CeA (Nie et al., 2004). Thus, endogenous transmitters in the amygdala may exert their effects at different receptor locations depending on the synaptic pathway involved.
BLA stimulation results in inhibition of medial CeA (mCeA) (Royer et al., 1999; Rosenkranz et al., 2006), which is the primary CeA output subnucleus involved in autonomic arousal and emotional states. Inputs from BLA synapse on MPCs and inhibit mCeA neurons (Royer et al., 1999) and lcCeA neurons (Savander et al., 1995) (Fig.12) which are GABAergic (Cassell et al., 1999). BLA inputs also synapse with mCeA neurons and lCeA neurons (Jolkkonen and Pitkanen, 1998). Thus, the functional outcome of enhanced CRF-induced LTP after cocaine withdrawal would be stimulation of GABAergic lcCeA neurons to inhibit directly the output of non-GABAergic mCeA neurons. Alternatively, persistent stimulation of inhibitory lcCeA GABAergic neurons could inhibit GABAergic lCeA neurons (Sun et al., 1994) and would result in the disinhibition of non-GABAergic mCeA projection neurons (McDonald, 1982) along with downstream stimulation of target neurons involved in reward and emotion.
Thus, enhanced CRF-induced LTP after cocaine withdrawal mediated through D1- and D2-like receptors shows that a neurotransmitter system involved in the neuroendocrine and behavioral mechanisms underlying the stress response (Bale and Vale, 2004) converge with those mediating reward pathways through activation of endogenous DA receptors. Study of such interactions will be crucial in developing new insights towards pharmacological intervention associated with relapse to cocaine.
ACKNOWLEDGEMENTS
This work was supported by National Institute of Health Grant DA017727 to P.S.G and by NRSA F32 Ruth L. Kirschstein postdoctoral grant DA023316 to B.K. We thank Marcy B. Jordan, Jose A. Morón, Sophie Grimond-Billa and Nicole Bjorklund for their suggestions and critical scientific reading of the manuscript. We thank Joanna Bremer, Marcy B. Jordan and Nicole Bjorklund for their critical reading of English usage in this manuscript.
ABBREVIATIONS
- ACSF
artificial cerebrospinal fluid
- BLA
basolateral amygdala
- CeA
central amygdala
- lCeA
lateral region of the central amygdala
- lcCeA
lateral capsula central amygdala
- CRF
corticotropin releasing factor
- CRF1
CRF 1 receptor
- CRF2
CRF 2 receptor
- DA
dopamine
- D1R
dopamine 1 receptor
- D2R
dopamine 2 receptor
- D3R
dopamine 3 receptor
- D4R
dopamine 4 receptor
- D5R
dopamine 5 receptor
- fEPSP
field excitatory post synaptic potential
- GABA
gamma amino butyric acid
- GPCR
G-protein coupled receptors
- HFS
high frequency stimulation
- LA
lateral amygdala
- LTP
long-term potentiation
- L-VGCCs
L-type voltage gated calcium channels
- mCeA
medial region of the central amygdala
- NMDA
N-methyl-D-aspartate
- VTA
ventral tegmental area
REFERENCES
- Alfarez DN, Joels M, Krugers HJ. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur.J.Neurosci. 2003;17:1928–1934. doi: 10.1046/j.1460-9568.2003.02622.x. [DOI] [PubMed] [Google Scholar]
- Alfarez DN, Wiegert O, Joels M, Krugers HJ. Corticosterone and stress reduce synaptic potentiation in mouse hippocampal slices with mild stimulation. Neuroscience. 2002;115:1119–1126. doi: 10.1016/s0306-4522(02)00483-9. [DOI] [PubMed] [Google Scholar]
- Asan E. The catecholaminergic innervation of the rat amygdala. Adv.Anat.Embryol.Cell Biol. 1998;142:1–118. doi: 10.1007/978-3-642-72085-7. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Eghbal-Ahmadi M, Tabachnik E, Brunson KL, Baram TZ. Down-regulation of hypothalamic corticotropin-releasing hormone messenger ribonucleic acid (mRNA) precedes early-life experience-induced changes in hippocampal glucocorticoid receptor mRNA. Endocrinology. 2001;142:89–97. doi: 10.1210/endo.142.1.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai M. Dimerization of G-protein-coupled receptors: roles in signal transduction. Cell Signal. 2004;16:175–186. doi: 10.1016/s0898-6568(03)00128-1. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF Receptors: Role in Stress Responsivity and Other Behaviors. Annu.Rev.Pharmacol.Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Baram TZ, Yi S, Avishai-Eliner S, Schultz L. Development neurobiology of the stress response: multilevel regulation of corticotropin-releasing hormone function. Ann.N.Y.Acad.Sci. 1997;814:252–265. doi: 10.1111/j.1749-6632.1997.tb46161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissiere S, Humeau Y, Luthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat.Neurosci. 2003;6:587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- Breitwieser GE. G protein-coupled receptor oligomerization: implications for G protein activation and cell signaling. Circ.Res. 2004;94:17–27. doi: 10.1161/01.RES.0000110420.68526.19. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann.N.Y.Acad.Sci. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- Centonze D, Grande C, Usiello A, Gubellini P, Erbs E, Martin AB, Pisani A, Tognazzi N, Bernardi G, Moratalla R, Borrelli E, Calabresi P. Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. Journal of Neuroscience. 2003;23:6245–6254. doi: 10.1523/JNEUROSCI.23-15-06245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzaki E, Murphy BJ, Wang LX, Million M, Ohning GV, Crowe PD, Petroski R, Tache Y, Grigoriadis DE. Differential profile of CRF receptor distribution in the rat stomach and duodenum assessed by newly developed CRF receptor antibodies. Journal of Neurochemistry. 2004;88:1–11. doi: 10.1046/j.1471-4159.2003.02078.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Brunson KL, Muller MB, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF(1))-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. J.Comp Neurol. 2000;420:305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza EB. Corticotropin-releasing factor receptors in the rat central nervous system: characterization and regional distribution. J.Neurosci. 1987;7:88–100. doi: 10.1523/JNEUROSCI.07-01-00088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza EB, Insel TR, Perrin MH, Rivier J, Vale WW, Kuhar MJ. Corticotropin-releasing factor receptors are widely distributed within the rat central nervous system: an autoradiographic study. J.Neurosci. 1985;5:3189–3203. doi: 10.1523/JNEUROSCI.05-12-03189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliava M, Yilmazer-Hanke D, Asan E. Interrelations between monoaminergic afferents and corticotropin-releasing factor-immunoreactive neurons in the rat central amygdaloid nucleus: ultrastructural evidence for dopaminergic control of amygdaloid stress systems. Histochem.Cell Biol. 2003;120:183–197. doi: 10.1007/s00418-003-0557-9. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J.Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Avishai-Eliner S, Chen Y, Baram TZ. Region-specific onset of handling-induced changes in corticotropin-releasing factor and glucocorticoid receptor expression. Endocrinology. 2004;145:2702–2706. doi: 10.1210/en.2004-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Fu Y, Shinnick-Gallagher P. Two intra-amygdaloid pathways to the central amygdala exhibit different mechanisms of long-term potentiation. J.Neurophysiol. 2005;93:3012–3015. doi: 10.1152/jn.00871.2004. [DOI] [PubMed] [Google Scholar]
- Gao L, Lu CM, Xu C, Tao Y, Cong BH, Ni X. Differential regulation of prostaglandin production mediated by corticotropin-releasing hormone receptor type 1 and type 2 in cultured human placental Trophoblasts. Endocrinology. 2008;149:2866–2876. doi: 10.1210/en.2007-1377. [DOI] [PubMed] [Google Scholar]
- Goeders NE. Stress and cocaine addiction. Journal of Pharmacology and Experimental Therapeutics. 2002;301:785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Effects of the CRH receptor antagonist CP-154,526 on intravenous cocaine self-administration in rats. Neuropsychopharmacology. 2000;23:577–586. doi: 10.1016/S0893-133X(00)00148-2. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron. 2005;47:255–266. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Gounko NV, Kalicharan D, Rybakin V, Gramsbergen A, Van der Want JJL. The dynamic developmental localization of the full-length corticotropin-releasing factor receptor type 2 in rat cerebellum. European Journal of Neuroscience. 2006;23:3217–3224. doi: 10.1111/j.1460-9568.2006.04869.x. [DOI] [PubMed] [Google Scholar]
- Gray TS, Bingaman EW. The amygdala: corticotropin-releasing factor, steroids, and stress. Crit Rev.Neurobiol. 1996;10:155–168. doi: 10.1615/critrevneurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn.Mem. 1994;1:74–82. [PubMed] [Google Scholar]
- Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc.Natl.Acad.Sci.U.S.A. 1995;92:2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Betancourt L, Smith RG. Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Mol.Endocrinol. 2006;20:1772–1785. doi: 10.1210/me.2005-0084. [DOI] [PubMed] [Google Scholar]
- Jolkkonen E, Pitkanen A. Intrinsic connections of the rat amygdaloid complex: projections originating in the central nucleus. J Comp Neurol. 1998;395:53–72. doi: 10.1002/(sici)1096-9861(19980525)395:1<53::aid-cne5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Alesdatter JE. Involvement of N-methyl-D-aspartate receptor stimulation in the ventral tegmental area and amygdala in behavioral sensitization to cocaine. J.Pharmacol.Exp.Ther. 1993;267:486–495. [PubMed] [Google Scholar]
- Karteris E, Vatish M, Hillhouse EW, Grammatopoulos DK. Preeclampsia is associated with impaired regulation of the placental nitric oxide-cyclic guanosine monophosphate pathway by corticotropin-releasing hormone (CRH) and CRH-related peptides. Journal of Clinical Endocrinology and Metabolism. 2005;90:3680–3687. doi: 10.1210/jc.2004-2210. [DOI] [PubMed] [Google Scholar]
- Kraetke O, Wiesner B, Eichhorst J, Furkert J, Bienert M, Beyermann M. Dimerization of corticotropin-releasing factor receptor type 1 is not coupled to ligand binding. J.Recept.Signal.Transduct.Res. 2005;25:251–276. doi: 10.1080/10799890500468838. [DOI] [PubMed] [Google Scholar]
- Kroner S, Rosenkranz JA, Grace AA, Barrionuevo G. Dopamine modulates excitability of basolateral amygdala neurons in vitro. J.Neurophysiol. 2005;93:1598–1610. doi: 10.1152/jn.00843.2004. [DOI] [PubMed] [Google Scholar]
- Lee KH, Kim MY, Kim DH, Lee YS. Syntaxin 1A and receptor for activated C kinase interact with the N-terminal region of human dopamine transporter. Neurochem.Res. 2004;29:1405–1409. doi: 10.1023/b:nere.0000026404.08779.43. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lin CH, Lu KT, Leu TH, Chang WC, Gean PW. A role for the PI-3 kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdala. Neuron. 2001;31:841–851. doi: 10.1016/s0896-6273(01)00433-0. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A, Skagerberg G. Selective histochemical demonstration of dopamine terminal systems in rat di- and telencephalon: new evidence for dopaminergic innervation of hypothalamic neurosecretory nuclei. Brain Res. 1984;306:19–30. doi: 10.1016/0006-8993(84)90352-4. [DOI] [PubMed] [Google Scholar]
- Liu J, Yu B, Neugebauer V, Grigoriadis DE, Rivier J, Vale WW, Shinnick-Gallagher P, Gallagher JP. Corticotropin-releasing factor and Urocortin I modulate excitatory glutamatergic synaptic transmission. J.Neurosci. 2004;24:4020–4029. doi: 10.1523/JNEUROSCI.5531-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin SE, Fallon JH. Dopaminergic and non-dopaminergic projections to amygdala from substantia nigra and ventral tegmental area. Brain Res. 1983;262:334–338. doi: 10.1016/0006-8993(83)91029-6. [DOI] [PubMed] [Google Scholar]
- Lu L, Liu Z, Huang M, Zhang Z. Dopamine-dependent responses to cocaine depend on corticotropin-releasing factor receptor subtypes. J.Neurochem. 2003;84:1378–1386. doi: 10.1046/j.1471-4159.2003.01635.x. [DOI] [PubMed] [Google Scholar]
- Markovic D, Punn A, Lehnert H, Grammatopoulos DK. Intracellular mechanisms regulating corticotropin-releasing hormone receptor-2 beta endocytosis and interaction with extracellularly regulated kinase 1/2 and p38 mitogen-activated protein kinase signaling cascades. Molecular Endocrinology. 2008;22:689–706. doi: 10.1210/me.2007-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Matsukawa N, Maki M, Yasuhara T, Hara K, Yu G, Xu L, Kim KM, Morgan JC, Sethi KD, Borlongan CV. Overexpression of D2/D3 receptors increases efficacy of ropinirole in chronically 6-OHDA-lesioned Parkinsonian rats. Brain Res. 2007;1160:113–123. doi: 10.1016/j.brainres.2007.05.030. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cytoarchitecture of the central amygdaloid nucleus of the rat. J.Comp Neurol. 1982;208:401–418. doi: 10.1002/cne.902080409. [DOI] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J.Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez d.F., Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J.Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng GY, O'Dowd BF, Lee SP, Chung HT, Brann MR, Seeman P, George SR. Dopamine D2 receptor dimers and receptor-blocking peptides. Biochem.Biophys.Res.Commun. 1996;227:200–204. doi: 10.1006/bbrc.1996.1489. [DOI] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- O'Dowd BF, Ji X, Alijaniaram M, Rajaram RD, Kong MM, Rashid A, Nguyen T, George SR. Dopamine receptor oligomerization visualized in living cells. J.Biol.Chem. 2005;280:37225–37235. doi: 10.1074/jbc.M504562200. [DOI] [PubMed] [Google Scholar]
- Orozco-Cabal L, Liu J, Pollandt S, Schmidt K, Shinnick-Gallagher P, Gallagher JP. Dopamine and corticotropin-releasing factor synergistically alter basolateral amygdala-to-medial prefrontal cortex synaptic transmission: functional switch after chronic cocaine administration. J.Neurosci. 2008;28:529–542. doi: 10.1523/JNEUROSCI.2666-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou N, Chen J, Randeva HS, Levine MA, Hillhouse EW, Grammatopoulos DK. Protein kinase A-induced negative regulation of the corticotropin-releasing hormone R1alpha receptor-extracellularly regulated kinase signal transduction pathway: the critical role of Ser301 for signaling switch and selectivity. Mol.Endocrinol. 2004;18:624–639. doi: 10.1210/me.2003-0365. [DOI] [PubMed] [Google Scholar]
- Pape HC. GABAergic neurons: gate masters of the amygdala, mastered by dopamine. Neuron. 2005;48:877–879. doi: 10.1016/j.neuron.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Perrin M, Donaldson C, Chen R, Blount A, Berggren T, Bilezikjian L, Sawchenko P, Vale W. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc.Natl.Acad.Sci.U.S.A. 1995;92:2969–2973. doi: 10.1073/pnas.92.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Quick EA, Reeder DC, Morgan ZR, Kalivas PW. Calcium-mediated second messengers modulate the expression of behavioral sensitization to cocaine. J.Pharmacol.Exp.Ther. 1998;286:1171–1176. [PubMed] [Google Scholar]
- Pollandt S, Liu J, Orozco-Cabal L, Grigoriadis DE, Vale WW, Gallagher JP, Shinnick-Gallfagher P. Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur.J.Neurosci. 2006;24:1733–1743. doi: 10.1111/j.1460-9568.2006.05049.x. [DOI] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, El Ghundi M, Cheng R, O'Dowd BF, George SR. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc.Natl.Acad.Sci.U.S.A. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter RM, Pich EM, Koob GF, Weiss F. Sensitization of cocaine-stimulated increase in extracellular levels of corticotropin-releasing factor from the rat amygdala after repeated administration as determined by intracranial microdialysis. Neurosci.Lett. 1995;187:169–172. doi: 10.1016/0304-3940(95)11365-4. [DOI] [PubMed] [Google Scholar]
- Richter RM, Weiss F. In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse. 1999;32:254–261. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Rios CD, Jordan BA, Gomes I, Devi LA. G-protein-coupled receptor dimerization: modulation of receptor function. Pharmacol.Ther. 2001;92:71–87. doi: 10.1016/s0163-7258(01)00160-7. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Buffalari DM, Grace AA. Opposing influence of basolateral amygdala and footshock stimulation on neurons of the central amygdala. Biol.Psychiatry. 2006;59:801–811. doi: 10.1016/j.biopsych.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Modulation of basolateral amygdala neuronal firing and afferent drive by dopamine receptor activation in vivo. J.Neurosci. 1999;19:11027–11039. doi: 10.1523/JNEUROSCI.19-24-11027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Martina M, Pare D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J.Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z, Hohn J, Szabo G, Penke B. Critical role of endogenous corticotropin-releasing factor (CRF) in the mediation of the behavioral action of cocaine in rats. Life Sci. 1992;51:2019–2024. doi: 10.1016/0024-3205(92)90151-e. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol.Rev. 2001;53:209–243. [PubMed] [Google Scholar]
- Savander V, Go CG, LeDoux JE, Pitkanen A. Intrinsic connections of the rat amygdaloid complex: projections originating in the basal nucleus. J.Comp Neurol. 1995;361:345–368. doi: 10.1002/cne.903610211. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol.Biochem.Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- See RE, Fuchs RA, Ledford CC, McLaughlin J. Drug addiction, relapse, and the amygdala. Ann.N.Y.Acad.Sci. 2003;985:294–307. doi: 10.1111/j.1749-6632.2003.tb07089.x. [DOI] [PubMed] [Google Scholar]
- See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 2001;154:301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Allen PB, Fienberg AA, Valle CG, Huganir RL, Nairn AC, Greengard P. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J.Neurosci. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Yi A, Cassell M. Evidence for a GABAergic interface between cortical afferents and brainstem projection neurons in the rat central extended amygdala. J.Comp.Neurol. 1994;340:43–64. doi: 10.1002/cne.903400105. [DOI] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J.Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HW, Hu XD, Zhang HM, Xin WJ, Li MT, Zhang T, Zhou LJ, Liu XG. Roles of CaMKII, PKA, and PKC in the induction and maintenance of LTP of C-fiber-evoked field potentials in rat spinal dorsal horn. J.Neurophysiol. 2004;91:1122–1133. doi: 10.1152/jn.00735.2003. [DOI] [PubMed] [Google Scholar]
- Young SF, Griffante C, Aguilera G. Dimerization between vasopressin V1b and corticotropin releasing hormone type 1 receptors. Cell Mol.Neurobiol. 2007;27:439–461. doi: 10.1007/s10571-006-9135-8. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Schlussman SD, Ho A, Kreek MJ. Alterations in hypothalamic-pituitary-adrenal axis activity and in levels of proopiomelanocortin and corticotropin-releasing hormone-receptor 1 mRNAs in the pituitary and hypothalamus of the rat during chronic ‘binge’ cocaine and withdrawal. Brain Res. 2003;964:187–199. doi: 10.1016/s0006-8993(02)03929-x. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]