Abstract
Here we unravel the structural features of human IgM and IgA that govern their interaction with the human Fcα/μ receptor (hFcα/μR). Ligand polymerization status was crucial for the interaction, because hFcα/μR binding did not occur with monomeric Ab of either class. hFcα/μR bound IgM with an affinity in the nanomolar range, whereas the affinity for dimeric IgA (dIgA) was tenfold lower. Panels of mutant IgM and dIgA were used to identify regions critical for hFcα/μR binding. IgM binding required contributions from both Cμ3 and Cμ4 Fc domains, whereas for dIgA, an exposed loop in the Cα3 domain was crucial. This loop, comprising residues Pro440–Phe443, lies at the Fc domain interface and has been implicated in the binding of host receptors FcαRI and polymeric Ig receptor (pIgR), as well as IgA-binding proteins produced by certain pathogenic bacteria. Substitutions within the Pro440–Phe443 loop resulted in loss of hFcα/μR binding. Furthermore, secretory component (SC, the extracellular portion of pIgR) and bacterial IgA-binding proteins were shown to inhibit the dIgA–hFcα/μR interaction. Therefore, we have identified a motif in the IgA–Fc inter-domain region critical for hFcα/μR interaction, and highlighted the multi-functional nature of a key site for protein–protein interaction at the IgA Fc domain interface.
Keywords: Human Fcα/μ receptor, IgA, IgM
Introduction
A receptor for IgM and IgA, termed Fcα/μ receptor (Fcα/μR), was first described in the mouse where it is constitutively expressed on the majority of B cells and macrophages [1]. Mouse Fcα/μR (mFcα/μR) can mediate endocytosis of IgM-coated targets, and may contribute to the primary stages of the immune response to microbes. It has been suggested that mFcα/μR, due to its high affinity for IgM and intermediate affinity for IgA, may assist also in the maintenance of plasma concentrations of IgM and IgA [2]. Although there is no significant homology between Fcα/μR and other known proteins, the N-terminal Ig-like domain of Fcα/μR shares common sequence features with domain 1 (D1) of the polymeric Ig receptor (pIgR), the receptor responsible for transport of pentameric IgM and dimeric IgA (dIgA) into mucosal secretions [1]. In particular, the receptors carry a related motif, which in pIgR appears critical for interaction with IgM and dIgA [3–5].
The human form of Fcα/μR (hFcα/μR) shares 49% amino acid identity with mFcα/μR [1]. However, the human and mouse receptors have very different expression profiles. hFcα/μR is absent from circulating B cells and macrophages and instead is expressed on a subpopulation of pre-germinal B cells (IgD+/CD38+) and on follicular DC (FDC) in tonsillar tissues [2]. Thus in humans, the receptor may play a role in trapping IgM or IgA immune complexes and in presentation of intact antigens to B cells in germinal centers, a process known to occur for IgG immune complexes through binding to the IgG-specific receptor FcγRII (CD32) on FDC. hFcα/μR may be particularly important in the development of lymphoid follicles in secondary lymphoid tissues during early immune responses, when IgM is the predominant Ig subclass. In contrast to its murine counterpart, hFcα/μR exhibits unusual biochemical features, including the formation of stable homodimers in the cell membrane that are resistant to SDS and 2-mercaptoethanol [1, 2].
Certain mAb raised against mFcα/μR have been found to bind to a peptide encompassing the region from mFcα/μR’s Ig-like domain that corresponds to the Ig-binding motif in domain 1 of pIgR [6]. Interestingly, these mAb can block binding of IgA and IgM to mFcα/μR. The fact that these same mAb cross-react with hFcα/μR, which carries the conserved motif in its Ig-like domain, suggests that the site for IgM and IgA on hFcα/μR lies in this same region. In contrast, the site(s) on human IgM and IgA that interacts with hFcα/μR is unknown. To gain a better understanding of the structural requirements for the interaction of human IgA and IgM with hFcα/μR, we have produced recombinant dIgA and pentameric IgM and have adopted a mutational approach to identify individual residues on the Fc involved in interaction with hFcα/μR. We demonstrate that both the Cμ3 and Cμ4 domains of IgM are required for binding to the receptor, and that residues at the Fc inter-domain region of dIgA are critical for hFcα/μR interaction. Our studies reveal that hFcα/μR interacts with the same Fc regions that form interaction sites for other host receptors as well as for Ig-binding proteins produced by certain important human pathogens.
Results
hFcα/μR expressed following transfection into COS-7 cells binds human IgM
To determine whether hFcα/μR is indeed a receptor for IgM and IgA like its murine counterpart, the full-length receptor was cloned into the pSecTag2/hygro expression vector and expressed in COS-7 cells. Expression of the construct was determined by immunofluorescent assay (IFA) with mAb 9E10 specific for the myc tag expressed C-terminally to the receptor. Although much of the hFcα/μR remains intracellular and appears localized into certain subcompartments, surface expression was evident (Fig. 1). Significant IgM binding was detected by IFA using a goat anti-mouse λ L chain conjugated to PE either with unfixed cells (data not shown) or with fixed and permabilized cells (Fig. 1).
Figure 1.
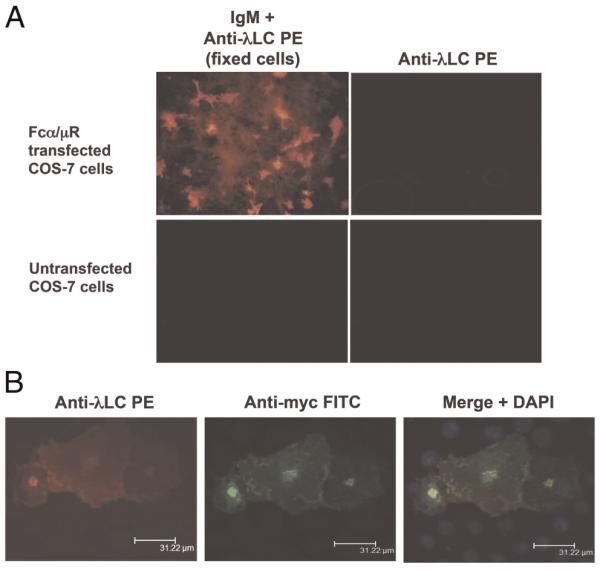
(A) Binding of anti-NIP IgM to hFcα/μR-transfected COS-7 cells assessed by IFA. IgM binding was detected using an anti-λ L chain-PE conjugate. No binding of IgM was seen to untransfected COS-7 cells, and no binding of the anti-λ L chain detecting Ab to Fcα/μR-positive transfectants was seen in the absence of IgM. (B) Bound IgM co-localizes with hFcα/μR on COS-7 transfectants. hFcα/μR was detected by mouse anti-myc Ab followed by an anti-mouse IgG-FITC conjugate (green), and DAPI staining (blue) revealed cell nuclei. Transfected cells were fixed and permeabilized prior to staining with Ab.
The Cμ3 and Cμ4 domains of IgM contribute to hFcα/μR binding
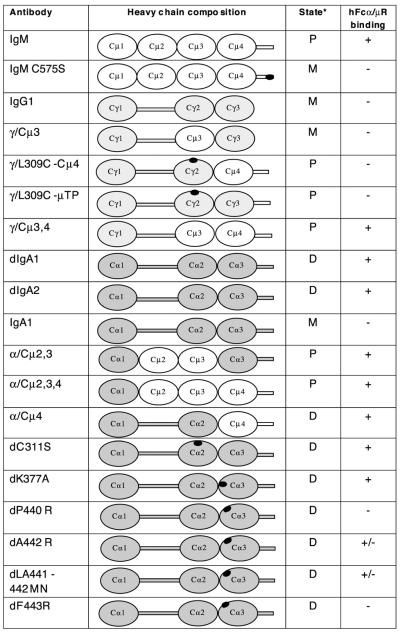
To determine the region of the IgM molecule critical for interaction with hFcα/μR, we used a panel of domain-swapped Ab described in a previous study [7], in which homologous domains are exchanged between IgG and IgM. These 3-nitro-4-hydroxy-5-iodophenylacetyl (NIP)-specific domain-swapped Ab consist of mouse λ L chain and a heavy chain comprising a mouse variable domain linked to human heavy chain constant regions. The domain-swapped Ab are designated as in Fig. 2. They are composed of mixtures of monomeric and polymeric forms. Ab with the L309C mutation exist predominantly in higher polymeric forms, including pentamers and hexamers [7–10]. The ability of the transfected hFcα/μR to bind the domain-swapped Ab was analyzed in IFA experiments (Figs. 3 and 6). We observed that those Ab that contained both Cμ3 and Cμ4 domains were able to interact with hFcα/μR. In contrast, no binding was observed with human IgG or IgM/IgG domain-swaps lacking either the Cμ3 or the Cμ4 domains. As all these domain-swapped Ab share identical Fab regions, we can also deduce that the Fc region mediates all of the binding to Fcα/μR, a result confirmed by the observation that human IgM Fab failed to bind (data not shown).
Figure 2.
The nomenclature, heavy chain composition, polymerization state, and hFcα/μR-binding ability of the anti-NIP Ab used in this study. The domain arrangements are shown diagrammatically with heavy chain regions derived from human IgM shown in white, those from human IgG in pale gray, and those from human IgA in dark gray. Hinge regions and tailpieces are shown as bars between domains and C-terminals, respectively. The positions of mutations are indicated by black ovals. Specific details of each mutation have been published elsewhere [8–13]. hFcα/μR-binding data were derived from IFA and/or rosetting experiments. *Predominant polymerization state present: P, polymeric; D, dimeric; M, monomeric.
Figure 3.
Binding of IgG/IgM domain-swap and monomeric point mutants to hFcα/μR transfectants assessed by IFA on fixed and permeabilized cells. Fcα/μR expression (green) was detected as in Fig. 1. Binding of test Ab was detected with an anti-mouse λ L chain-PE conjugate (red) that bound to the common L chain shared by all the test Ab. hFcα/μR bound only WT IgM and the γ/Cμ3,4 domain-swap Ab.
Figure 6.
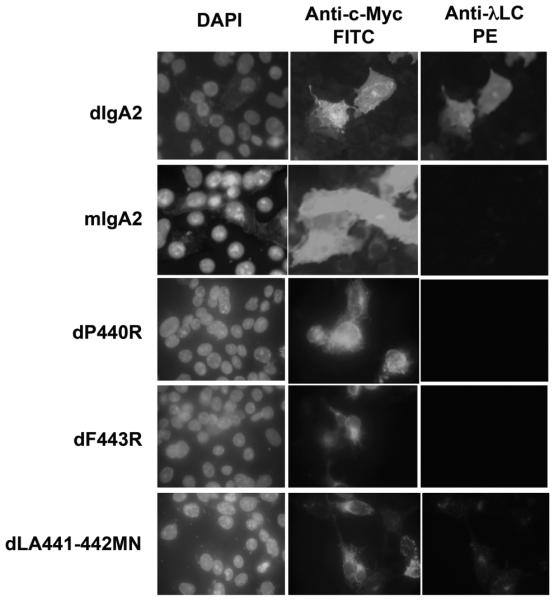
Binding of WT and mutant dIgA2 to fixed and permeabilized hFcα/μR transfectants assessed by IFA. dIgA2 binding was detected using anti-λL chain-PE conjugate. Fcα/μR expression was detected as in Fig. 2. Only WT dIgA2 and the dLA441-442MN mutant bound hFcα/μR. Note the lack of binding by the monomeric IgA2 (mIgA2).
We next investigated whether the binding of hFcα/μR was dependent on the ability of IgM to polymerize. We examined the binding of an IgM point mutant (IgM C575S) with disrupted capability to polymerize and which is secreted from J558L cells principally as monomers [8–11]. The IgM C575S mutant failed to bind to hFcα/μR-transfected COS-7 cells (Fig. 3). However, polymerization per se was not, the only dictator of whether an Ab binds to Fcα/μR, because γL309C-μTP, an IgG molecule that carries the IgM tailpiece and a Cys residue important for inter-monomer disulfide bridges and assembles into pentamers and hexamers, did not bind to COS-7 cells transfected with hFcα/μR (Fig. 2). Therefore, the above data show that the interaction of hFcα/μR with human IgM is dependent on amino acid contributions made from both the Cμ3 and Cμ4 domains, and on the polymeric nature of IgM.
Preincubation with secretory component (SC) blocks the ability of IgM to bind Fcα/μR
Given the similarity between the sequences of Fcα/μR and domain 1 of pIgR, we wondered whether the extracellular portion of the pIgR (SC) might be able to block binding of IgM to hFcα/μR. SC complexed with IgM was found to be no longer able to bind Fcα/μR (Fig. 4, top panel). This result suggests that the binding site for hFcα/μR on IgM overlaps with that for human pIgR.
Figure 4.
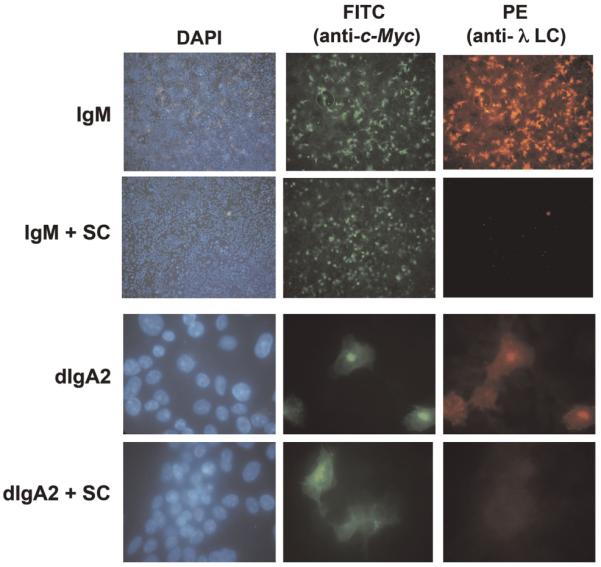
Inhibition of IgM and dIgA2 binding by SC. The binding of IgM (upper panel, ×10 magnification) and dIgA2 (lower panel, ×40 magnification) to hFcα/μR transiently expressed on COS-7 cells could be blocked with saturating levels of free SC. Fcα/μR expression and IgM or dIgA2 binding were detected as in Fig. 1.
Binding of human pIgM to hFcα/μR can be blocked with mAb to the Cμ3 or Cμ4 domains
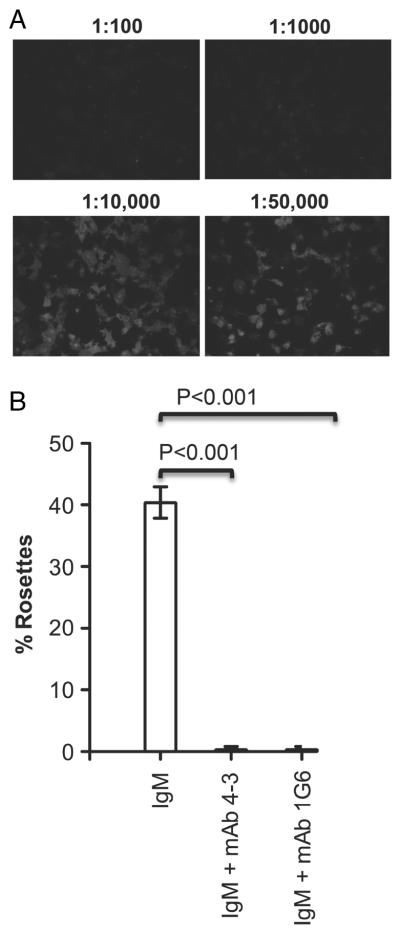
To address the relative importance of the Cμ3 and Cμ4 domains in hFcα/μR binding, we investigated whether mAb specific for epitopes in the Cμ3 (mAb 4–3) or Cμ4 (mAb 1G6) domain could inhibit the interaction of IgM-opsonized red cells with hFcα/μR transfectants (Fig. 5). Both mAb completely blocked human IgM binding as assessed by IFA when used at concentrations as low as 10 ng/mL (Fig. 5A), and completely disrupted rosette formation at 100μg/mL of mAb, providing additional support to the notion that both Cμ3 and Cμ4 domains play a role in binding to hFcα/μR (Fig. 5B).
Figure 5.
Inhibition of IgM binding to hFcα/μR by anti-IgM-Fc-specific Ab. (A) IgM binding to fixed and permeabilized COS-7 cells transfected with human FcαμR can be inhibited by a 1:1000 dilution of mAb 4–3 (anti-Cμ3). Similar results were obtained with mAb 1G6 (anti-Cμ4). (B) Erythrocytes opsonized with IgM (~133 μg/mL) and pre-incubated with 100 μg/mL of either anti-IgM Fc mAb 4–3 or 1G6 fail to rosette with hFcαμR transfectants. Figure shows the mean±SD of two independent experiments. Differences between the untreated control group and erythrocytes pre-incubated with mAb 4-3 or 1G6 were analyzed using Dunnett’s multiple comparison test. Statistically significant differences between the groups are indicated.
hFcα/μR binds dimeric human IgA
Next, we turned to human IgA and found in IFA experiments that hFcα/μR can bind human IgA. As with IgM, the binding of IgA to hFcα/μR was dependent on the polymerization status of the Ab. Only dimeric versions of human IgA1 or IgA2 were capable of binding to hFcα/μR-transfected COS-7 cells, in agreement with previous observations [2] (Fig. 6). The intensity of staining seen with IgM in IFA was always stronger than that seen with dIgA, even when dIgA was used at tenfold higher concentrations than IgM. No binding to hFcα/μR was seen with monomeric versions of either human IgA2 (Fig. 6) or IgA1 (data not shown) from two different sources.
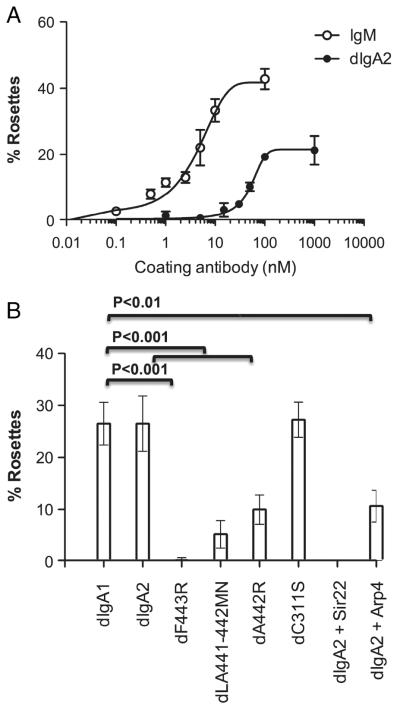
As direct IFA experiments do not give any quantitative indication of the affinities of IgM and dIgA for hFcα/μR, we compared the ability of both Ab to interact with hFcα/μR in a rosetting assay with hFcα/μR-transfected SGHPL-4 cells (Fig. 7). We observed that a concentration of approximately 3 nM of IgM was required to give half maximal rosette formation with IgM, whereas a concentration of around 40 nM was required for dIgA2 to give half maximal rosette formation (Fig. 7A). These findings are consistent with IgM binding hFcα/μR with an approximately tenfold higher affinity than dIgA2.
Figure 7.
Binding of IgM and dIgA to hFcα/μR-transfected SGHPL-4 cells assessed by rosette formation. (A) IgM-opsonized erythrocytes bind hFcαμR with greater affinity than erythrocytes opsonized with equimolar concentrations of dIgA2. (B) Rosette formation was assessed with erythrocytes opsonized with dIgA or various dIgA1 point mutants (all at ~333 μg/mL) purified by size exclusion chromatography. Rosette formation by dIgA2-opsonized erythrocytes could be completely ablated by Sir22 and partially by Arp4 when the bacterial IgA-binding proteins were pre-incubated with dIgA2-opsonized erythrocytes at 100 μg/mL. Figures show the mean±SD of two independent experiments. Differences between %rosettes obtained with dIgA1 and the various test groups were analyzed using Dunnett’s multiple comparison test. Statistically significant differences between the groups are indicated.
Sites in the Cα2/Cα3 inter-domain region contribute to Fcα/μR binding
To investigate the dIgA site(s) involved in hFcα/μR binding, first we made use of domain-swapped Ab in which homologous domains are exchanged between IgA and IgM [7–11] (Fig. 2). Whereas the IgG/IgM domain-swapped Ab γ/L309C-Cμ4 and γ/Cμ3 were unable to bind hFcα/μR, the equivalent domain swaps in an IgA backbone (α/Cμ4 and α/Cμ2,3 respectively) were found to bind to hFcα/μR (Fig. 2). This finding suggests that, in terms of hFcα/μR interaction, residues in the IgA Cα2 and Cα3 domains can substitute for the equivalent regions in the IgM Cμ3 and Cμ4 domains respectively.
To identify individual IgA heavy chain residues involved in the interaction with hFcα/μR, we made use of a panel of mutant dIgA1 Ab which had been previously generated to investigate the interaction of human IgA with pIgR [12]. The mutants carried single- or double-point mutations in the Cα3 domain, with the exception of C311S, in which Cys311 on the surface of the Cα2 domain is mutated to Ser. Using IFA, the dIgA1 mutant’s dP440R and dF443R showed no detectable binding to Fcα/μR whereas dLA441-442MN appeared to bind less well than WT dIgA (Fig. 6). In the rosetting assay, which appears to be more sensitive, the dIgA1 mutant dF443R was observed to be unable to rosette to hFcα/μR transfectants (Fig. 7B). The dLA441-442MN mutant displayed a significantly reduced ability to interact with the receptor compared with WT dIgA1, whereas mutant dA442R showed an intermediate binding capacity (Fig. 7B). Mutation of Cys311 to Ser had no noticeable effect on binding, with the C311S mutant showing rosetting frequencies equivalent to those seen with WT dIgA1 or dIgA2 (Fig. 7B). These findings suggest that residues in the Pro440-Leu441-Ala442-Phe443 (PLAF) loop of the Cα3 domain, which lies at the interface with the Cα2 domain, play a critical role in the Fcα/μR interaction.
Interaction of dIgA with Fcα/μR can be blocked with SC or IgA-binding proteins
As residues in the PLAF loop of the Cα3 domain are known to play a role in binding pIgR [12], we investigated whether SC could inhibit binding of dIgA to hFcα/μR. High backgrounds precluded the use of rosetting in this analysis, but using IFA, we found that SC could indeed inhibit the interaction (Fig. 4, lower panel). Residues in the PLAF loop are known to be involved also in binding to the streptococcal IgA-binding proteins Sir22 and Arp4 [14]. Hence we tested the ability of purified forms of these bacterial proteins to perturb binding of dIgA to hFcα/μR, as they are known to do for binding of IgA to FcαRI (CD89) [14]. Preincubation with Sir22 was found to completely inhibit binding of dIgA2-coated erythrocytes to hFcα/μR (Fig. 7B). Equivalent concentrations of Arp4 resulted in around 50% inhibition of dIgA binding to hFcα/μR. Taken together, these analyses indicate that residues lying at the Cα2/Cα3 domain interface of IgA Fc are critical for interaction with hFcα/μR. Residues in the PLAF loop of the Cα3 domain are particularly implicated.
Discussion
We attempted to express a functional soluble form of hFcα/μR in E. coli but despite producing pure hFcα/μR protein and testing a variety of refolding conditions, we were unable to generate soluble receptor capable of binding IgM (data not shown). Expression of full-length hFcα/μR in COS and SGHPL-4 cells proved successful and we were able to gather information on ligand binding using IFA and rosetting techniques. With both IgM and IgA, we observed that polymerization (predominantly as pentamers for IgM and dimers for IgA) was required for binding to hFcα/μR. This finding is in agreement with observations by others using IgM monomers produced by chemical reduction of pentameric IgM [2]. We note that J chain was present in all the polymeric Ig that were observed to bind hFcα/μR. At this stage, it is not clear whether the requirement for polymerization reflects (i) a need for the presence in the appropriate proximity and relative orientation of at least two Fc regions to enable receptor engagement of sufficient avidity for detection, or (ii) a direct role for J chain in receptor binding, or (iii) both of these as is the case for pIgR. Further experimentation, possibly inhibition studies using anti-J chain Ab, will be required to tease out precisely why ligand polymerization is necessary for receptor binding. In any case, it is striking that the hFcα/μR-ligand interaction requires polymerization of ligand and possible dimerization of hFcα/μR, suggesting that receptor signalling may proceed, at least in part, via coordination of the oligomerization states of both ligand and receptor.
IgM was observed to bind hFcα/μR with high affinity. The finding that half maximal rosette formation was seen with 3 nM IgM suggests a KD in the nanomolar range. The receptor showed an intermediate affinity for dIgA, around tenfold lower than that for IgM, in agreement with observations made for murine Fcα/μR and the human pIgR [1, 5]. The higher affinity seen with IgM may reflect an avidity effect due to engagement of more than one receptor by the IgM pentamer. Additional experiments using chemical cross-linking of IgM C575S and of monomeric IgA to generate J-chain-free dimers, trimers, and tetramers of IgM and IgA might be informative in terms of distinguishing between an avidity effect or an intrinsically greater affinity of individual receptors for IgM than IgA [15].
The domain-swap experiments indicate that interaction of hFcα/μR with IgM requires the presence of both the Cμ3 and Cμ4 domains. One interpretation is that regions in both domains are directly involved in interaction with hFcα/μR. Our demonstration that mAb specific for epitopes within both domains can block interaction with hFcα/μR tends to support this possibility. One might envisage a docking site comprising regions from the two domains that lie close together in three-dimensional space. The Cμ3/Cμ4 domain interface would be one obvious surface to fulfil such a requirement. However, we cannot rule out the alternative interpretation that the interaction site might lie on only one of the Fc domains, with the other domain serving to stabilize the overall Fc conformation. Further information on the Fcα/μR interaction site on IgM was provided by the observation that SC (the extracellular portion of pIgR) is capable of blocking the Fcα/μR–IgM interaction. Although the interaction site for pIgR on IgM has not been definitively defined, the available evidence is consistent with a role for the Cμ4 domain [11]. Taken together, our findings suggest that hFcα/μR binding requires distinct contributions from both the Cμ3 and Cμ4 domains of IgM. Intriguingly, the Cμ4 domain of pentameric IgM has recently been shown to be involved in binding Plasmodium falciparum erythrocyte membrane protein 1 [7]. It remains to be determined if non-specific binding of IgM by infected erythrocytes can block IgM binding to hFcα/μR. This may be crucial for the parasite, if the hFcα/μR does turn out to play a role in the presentation of immune complexes to B cells in germinal centers.
Turning to the interaction of hFcα/μR with dIgA, we probed the role of a surface-exposed loop (PLAF) in Cα3, which lies at the domain interface. We found that mutation of residues Pro440, Ala442, and Phe443 to Arg, and joint mutation of Leu441 and Ala442 to Met and Asn, respectively, resulted in loss or reduction of binding to hFcα/μR. Interestingly, we found also in IFA that SC was capable of inhibiting the hFcα/μR-dIgA interaction. The PLAF loop has previously been shown to play a role in the binding of SC (pIgR) to dIgA [12], so this finding adds weight to the idea that the PLAF loop is critical for hFcα/μR interaction. Further, two streptococcal IgA-binding proteins, Sir22 and Arp4, which also interact with the PLAF loop [14] were found to inhibit binding of dIgA to hFcα/μR. Thus, several lines of evidence point toward the PLAF loop in the inter-domain region making an important contribution to the hFcα/μR-binding site.
Given the structural similarities between the N-terminal domains of Fcα/μR and pIgR, and in particular the motifs implicated in ligand binding [1, 6], there might be some expectation of similar modes of interaction of the receptors with their shared ligands. However, the fact that there are important differences between the interaction of pIgR with IgM and dIgA [16] calls for caution in any presumptions. In any case, it is well recognized that even subtle differences in the contact surfaces involved in FcR-Ig interactions may have profound effects on binding events [17, 18]. Nevertheless, we can see some common features between the binding of dIgA to hFcα/μR and pIgR. The pIgR-binding site on dIgA has been localized to the upper surface of the Cα3 domain [11, 12, 19, 20] with contributions from the Cα3 domain PLAF loop [12]. It would be of interest to probe the potential role of the Gln402–Thr414 region of the IgA Cα3 domain in hFcα/μR binding, because this region has been implicated in pIgR binding [12, 19]. A further similarity between the receptors is the requirement for polymerization of IgA for binding. J chain is required for pIgR interaction, and evidence points toward a direct interaction between J chain and pIgR [21–23]. Despite having shown that IgA polymerization is required for hFcα/μR binding, we have not investigated whether there is a strict requirement for J chain to be present. Thus, although some questions remain, we have shown a degree of overlap between the interaction sites for dIgA on hFcα/μR and pIgR.
The PLAF loop is exposed at the IgA Fc domain interface (Fig. 8). In a solution structure model of dIgA1 [24], where the two monomers are predicted to lie in a near-planar arrangement, the PLAF loops on the same sides of the two IgA monomers can be seen to lie relatively close to each other. The J chain, which is not included in the model, is thought to occupy a position near the C-termini of the Fc regions, and thus would be ideally positioned for possible involvement in the interaction with hFcα/μR. One might speculate that a receptor molecule could readily form contacts with a surface comprising the PLAF loop and the neighboring J chain (Fig. 8B). If receptor dimerization does facilitate interaction with dIgA, one could envisage two receptors interacting with regions encompassing the two PLAF loops, and possibly also J chain elements, lying on the same side of the dimer. Alternatively, two receptors might dock on opposites sides of the dimer. Whatever the precise arrangement, the four potential interaction sites within the Fc region in close proximity to J chain appear to provide suitable possibilities for high avidity interaction.
Figure 8.
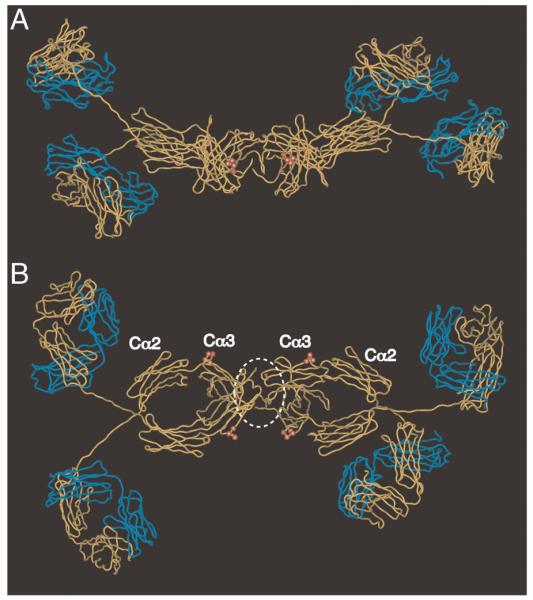
Molecular model of dIgA1 showing residues predicted to be critical for interaction with hFcα/μR. The figure shows two views of the solution structure of human dIgA1 [24] (PDB accession: 2qtj) in which the heavy chains are shown in yellow, and the L chains in blue. The tailpieces are omitted for clarity. The heavy chain residues Pro440–Phe443 implicated in hFcα/μR interaction are shown as red spheres. J chain is not included in the model, but we have added a white dotted line to indicate a possible position. (A) side view; (B) view from above.
Remarkably, the proposed hFcα/μR site on dIgA, involving the Cα3 domain PLAF loop, overlaps with the interaction sites for not only pIgR, as mentioned earlier, but also for the well-characterized Fc receptor of IgA, FcαRI (CD89) [13, 25, 26]. Further, the interaction sites for a number of unrelated bacterial IgA-binding proteins produced by important human pathogens have been localized to the same inter-domain region [14, 27, 28]. In addition, the PLAF loop influences the sensitivity of IgA1 to cleavage by a type of IgA1 protease secreted by Neisseria meningitidis, presumably through forming a point of contact between the IgA1 substrate and the protease [29]. Interestingly, the equivalent inter-domain region on IgG Fc is also involved in interaction with a variety of molecules [7, 30, 31], and has been recognized to be one of only a limited number of surface-exposed regions on IgG that are especially suited to protein–protein interaction [32, 33]. Presumably, such regions are conserved because they mediate essential interactions with key host receptors, but they obviously represent points of vulnerability that may be exploited by co-evolving pathogens [34].
The apparently restricted expression profile of hFcα/μR (on a subpopulation of pre-germinal B cells and FDC in tonsillar tissues) [2] suggests that it is unlikely to be co-expressed on the same cells as either FcαRI, which is found on neutrophils, monocytes, some macrophages, eosinophils, interstitial DC and Kupffer cells, or pIgR, which is expressed on mucosal epithelial cells. Thus, the overlapping nature of the binding sites on dIgA for these different receptors appears unlikely to have a major physiological impact. The potential effects of site overlap with bacterial IgA-binding or malaria IgM-binding proteins are more difficult to predict. However, if sufficient concentrations of the bacterial IgA-binding proteins were able to penetrate into secondary lymphoid organs during certain phases of infection, it is conceivable that some inhibition of hFcα/μR function might ensue because, for example, Arp4 has been shown to inhibit the ability of IgA to trigger immune cell responses via FcαRI [14]. As it has been postulated that hFcα/μR may play a role in trapping IgM or IgA immune complexes and in presenting intact antigens to B cells in germinal centers, any perturbation of this role by bacterial IgA-binding proteins might have the capacity to impact on the subsequent immune response.
In summary, through analysis of the effects of different directed alterations to the Fc, we have shown that binding of hFcα/μR to IgM is governed by the Cμ3 and Cμ4 domains, and we have identified residues at the IgA Fc domain interface that are critical for binding of dIgA to hFcα/μR. This interaction site on dIgA overlaps with the regions bound by other host Fc receptors, and is known to be a region targeted by pathogens as a means to circumvent the immune response. The study not only provides new insights into the molecular basis of hFcα/μR function, but also serves to highlight the key role that the IgA Fc domain interface plays in the function of this important Ig isotype.
Materials and methods
Cloning and expression of a membrane anchored version of hFcα/μR
To characterize hFcα/μR, and to establish its ability to bind human IgM or IgA, a full-length construct of hFcα/μR was amplified from an Incyte EST clone (Open Biosystems, accession number: AY063125) by PCR using forward (5′-tcttggcgcgcccttccacaaaaaagaccc-3′) and reverse (5′-ctgtccctcgaggtcctggatttctctctg-3′) primers containing AscI and XhoI restriction enzyme sites (underlined) respectively. The PCR product was cloned into the pCR2.1 TOPO vector. An AscI/XhoI fragment was then subcloned into the mammalian expression vector pSecTag2/Hygro (Invitrogen) upstream of a myc tag, and sequenced in both directions to confirm that no errors had been introduced. The resultant plasmid was transiently expressed following transfection into either COS-7 cells or the human extravillous trophoblast cell line SGHPL-4 (formerly known as MC4; [35]) using Fugene 6 (Roche) as described by the manufacturer. Transfection efficiency was assessed by IFA detecting with mAb 9E10 (anti-myc). Ability to bind IgM was assessed by incubation with IgM followed by detection with goat anti-mouse λ L chain-PE, as described previously [7].
Ab and other proteins
IgM/IgG and IgM/IgA1 domain-swap, point-mutants, and IgG1 or IgA1 Ab specific for the hapten NIP were purified from tissue culture supernatants as previously described [8–13]. For certain IgA1 mutants (dP440R, dF443R, and dLA441-442MA) dimers were separated from contaminating monomers by size exclusion chromatography. Other Ab used for investigating binding to hFcα/μR were obtained from commercial sources as follows: human dIgA2 anti-NIP and human IgM anti-NIP (Serotec); mouse IgMλ (Pharmingen); and human IgM Fabμ fragment (Rockland). Detecting Ab used in immunoblotting and IFA include anti-polyhistidine-HRP mAb (HIS-1, Sigma), goat anti-mouse λ L chain-RPE (Southern Biotech), mouse mAb 9E10 anti-myc (Sigma), goat anti-mouse IgG Fab-FITC (Sigma), goat F(ab’)2 anti-human IgM Fcμ-RPE (Caltag laboratories), and mouse anti-human IgM κ-chain specific (Sigma). A panel of anti-IgM μ-chain-specific mAb used in blocking studies (1F11, 4-3, 1G6, 5D7, 196.6b, HB57, 1X11) have been described previously [7, 36, 37]. SC was prepared as previously described [11]. The streptococcal IgA-binding M proteins Arp4 (M4) and Sir22 (M22) were purified as described [38, 39]. Purification of the malaria parasite IgM-binding protein DBL4β has been described recently [7].
Analysis of Ig-binding to hFcα/μR expressed in COS-7 cells by IFA
COS-7 cells transfected with hFcα/μR were grown on 12 mm circular coverslips (Raymond A Lamb). Cells were washed in PBS and fixed in ice-cold 50:50 methanol:acetone for 20 min. After washing in PBS, cells were blocked in PBS/5% FBS for 1 h. After washing, 7.5 ng of mAb 9E10 to detect the myc tag as a marker of transfection efficiency, or test Ab (10 ng of IgM, 100 ng of dIgA2, or up to 15 μg for domain-swapped or point-mutated Ab) in a final volume of 50 μL PBS/1% FBS were spotted onto parafilm and laid over a coverslip for 1 h in a humid chamber. For blocking experiments, IgM or dIgA2 were pre-incubated with 10.5 μg SC for 1 h at room temperature prior to the assay. After incubation cells were washed three times in PBS/1% FBS prior to incubation for 1 h in the dark with a 1:100 dilution of goat anti-mouse IgG-FITC for the 9E10 coverslips or a similar dilution of goat anti-mouse λ L chain-RPE for the test coverslips. Cells were washed as above with a final wash in PBS and mounted with Prolong Gold antifade reagent containing DAPI (Invitrogen) before fluorescence microscopic analysis.
Rosetting assay
Human erythrocytes were derivatized with NIP and sensitized with varying concentrations of anti-NIP IgM, dIgA1, dIgA2, or dIgA1 mutants as described [7]. After 24 h in culture, SGHPL-4 cells transfected with hFcα/μR were resuspended in RPMI 1640 culture medium (Gibco) containing 10% Ig-stripped FBS (PAA Laboratories, GmbH). In the inhibition assay, which was essentially a modification of a previously described method [14], 100 μL diluted sensitized erythrocytes and inhibitor (bacterial Ig-binding proteins Sir22 and Arp4 [38, 39]) at 100 μg/mL or anti-IgM mAb at 1:1000] in RPMI/FBS, were incubated at room temperature for 1 h in wells of a round-bottomed microtiter plate. 50 μL of SGHPL-4 cells expressing the hFcα/μR (~50 000 cells) in RPMI/FBS were added and mixed carefully; the plates were incubated for 10 min, centrifuged at 50g for 5 min, and further incubated for 50 min. Following the addition of acridine orange solution (6 μg/mL final concentration) to stain nucleated cells, the suspensions were examined by fluorescence microscopy, defining a rosette as a fluorescent SGHPL-4 cell with three or more erythrocytes attached.
Acknowledgements
This work was supported by a Medical Research Council Career Establishment Award (G0300145) and a European union Marie Curie Excellence Grant, Antibody Immunotherapy for Malaria (MEXT-CT-2003-509670) to RJP. JMW and RP thank the Wellcome Trust for support.
Abbreviations
- dIgA
dimeric IgA
- FDC
follicular DC
- IFA
immunofluorescent assay
- hFcα/μR
human Fcα/μ receptor
- mFcα/μR
mouse Fcα/μ receptor
- NIP
3-nitro-4-hydroxy-5-iodophenylacetyl
- pIgR
polymeric Ig receptor
- PLAF
Pro440-Leu441-Ala442-Phe443
- SC
secretory component
Footnotes
Conflict of interest: The authors have declared no financial or commercial conflict of interest.
References
- 1.Shibuya A, Sakamoto N, Shimizu Y, Shibuya K, Osawa M, Hiroyama T, Eyre G, et al. Fcα/μ receptor mediates endocytosis of IgM-coated microbes. Nat. Immunol. 2000;1:441–446. doi: 10.1038/80886. [DOI] [PubMed] [Google Scholar]
- 2.Kikuno K, Kang D-W, Tahara K, Torii I, Kubagawa HM, Ho KJ, Baudino L, et al. Unusual biochemical features and follicular dendritic cell expression of human Fcα/μ receptor. Eur. J. Immunol. 2007;37:1–11. doi: 10.1002/eji.200737655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakos M, Kurosky AA, Cwerwinski EW, Goldblum RM. A conserved binding site on the receptor for polymeric Ig is homologous to CDR1 of Ig V kappa domains. J. Immunol. 1993;151:1346–1352. [PubMed] [Google Scholar]
- 4.Coyne RS, Siebrecht M, Peitsch MC, Casanova JE. Mutational analysis of polymeric immunoglobulin receptor/ligand interactions. Evidence for the involvement of multiple complementarity determining region (CDR)-like loops in receptor domain I. J. Biol. Chem. 1994;269:31620–31625. [PubMed] [Google Scholar]
- 5.Roe M, Norderhaug IN, Brandtzaeg P, Johansen F-E. Fine specificity of ligand binding domain I in the polymeric Ig receptor: importance of the CDR2-containing region for IgM interaction. J. Immunol. 1999;162:6046–6052. [PubMed] [Google Scholar]
- 6.Cho Y, Usui K, Honda S-I, Tahara-Hanaoka S, Shibuya K, Shibuya A. Molecular characteristics of IgA and IgM Fc binding to the Fcα/μR. Biochem. Biophys. Res. Commun. 2006;345:474–478. doi: 10.1016/j.bbrc.2006.04.084. [DOI] [PubMed] [Google Scholar]
- 7.Ghumra A, Semblat J-P, McIntosh RS, Raza A, Rasmussen IB, Braathen R, Johansen F-E, et al. Identification of residues in the Cμ4 domain of polymeric IgM essential for interaction with P. falciparum erythrocyte membrane protein 1 (PfEMP1) J. Immunol. 2008;181:2019–2027. doi: 10.4049/jimmunol.181.3.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sørensen V, Rasmussen IB, Norderhaug L, Natvig I, Michaelsen TE, Sandlie I. Effect of the IgM and IgA secretory tailpieces on polymerization and secretion of IgM and IgG. J. Immunol. 1996;156:2858–2865. [PubMed] [Google Scholar]
- 9.Sørensen V, Sundvold V, Michaelsen TE, Sandlie I. Polymerization of IgA and IgM: roles of Cys309/Cys414 and the secretory tailpiece. J. Immunol. 1999;162:3448–3455. [PubMed] [Google Scholar]
- 10.Sørensen V, Rasmussen IB, Sundvold V, Michaelsen TE, Sandlie I. Structural requirements for incorporation of J chain into human IgM and IgA. Int. Immunol. 2000;12:19–27. doi: 10.1093/intimm/12.1.19. [DOI] [PubMed] [Google Scholar]
- 11.Braathen R, Sorensen V, Brandtzaeg P, Sandlie I, Johansen F-E. The carboxyl-terminal domains of IgA and IgM direct isotype-specific polymerization and interaction with the polymeric immunoglobulin receptor. J. Biol. Chem. 2002;277:42755–42762. doi: 10.1074/jbc.M205502200. [DOI] [PubMed] [Google Scholar]
- 12.Lewis MJ, Pleass RJ, Batten MR, Atkin JD, Woof JM. Structural requirements for the interaction of human IgA with the human polymeric Ig receptor. J. Immunol. 2005;175:6694–6701. doi: 10.4049/jimmunol.175.10.6694. [DOI] [PubMed] [Google Scholar]
- 13.Pleass RJ, Dunlop JI, Anderson CM, Woof JM. Identification of residues in the CH2/CH3 domain interface of IgA essential for interaction with the human Fcα receptor (FcαR) CD89. J. Biol. Chem. 1999;274:23508–23514. doi: 10.1074/jbc.274.33.23508. [DOI] [PubMed] [Google Scholar]
- 14.Pleass RJ, Areschoug T, Lindahl G, Woof JM. Streptococcal IgA-binding proteins bind in the Cα2–Cα3 interdomain region and inhibit binding of IgA to human CD89. J. Biol. Chem. 2001;276:8197–8204. doi: 10.1074/jbc.M009396200. [DOI] [PubMed] [Google Scholar]
- 15.Kagey-Sobotka A, Dembo M, Goldstein B, Metzger H, Lichtenstein LM. Qualitative characteristics of histamine release from human basophils by covalently cross-linked IgE. J. Immunol. 1981;127:2285–2291. [PubMed] [Google Scholar]
- 16.Norderhaug IN, Johansen F-E, Krajci P, Brandtzaeg P. Domain deletions in the human polymeric Ig receptor disclose differences between its dimeric IgA and pentameric IgM interaction. Eur. J. Immunol. 1999;29:3401–3409. doi: 10.1002/(SICI)1521-4141(199910)29:10<3401::AID-IMMU3401>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 17.Duncan AR, Woof JM, Partridge LJ, Burton DR, Winter G. Localization of the binding site for the human high-affinity Fc receptor on IgG. Nature. 1988;332:563–564. doi: 10.1038/332563a0. [DOI] [PubMed] [Google Scholar]
- 18.Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int. Immunol. 2001;13:1551–1559. doi: 10.1093/intimm/13.12.1551. [DOI] [PubMed] [Google Scholar]
- 19.Hexham JM, White KD, Carayannopoulous LN, Mandecki W, Brisette R, Yang Y-S, Capra JD. A human immunoglobulin (Ig) A Cα3 domain motif directs polymeric Ig receptor-mediated secretion. J. Exp. Med. 1999;189:747–752. doi: 10.1084/jem.189.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White KD, Capra JD. Targeting mucosal sites by polymeric immunoglobulin receptor-directed peptides. J. Exp. Med. 2002;196:551–555. doi: 10.1084/jem.20020581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandtzaeg P, Prydz H. Direct evidence for an integrated function of J chain and secretory component in epithelial transport of immunoglobulins. Nature. 1984;311:71–73. doi: 10.1038/311071a0. [DOI] [PubMed] [Google Scholar]
- 22.Johansen F-E, Braathen R, Brandtzaeg P. The J chain is essential for polymeric Ig receptor-mediated epithelial transport of IgA. J. Immunol. 2001;167:5185–5192. doi: 10.4049/jimmunol.167.9.5185. [DOI] [PubMed] [Google Scholar]
- 23.Braathen R, Hohman VS, Brandtzaeg P, Johansen F-E. Secretory antibody formation: conserved binding interactions between J chain and polymeric receptor from humans and amphibians. J. Immunol. 2007;178:1589–1597. doi: 10.4049/jimmunol.178.3.1589. [DOI] [PubMed] [Google Scholar]
- 24.Bonner A, Furtado PB, Almogren A, Kerr MA, Perkins SJ. Implications of the near-planar solution structure of human myeloma dimeric IgA1 for mucosal immunity and IgA nephropathy. J. Immunol. 2008;180:1008–1018. doi: 10.4049/jimmunol.180.2.1008. [DOI] [PubMed] [Google Scholar]
- 25.Carayannopolous L, Hexham JM, Capra JD. Localization of the binding site for the monocyte immunoglobulin (Ig) A-Fc receptor (CD89) to the domain boundary between Cα2 and Cα3 in human IgA1. J. Exp. Med. 1996;183:1579–1586. doi: 10.1084/jem.183.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herr AB, Ballister ER, Bjorkman PJ. Insights into IgA-mediated immune responses from the crystal structures of human FcαR1 and its complex with IgA1-Fc. Nature. 2003;423:614–620. doi: 10.1038/nature01685. [DOI] [PubMed] [Google Scholar]
- 27.Wines BD, Willoughby N, Fraser JD, Hogarth PM. A competitive mechanism for staphylococcal toxin SSL7 inhibiting the leukocyte IgA receptor, FcαRI, is revealed by SSL7 binding at the Cα3/Cα3 interface of IgA. J. Biol. Chem. 2006;281:1389–1393. doi: 10.1074/jbc.M509334200. [DOI] [PubMed] [Google Scholar]
- 28.Ramsland PA, Willoughby N, Trist HM, Farrugia W, Hogarth PM, Fraser JD, Wines BD. Structural basis for evasion of IgA immunity by Staphylococcal aureus revealed in the complex of SSL7 with Fc of human IgA1. Proc. Natl. Acad. Sci. USA. 2007;104:15051–15056. doi: 10.1073/pnas.0706028104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senior BW, Woof JM. Sites in the CH3 domain of human IgA1 that influence sensitivity to bacteria IgA1 proteases. J. Immunol. 2006;177:3913–3919. doi: 10.4049/jimmunol.177.6.3913. [DOI] [PubMed] [Google Scholar]
- 30.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 31.Lewis MJ, Meehan M, Owen P, Woof JM. A common theme in interaction of bacterial immunoglobulin-binding proteins with immunoglobulins illustrated in the equine system. J. Biol. Chem. 2008;283:17615–17623. doi: 10.1074/jbc.M709844200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burton DR. Immunoglobulin G: functional sites. Mol. Immunol. 1985;22:161–206. doi: 10.1016/0161-5890(85)90151-8. [DOI] [PubMed] [Google Scholar]
- 33.DeLano WL, Ultsch MH, De Vos AM, Wells JA. Convergent solutions to binding at a protein–protein interface. Science. 2000;287:1279–1283. doi: 10.1126/science.287.5456.1279. [DOI] [PubMed] [Google Scholar]
- 34.Abi-Rached L, Dorighi K, Norman PJ, Yawata M, Parham P. Episodes of natural selection shaped the interactions of IgA-Fc with FcαRI and bacterial decoy proteins. J. Immunol. 2007;178:7943–7954. doi: 10.4049/jimmunol.178.12.7943. [DOI] [PubMed] [Google Scholar]
- 35.Choy MY, Manyonda IT. The phagocytic activity of human first trimester extravillous trophoblasts. Hum. Repro. 1998;13:2941–2949. doi: 10.1093/humrep/13.10.2941. [DOI] [PubMed] [Google Scholar]
- 36.Rudich SM, Winchester R, Mongini PK. Human B-cell activation. Evidence for diverse signals provided by various monoclonal anti-IgM antibodies. J. Exp. Med. 1985;162:1236–1255. doi: 10.1084/jem.162.4.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudich SM, Mihaesco E, Winchester R, Mongini PK. Analysis of the domain specificity of various murine anti-human IgM monoclonal antibodies differing in human B-lymphocyte signaling activity. Mol. Immunol. 1987;24:809–820. doi: 10.1016/0161-5890(87)90183-0. [DOI] [PubMed] [Google Scholar]
- 38.Stenberg L, O’ Toole PW, Mestecky J, Lindahl G. Molecular characterization of protein Sir, a streptococcal cell surface protein that binds both immunoglobulin A and immunoglobulin G. J. Biol. Chem. 1994;269:13458–13464. [PubMed] [Google Scholar]
- 39.Frithz E, Hedén L-O, Lindahl G. Extensive sequence homology between IgA receptor and M proteins in Streptococcus pyogenes. Mol. Microbiol. 1989;3:1111–1119. doi: 10.1111/j.1365-2958.1989.tb00261.x. [DOI] [PubMed] [Google Scholar]