Abstract
Objective
There is increased interest in measuring peripheral oxytocin levels to better understand the role of this peptide in mammalian behavior, physiology, and disease. The purpose of this study was to compare methods for plasma oxytocin measurement using a commercially available enzyme immunoassay (EIA) and radioimmunoassay (RIA), to evaluate the need for sample extraction, and to assess the immunospecificity of the assays.
Methods
Oxytocin was measured in extracted and unextracted human plasma samples (n = 39). Oxytocin and its degradation products were separated by high performance liquid chromatography and gel filtration chromatography and then assayed by EIA or RIA to identify oxytocin immunoreactive peaks.
Results
Without extraction, plasma measured by EIA was more than 100-fold higher than in extracted plasma, and the correlation between oxytocin levels in extracted and unextracted plasma was minimal (Spearman’s rho = −0.10, p = 0.54). Using the RIA, most samples (> 90%) were below the level of detection with or without extraction. Following chromatographic fractionation of sample extracts, multiple immunoreactive products were found to be present in addition to oxytocin, which casts doubts on the specificity of the assays.
Conclusions
Changes in oxytocin levels have been reported in social and behavioral challenge studies. This study indicates that sample extraction is necessary to obtain valid assay results. Changes in oxytocin degradation products are likely to contribute to the previously observed responses in circulating oxytocin levels to behavioral and social challenge. There is a critical need for validated and reliable methods to measure oxytocin in biological samples.
Keywords: Oxytocin, Extraction, Immunoassays
Introduction
The neurohypophyseal peptide oxytocin, which acts in the central nervous systems of males and females, plays an important role in a variety of complex social behaviors including affiliation, sexual behavior, social recognition, stress buffering, aggression, and trust (1-9). There has been increased interest in quantifying peripheral oxytocin levels to better understand oxytocin’s role in human and animal social behavior. Oxytocin has been measured by immunoassays in biological fluids including plasma, saliva, urine, and cerebrospinal fluid in different mammalian species. Sample preparation often involves extraction (10-13), but several reports have utilized direct measurement (9, 14). Reported plasma oxytocin levels vary from 1 to 300 pg/ml in human and mammalian species, however, significant differences exist between values observed in similar populations and are likely due to methodological differences, making comparison between studies difficult (Table 1). Lower oxytocin values have usually been obtained from samples subjected to extraction, while higher values occurred in samples assayed directly, suggesting that sample preparation influences quantification. The purpose of this study was to compare two commercially available methods1 for oxytocin measurement using enzyme immunoassay (EIA) or radioimmunoassay (RIA), to determine the stability of plasma oxytocin, to evaluate the need for sample extraction, and to assess the immunospecificity of the assays.
Table 1.
Reported Oxytocin Levels Measured Using Different Methods
| Species | Sex | Sample | State | Concentration (pg/ml) |
Measurement | Extraction | Reference |
|---|---|---|---|---|---|---|---|
| Humans | F | Plasma | Baseline | 23 ± 11.2 | EIA | Y | (24) |
| Humans | F/M | Plasma Saliva |
Control | 7.5 ± 1.4 6.5 ± 1.8 |
EIA | Y | (18) |
| Humans | F | Plasma Saliva |
Baseline | 4.8 ± 0.5 4.7 ± 0.5 |
EIA | Y | (11) |
| Humans | F/M | Saliva | Control | 31 ± 12.7 24.4 ± 32 |
EIA | N Y |
(21) |
| Humans | F/M | Plasma | Control | 198 ± 165 | EIA | N | (9) |
| Humans | M | Plasma | Baseline | 99 – 421 | EIA | N | (25) |
| Humans | F/M | Plasma | Control | 240 ± 224 | EIA | N | (26) |
| Humans | F/M | Plasma | Baseline | 259 ± 38 | EIA | N | (27) |
| Humans | F | Saliva | 6.44 - 61.05 | EIA | N | (28) | |
| Humans | M | Plasma Saliva |
Baseline | 405 ± 152 7.1 ± 4.0 |
EIA | N | (17) |
| Humans | F M |
Plasma | Baseline | 249 ± 180 273 ± 234 |
EIA | N | (29) |
| Rhesus Monkey |
M | Plasma | Nurse-reared Mother-reared |
225 275 |
EIA | N | (30) |
| Prairie voles Rats |
F M F M |
Plasma | Baseline | 488 ± 88 264 ± 31 187 ± 53 79 ± 6 |
EIA | N | (14) |
| Rats | M | Plasma | 0800 | 450 – 700 | EIA | N | (31) |
| Rats | M | Plasma | Control | 200 | EIA | N | (32) |
| Rats Mice |
F | Plasma | Control | 60 120 |
EIA | N | (33) |
| Mice | F/M | Plasma | CD38−/− | 200 | EIA | N | (34) |
| Mice | F/M | Plasma | Control | 100 – 120 | EIA | N | (35) |
| Humans | M/F | Plasma | Baseline | 4.0 | RIA | Y | (10) |
| Humans | M | Plasma | Control Autistic |
1.2 ± 0.8 0.6 ± 0.6 |
RIA | Y | (36) |
| Humans | M | Plasma | Normal | 0.17 | RIA | Y | (37) |
| Humans | M | Plasma | Control Autistic |
1.4 ± .10 0.8 ± .09 |
RIA | Y | (38) |
| Humans | F | Plasma | Baseline | 3.5 ± 3.5 | RIA | Y | (39) |
| Humans | M | Plasma | Control | 2-4 | RIA | Y | (40) |
| Humans | M | Plasma | Baseline | 71 | RIA | Y | (41) |
| Humans | F M |
Plasma | Baseline | 1.7 ± 0.2 1.5 ± 0.2 |
RIA | Y | (42) |
| Humans | F | Plasma | Control Birth Control |
1.5 - 2.6 2 |
RIA | Y | (12) |
| Humans | F/M | Plasma | Control Autistic |
5 – 8 8 – 12 |
RIA | Y | (43) |
| Humans | F/M | Plasma | Control | 0.1 – 4.6 | RIA | Y | (16) |
| Humans | F/M | Plasma | Baseline | 2.17 ± 0.4 | RIA | Y | (44) |
| Humans | F | Plasma | Baseline | 50 ± 9.6 | RIA | Y | (45) |
| Humans | F | Plasma | Control | 1 – 3 | RIA | Y | (46) |
| Humans | F | Plasma | Baseline | 5.7 ± 8.1 | RIA | Y | (47) |
| Humans | F/M | Plasma | Control | 1 | RIA | Y | (48) |
| Humans | F | Plasma | Baseline | 1.6 ± 2.8 | RIA | Y | (13) |
| Cynomolgus Monkey |
F | Plasma | Prepartum Parturition |
5 22 ± 7 |
RIA | Y | (49) |
| Heifers | F | Plasma | Pregnant Baseline | 1 - 10 | RIA | Y | (50) |
| Rabbit | M | Plasma | Control | 8.8 ± 2.1 | RIA | Y | (51) |
| Rats | M | Plasma Urine |
Control | 17 ± 22 1.4 ± 0.1 |
RIA | Y | (52) |
| Rats | M | Plasma | 0800-0900 1700-1800 |
3.0 ± 0.6 6.6 ± 1.0 |
RIA | Y | (53) |
| Rats | M | Plasma | Control | 1 – 2 | RIA | Y | (54) |
| Rats | F | Plasma | Control | 30.2 ± 8.5 | RIA | Y | (55) |
| Mice | M | Plasma Urine |
Baseline OT +/+ |
50 250.2 ± 35.3 |
RIA | Y | (20) |
| Rabbit | F | Plasma | During Birth & Parturition |
12 - 3030 | RIA | N | (56) |
| Humans | F/M | Urine | Baseline | 14-17 μg/mg creatinine | HPLC | Y | (19) |
Methods
Oxytocin Assays
Oxytocin EIA (Assay Designs, Ann Arbor, MI) and RIA (Phoenix Pharmaceuticals, Burlingame, CA) were performed following the manufacturers’ protocols. Limits of detection were 1.2 pg/well and 1.0 pg/tube. These values would equate to 0.12 and 0.1 pg/ml for unextracted samples or 1.2 and 1.0 pg/ml after extraction of 1 ml plasma. Intra- and inter-assay variability were 9% and 15%, 7% and 15%, for EIA and RIA, respectively, as reported by the manufacturers. Unextracted samples measured by EIA were diluted 1:4 as described by Kramer et al. (14).
Sample Extraction
Sample extraction is a procedure to eliminate the effect of potentially interfering molecules, reduce sample matrix effects, and concentrate and enrich the analyte of interest from the sample prior to analysis. Sample extraction was evaluated using two different methods: solid phase extraction (the method recommended by both oxytocin assay manufacturers), and solvent extraction (a method previously validated for the measurement of oxytocin (10). De-identified human EDTA plasma or serum samples were provided by the University of Miami Diabetes Research Institute Clinical Chemistry Laboratory and stored at −80°C until assay. Solid phase extraction of samples was performed using 200 mg C18 Sep-Pak columns (Bachem, San Carlos, CA). Columns were equilibrated with 3 ml of acetonitrile, then twice with 3 ml of 0.1% trifluoroacetic acid (TFA). Up to 1 ml of plasma was mixed with an equal volume of 0.1% TFA, centrifuged at 14,000 g for 20 minutes at 4°C, and the acidified and clarified plasma was then applied to the column. The flow-through fraction was discarded, columns were washed once with 3 ml of 0.1% TFA, then twice with 3 ml of water. Oxytocin was eluted with 3 ml of 60% acetonitrile. The solvent was evaporated under a stream of nitrogen gas, and the sample completely dried by lyophilization. In order to evaluate different extraction procedures, some samples were extracted using an organic solvent method as described previously (10). Briefly, 1 ml plasma was mixed with 2 ml of ice-cold acetone, centrifuged at 1,200 g for 20 minutes at 4°C, the supernatant was transferred, and washed with 2 ml anhydrous ether. The extract was dried under a stream of nitrogen gas. For immunoassay, samples were reconstituted in 0.12 ml of assay buffer provided by the EIA or RIA kits. For HPLC fractionation (see below), 4 or 10 ml of plasma were extracted (1 ml/column) and the eluates pooled to provide sufficient levels of oxytocin for detection by EIA or RIA, respectively.
Extraction efficiency was determined by spiking plasma with approximately 20,000 cpm/ml of 3H-oxytocin (Perkin Elmer, Waltham, MA) and fractions (i.e., flow-through, washes, eluate) were collected and 3H determined by scintillation counting.
Chromatography
Chromatography is a procedure that separates molecules based on their physical properties. High performance liquid chromatography (HPLC) separates molecules based on hyrodphobicity, such that polar compounds are eluted first. Gel filtration chromatography separates molecules based upon their molecular weight by utilizing a porous gel.
Oxytocin and its degradation products were separated by high performance liquid chromatography (HPLC) on a Phenomenex C18 (4.6×150 mm) column using a Hitachi LaChrom Elite HPLC system (Schaumburg, IL). Samples were eluted at a flow rate of 1 ml per min with 20% (v/v) acetonitrile/0.1% TFA for 9 minutes, then linearly increased to 100% acetonitrile/0.1% TFA over four minutes, and maintained in this buffer until the end of the run. Chromatography was monitored by ultraviolet absorbance at 280 nm. Plasma extracts (100 μl) were injected into the column and 0.75 ml fractions were collected.
Gel filtration chromatography was performed using a Superdex 75 10/300 GL column (GE Healthcare, Piscataway, NJ) eluted with ammonium acetate buffer (5 mM; pH = 7.8) at a flow rate of 1.0 ml per min and monitored at 280 nm. Plasma (400 μl) or plasma extracts (200 μl representing 4 ml of plasma) were injected into the column and 1.5 ml fractions were collected.
After chromatography, samples were lyophilized reconstituted with 0.12 ml assay buffer and assayed by EIA or RIA to identify oxytocin immunoreactive peaks.
Statistical analyses
Data were presented as mean ± standard error of the mean (SEM). Oxytocin values that were below the level of detection were assigned a value of 0.1 pg/ml for statistical analysis. Results were compared by paired t-tests between groups and relationships between variables were examined by Pearson product-moment and Spearman rank correlation coefficients using SPSS for Windows (Version 17, Chicago, IL). An alpha level of 0.05 was required for statistical significance.
Results
Extraction and Stability of Oxytocin
Two sample extraction methods were evaluated based on the recovery of added 3H-oxytocin to the sample. First, solid phase extraction on a C-18 column resulted in an extraction efficiency for plasma (n=6) and serum (n=3) samples of 92% ± 13% and 81% ± 5%, respectively. Second, using the organic solvent extraction method recovery of oxytocin in plasma (n=4) was 72% ± 2%. Given the higher recovery in plasma using the solid phase extraction, this method was used in subsequent experiments.
Initial studies assessed the stability of oxytocin in plasma. 3H-oxytocin was added to the samples, incubated at 0°, 22°, or 37°C for 2 or 18 hours, or subjected to several freeze/thaw cycles. The samples were then extracted and intact 3H-oxytocin was determined by HPLC. Extraction efficiency was similar for samples under the various conditions (95 ± 12%). HPLC analysis revealed that greater than 95% of the extracted 3H-oxytocin remained intact at all temperatures and incubation times (data not shown). After five freeze/thaw cycles, 87% of the oxytocin remained intact. These data indicate that these temperature conditions and extraction procedures had minimal effects on oxytocin stability.
Measurement of plasma oxytocin by EIA and RIA in extracted and unextracted samples
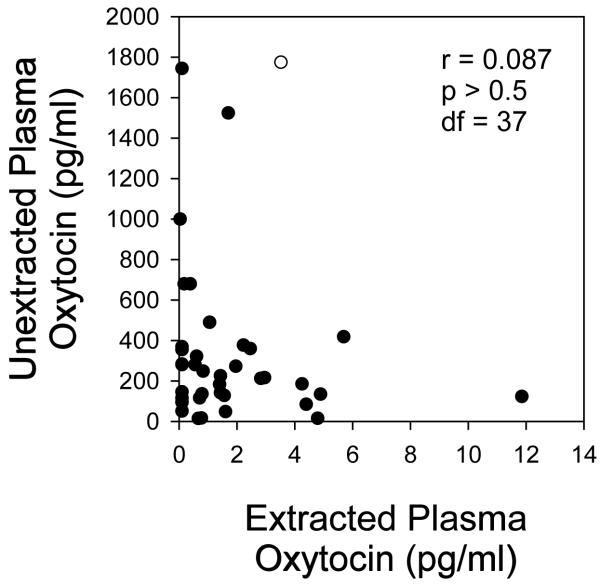
Oxytocin levels were compared between extracted and unextracted plasma from the same samples (n = 39) using EIA and RIA. For EIA, mean values were 1.8 ± 0.4 pg/ml in extracted samples and 358 ± 70 pg/ml in unextracted samples (p < 0.01 between groups). Oxytocin values measured in extracted samples did not correlate with values in unextracted samples by EIA using Pearson’s product moment correlation (r = 0.09, p = 0.60; Figure 1). Log transformation of the data did not result in a significant correlation (r = −0.14, p = 0.40). Given the non-normal distribution of values generated for extracted and unextracted samples, the data were also analyzed using nonparametric methods (Spearman rank correlation), which also revealed no significant association (r = −0.10, p = 0.54). By RIA, plasma oxytocin levels in 22 of 25 extracted samples and 19 of 25 unextracted samples had values below the limit of detection (<1.0 pg/tube). Thus, the RIA lacked sufficient sensitivity in more than 90% of samples to measure plasma oxytocin levels even after extraction and the resulting 10-fold concentration of the sample.
Figure 1.
Comparison of oxytocin levels in plasma (n = 39) with and without extraction. One milliliter of plasma was extracted using 200 mg C18 Sep-Pak columns as described in the Methods. Oxytocin levels in plasma (diluted 1:4 before assay) and the corresponding extract were assayed by the EIA method, and the results are expressed as pg/mL of the original plasma volume. Five of 39 samples had values below the limit of detection and were assigned a value of 0.1 pg/mL for the statistical analysis. Correlation was determined using the Pearson correlation coefficient.
Spike-and-recovery experiments were performed to determine whether analyte detection is affected by the biological sample matrix (in this case plasma) and analytical recovery in samples not extracted and to evaluate the effects of sample extraction on analytical recovery. To evaluate the accuracy of the measurement and to obtain concentrations measurable by both immunoassays, plasma samples were spiked with 50, 25 and 12.5 pg/ml oxytocin prior to assay with and without sample extraction. By EIA, spiking resulted in a linear increase in oxytocin concentrations (y = 1.16x − 2.51, r = 0.98, p < 0.02, where y is the measured oxytocin value in and x is the expected oxytocin value based on the added spike and recovery of the spike was 97 ± 6% in extracted samples. In unextracted, diluted plasma, spiking the samples resulted in a linear increase in oxytocin concentrations (y = 17.9x + 218, r = 0.98, p < 0.02), but in contrast to extracted samples, recovery of the spike was 364 ± 108%. Using RIA, spiking resulted in a linear increase in plasma oxytocin concentrations after solid phase extraction (y = 1.2x − 6.2, r = 0.99, p < 0.01), and recovery of the immunoreactivity was 85 ± 10%. In unextracted samples, there was no linear increase in oxytocin concentrations (y = −0.15x + 22.7, r = 0.33, p = 0.70) and recovery of the spike was 18.8 ± 9%. In spiked extracted samples that provided values in both EIA and RIA assays, results were highly correlated (r = 0.98, p < 0.01). These data demonstrate the linearity and accuracy of the measurements in extracted samples by both methods. Unextracted samples resulted in over-recovery of spiked oxytocin by EIA and under-recovery by RIA indicative of sample matrix effects affecting the measurement.
Fractionation of samples and characterization of immunoreactive products
As mentioned, observed oxytocin values were orders of magnitude higher in unextracted samples as opposed to extracted samples, which suggested the presence of non-oxytocin immunoreactive species that were removed by extraction. In order to evaluate the immunospecificity of the EIA, samples were subjected to chromatographic fractionation and immunoassay of the separated fractions. Using gel filtration chromatography, which separates molecules based upon size, at least three distinct peaks of immunoreactivity were observed in unextracted plasma (Figure 2A). Only the third peak eluted in the region that coincided with authentic oxytocin (MW=1009), and this peak accounted for approximately 55% of the total immunoreactivity. The same plasma was subjected to solid phase extraction followed by the same chromatography and immunoassay. After extraction, only a single peak of immunoreactivity, coincident with the elution of authentic oxytocin, was detected (Figure 2B). It is worth noting that this peak accounted for 20 to 40% of the immunoreactivity seen in the third peak of the unextracted sample, suggesting that extraction removed over 60% of the immunoreactivity in this “oxytocin peak”. These data demonstrate that immunoreactivity with molecular mass greater than that of oxytocin is present in plasma and contributes to the high values reported in unextracted plasma samples.
Figure 2.
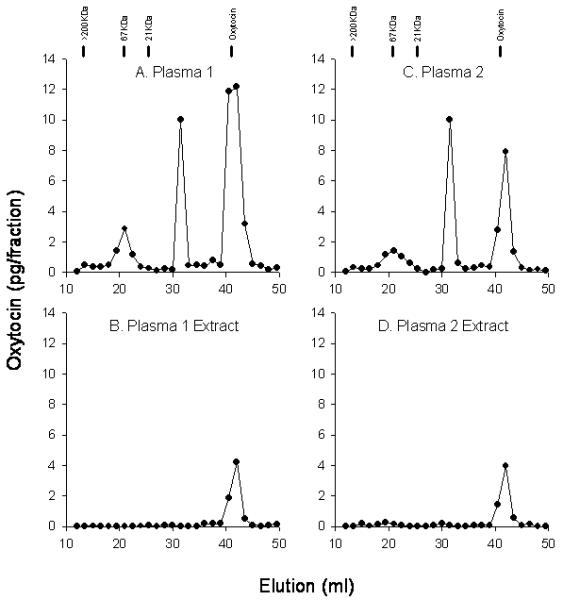
Oxytocin immunoreactivity in plasma and plasma extracts after gel filtration chromatography. Plasma (400 μL, A and C) or extracts of the same samples (B and D) were applied to a Superdex 75 10/300 GL column and eluted with 5-mM ammonium acetate buffer (pH 7.8) at a flow rate of 1.0 mL/min, and 1.5-mL fractions were collected. After collection, the fractions were lyophilized then reconstituted and assayed for oxytocin immunoreactivity by EIA. Samples 1 and 2 had plasma oxytocin levels of 442 and 281 pg/mL, respectively, and after extraction, 15.2 and 6.1 pg/mL, respectively. Elution of MW markers and of oxytocin is indicated above A and C.
To further evaluate the nature of the immunoreactive species present in the extracted samples, plasma extracts were subjected to reverse phase HPLC, which separates molecules based on their hydrophobicity, and the collected fractions tested for oxytocin by EIA (Figure 3). In three different plasma samples subjected to HPLC fractionation, two or three distinct peaks of oxytocin immunoreactivity were detected by EIA. In all cases, the peak that co-eluted with the position of authentic oxytocin was not the major peak of immunoreactivity. Oxytocin, based on elution position, accounted for only 25%, 15%, and 7% of the total immunoreactivity (Figure 3A, 3B, and 3C, respectively). Samples subjected to extraction with organic solvent yielded HPLC profiles with similar multiple immunoreactive peaks (data not shown). In two other samples analyzed by RIA following HPLC separation, multiple peaks of oxytocin immunity reactivity were again observed, and only 22% or 43% of the total immunoreactivity co-eluted with authentic oxytocin (Figure 3D, 3E, respectively).
Figure 3.
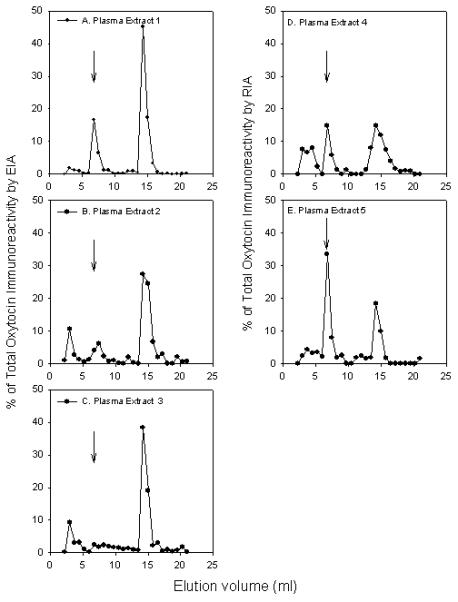
Oxytocin immunoreactivity profile in plasma extracts after high-pressure liquid chromatography fractionation. Plasma extracts of different samples were performed as described in the Methods. After collection, the fractions were lyophilized then reconstituted and assayed for oxytocin immunoreactivity by EIA (A–C) or RIA (D and E). Samples 1, 2, and 3 had plasma oxytocin levels of 15.5, 10.1, and 3.4 pg/mL, respectively. Samples 4 and 5 had plasma oxytocin levels of 13.3 and 4.1 pg/mL, respectively. Arrows indicate elution of the oxytocin standard.
Discussion
The results of the current study raise serious concerns regarding the use of commercially available assays for the measurement of plasma oxytocin. Assay results following chromatographic fractionation of unextracted plasma show the presence of multiple immunoreactive species. Extraction resulted in removal of larger molecular weight species that accounted for about half of the total immunoreactivity, as demonstrated by the gel filtration profile. In addition, oxytocin immunoreactivity of fractions eluting with the molecular size of oxytocin was reduced by 57-78% after extraction. While the reduction of oxytocin immunoreactivity from this peak varied between samples, these data suggest that there are also small molecular weight substances in plasma that contribute to the oxytocin immunoreactivity and are removed by extraction. Additional fractionation of extracted samples by HPLC, which separates molecules based on hydrophobicity and not size, clearly showed that the majority of immunoreactivity did not coincide with authentic oxytocin, and in the worst case the non-oxytocin peaks represented greater than 93% of the total immunoreactivity.
Preliminary studies from our laboratory examining oxytocin degradation suggest that at least one of the immunoreactive peaks identified by HPLC is consistent with an oxytocinase degradation product (unpublished observation). Results from several studies have shown that social and behavioral manipulations result in changes in plasma oxytocin immunoreactivity using the methods evaluated in this study and in the absence of sample extraction. It is possible that what is being measured is both plasma oxytocin and oxytocin degradation products, and that the degradation products contribute to the measured changes in circulating oxytocin levels. Given the short half-life of plasma oxytocin (3 to 6 minutes, (15)) it may be reasonable to assume that degradation products are more stable than the parent oxytocin, may accumulate in plasma, and are amenable to measurement. Clearly, the identity of the immunoreactive molecules and the specificity of the antibodies used in the immunoassays need to be elucidated if these assumptions are to be supported.
The present study suggests there are several other issues important in the measurement of plasma oxytocin. Two commercially available assays for plasma oxytocin were compared and it was found that sample extraction (strongly recommended by both manufacturers) is a critical variable for accurate measurement. Without extraction, plasma oxytocin levels by EIA were more than 100-fold higher compared to the same extracted sample, and there was no correlation between oxytocin levels in extracted and unextracted plasma. The effect of extraction was also evident in samples measured by RIA where unextracted plasma gave values that were at least 10-fold higher than the extracted sample.
The commercially available RIA lacks sensitivity to measure basal plasma oxytocin, resulting in values below the level of detection with or without extraction (<10% samples had detectable levels). In a recent study from our laboratory (13) using extracted human plasma samples, 49% of females and 67% of males had oxytocin values below the limit of detection by RIA. In another study that used the same RIA, greater than 6 ml of plasma had to be extracted in order to improve detection limits, which resulted in measured oxytocin levels between 0.1 and 4.6 pg/ml (16). In contrast, the present study showed less than 5% of the samples measured by EIA had undetectable levels.
In summary, the current study compared two commercially available immunoassays for the quantification of plasma oxytocin levels. Our data showed the importance of sample extraction for these measurements, and assessed the relative sensitivity between the assays. Importantly, further analysis showed the presence of multiple immunoreactive products in addition to oxytocin in sample extracts, which casts doubts on the specificity of the assays. Although the current study only examined oxytocin immunoreactivity in plasma samples, several published reports have assessed oxytocin in other biological fluids (e.g., saliva (17, 18), urine (19, 20)), and the validity of these methods has also been challenged (21-23). Given the increased interest and important role of this peptide in mammalian behavior, physiology and disease, there is a critical need to establish valid and reliable methods to measure oxytocin in plasma and other biological fluids.
Acknowledgments
This study was supported, in part, by Grants HL-36588 and HL-04726 from the National Heart, Lung, and Blood Institute and the National Institutes of Health.
Abbreviations
- EIA
enzyme immunoassay
- RIA
radioimmunoassay
- HPLC
high performance liquid chromatography
- SEM
standard error of the mean
- MW
molecular weight
Footnotes
At the time the study was conducted, the two oxytocin assay methods evaluated were the only commercially available kits on the market. Since that time, the manufacturer of the RIA kit (Phoenix Pharmaceuticals) has marketed two ELISA format kits that were not evaluated in this study.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23(8):779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol. 2002;23(2):200–24. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- 3.Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2(2):129–36. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 4.Light KC, Grewen KM, Amico JA. More frequent partner hugs and higher oxytocin levels are linked to lower blood pressure and heart rate in premenopausal women. Biological PsychologyCurrent Trends in Women’s Health Research. 2005;69(1):5–21. doi: 10.1016/j.biopsycho.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Neumann ID. Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2002;139:147–62. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- 6.Uvnas-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23(8):819–35. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- 7.Windle RJ, Shanks N, Lightman SL, Ingram CD. Central Oxytocin Administration Reduces Stress-Induced Corticosterone Release and Anxiety Behavior in Rats. Endocrinology. 1997;138(7):2829–34. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- 8.Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40(2):133–8. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- 9.Zak PJ, Kurzban R, Matzner WT. Oxytocin is associated with human trustworthiness. Hormones and Behavior. 2005;48(5):522–7. doi: 10.1016/j.yhbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Amico JA, Seif SM, Robinson AG. Oxytocin in human plasma: correlation with neurophysin and stimulation with estrogen. J Clin Endocrinol Metab. 1981;52(5):988–93. doi: 10.1210/jcem-52-5-988. [DOI] [PubMed] [Google Scholar]
- 11.Grewen KM, Davenport RE, Light KC. An investigation of plasma and salivary oxytocin responses in breast- and formula-feeding mothers of infants. Psychophysiology. 2010;47(4):625–32. doi: 10.1111/j.1469-8986.2009.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salonia A, Nappi RE, Pontillo M, Daverio R, Smeraldi A, Briganti A, Fabbri F, Zanni G, Rigatti P, Montorsi F. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm Behav. 2005;47(2):164–9. doi: 10.1016/j.yhbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Tabak BA, McCullough ME, Szeto A, Mendez AJ, McCabe PM. Oxytocin indexes relational distress following interpersonal harms in women. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2010.07.004. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer KM, Cushing BS, Carter CS, Wu J, Ottinger MA. Sex and species differences in plasma oxytocin using an enzyme immunoassay. Canad J Zool. 2004;82(8):1194–200. [Google Scholar]
- 15.Fabian M, Forsling ML, Jones JJ, Pryor JS. The clearance and antidiuretic potency of neurohypophysial hormones in man, and their plasma binding and stability. The Journal of Physiology. 1969;204(3):653–68. doi: 10.1113/jphysiol.1969.sp008937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marazziti D, Dell’Osso B, Baroni S, Mungai F, Catena M, Rucci P, Albanese F, Giannaccini G, Betti L, Fabbrini L, Italiani P, Del Debbio A, Lucacchini A, Dell’Osso L. A relationship between oxytocin and anxiety of romantic attachment. Clin Pract Epidemol Ment Health. 2006;2:28. doi: 10.1186/1745-0179-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent-infant contact. Psychoneuroendocrinology. 2010;35(8):1133–41. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Holt-Lunstad J, Birmingham WA, Light KC. Influence of a “warm touch” support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosom Med. 2008;70(9):976–85. doi: 10.1097/PSY.0b013e318187aef7. [DOI] [PubMed] [Google Scholar]
- 19.Fries ABW, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. From The Cover: Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. PNAS. 2005;102(47):17237–40. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polito AB3, Goldstein DL, Sanchez L, Cool DR, Morris M. Urinary oxytocin as a non-invasive biomarker for neurohypophyseal hormone secretion. Peptides. 2006;27(11):2877–84. doi: 10.1016/j.peptides.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Horvat-Gordon M, Granger DA, Schwartz EB, Nelson VJ, Kivlighan KT. Oxytocin is not a valid biomarker when measured in saliva by immunoassay. Physiology & Behavior. 2005;84(3):445–8. doi: 10.1016/j.physbeh.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Anderson G. Report of Altered Urinary Oxytocin and AVP Excretion in Neglected Orphans should be Reconsidered. Journal of Autism and Developmental Disorders. 2006;36(6):829–30. doi: 10.1007/s10803-006-0153-7. [DOI] [PubMed] [Google Scholar]
- 23.Young SN, Anderson GN. Bioanalytical inaccuracy: a threat to the integrity and efficiency of research. J Psychiatry Neurosci. 2010;35(1):3–6. doi: 10.1503/jpn.090171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Billhult A, Lindholm C, Gunnarsson R, Stener-Victorin E. The effect of massage on cellular immunity, endocrine and psychological factors in women with breast cancer -- a randomized controlled clinical trial. Auton Neurosci. 2008;140(1-2):88–95. doi: 10.1016/j.autneu.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Bello D, White-Traut R, Schwertz D, Pournajafi-Nazarloo H, Carter CS. An exploratory study of neurohormonal responses of healthy men to massage. J Altern Complement Med. 2008;14(4):387–94. doi: 10.1089/acm.2007.0660. [DOI] [PubMed] [Google Scholar]
- 26.Goldman M, Marlow-O’Connor M, Torres I, Carter CS. Diminished plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophr Res. 2008;98(1-3):247–55. doi: 10.1016/j.schres.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon I, Zagoory-Sharon O, Schneiderman I, Leckman JF, Weller A, Feldman R. Oxytocin and cortisol in romantically unattached young adults: associations with bonding and psychological distress. Psychophysiology. 2008;45(3):349–52. doi: 10.1111/j.1469-8986.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 28.White-Traut R, Watanabe K, Pournajafi-Nazarloo H, Schwertz D, Bell A, Carter CS. Detection of salivary oxytocin levels in lactating women. Dev Psychobiol. 2009;51(4):367–73. doi: 10.1002/dev.20376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor SE, Saphire-Bernstein S, Seeman TE. Are Plasma Oxytocin in Women and Plasma Vasopressin in Men Biomarkers of Distressed Pair-Bond Relationships? Psychological Science. 2010;21(1):3–7. doi: 10.1177/0956797609356507. [DOI] [PubMed] [Google Scholar]
- 30.Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28(5):910–8. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- 31.Devarajan K, Rusak B. Oxytocin levels in the plasma and cerebrospinal fluid of male rats: effects of circadian phase, light and stress. Neuroscience Letters. 2004;367(2):144–7. doi: 10.1016/j.neulet.2004.05.112. [DOI] [PubMed] [Google Scholar]
- 32.Thompson MR, Callaghan PD, Hunt GE, Cornish JL, McGregor IS. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”) Neuroscience. 2007;146(2):509–14. doi: 10.1016/j.neuroscience.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 33.Elabd C, Basillais A, Beaupied H, Breuil V, Wagner N, Scheideler M, Zaragosi LE, Massiera F, Lemichez E, Trajanoski Z, Carle G, Euller-Ziegler L, Ailhaud G, Benhamou CL, Dani C, Amri EZ. Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis. Stem Cells. 2008;26(9):2399–407. doi: 10.1634/stemcells.2008-0127. [DOI] [PubMed] [Google Scholar]
- 34.Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, Shnayder NA, Yamada K, Noda M, Seike T, Fujita K, Takasawa S, Yokoyama S, Koizumi K, Shiraishi Y, Tanaka S, Hashii M, Yoshihara T, Higashida K, Islam MS, Yamada N, Hayashi K, Noguchi N, Kato I, Okamoto H, Matsushima A, Salmina A, Munesue T, Shimizu N, Mochida S, Asano M, Higashida H. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446(7131):41–5. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- 35.Trainor BC, Takahashi EY, Silva AL, Crean KK, Hostetler C. Sex differences in hormonal responses to social conflict in the monogamous California mouse. Horm Behav. 2010;58(3):506–12. doi: 10.1016/j.yhbeh.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C, Levin H. Plasma oxytocin levels in autistic children. Biol Psychiatry. 1998;43(4):270–7. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- 37.Chicharro JL, Hoyos J, Bandrés F, Gallego F Gómez, Pérez M, Lucía A. Plasma Oxytocin during Intense Exercise in Professional Cyclists. Hormone Research. 2001;55(3):155–9. doi: 10.1159/000049988. [DOI] [PubMed] [Google Scholar]
- 38.Green L, Fein D, Modahl C, Feinstein C, Waterhouse L, Morris M. Oxytocin and autistic disorder: alterations in peptide forms. Biol Psychiatry. 2001;50(8):609–13. doi: 10.1016/s0006-3223(01)01139-8. [DOI] [PubMed] [Google Scholar]
- 39.Turner RA, Altemus M, Yip DN, Kupferman E, Fletcher D, Bostrom A, Lyons DM, Amico JA. Effects of emotion on oxytocin, prolactin, and ACTH in women. Stress. 2002;5(4):269–76. doi: 10.1080/1025389021000037586-1. [DOI] [PubMed] [Google Scholar]
- 40.Lee R, Garcia F, Van de Kar LD, Hauger RD, Coccaro EF. Plasma oxytocin in response to pharmaco-challenge to D-fenfluramine and placebo in healthy men. Psychiatry Res. 2003;118(2):129–36. doi: 10.1016/s0165-1781(03)00070-2. [DOI] [PubMed] [Google Scholar]
- 41.Uckert S, Becker AJ, Ness BO, Stief CG, Scheller F, Knapp WH, Jonas U. Oxytocin plasma levels in the systemic and cavernous blood of healthy males during different penile conditions. World J Urol. 2003;20(6):323–6. doi: 10.1007/s00345-002-0300-5. [DOI] [PubMed] [Google Scholar]
- 42.Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom Med. 2005;67(4):531–8. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- 43.Jansen LM, Gispen-de Wied CC, Wiegant VM, Westenberg HG, Lahuis BE, van Engeland H. Autonomic and neuroendocrine responses to a psychosocial stressor in adults with autistic spectrum disorder. J Autism Dev Disord. 2006;36(7):891–9. doi: 10.1007/s10803-006-0124-z. [DOI] [PubMed] [Google Scholar]
- 44.Wolff K, Tsapakis EM, Winstock AR, Hartley D, Holt D, Forsling ML, Aitchison KJ. Vasopressin and oxytocin secretion in response to the consumption of ecstasy in a clubbing population. J Psychopharmacol. 2006;20(3):400–10. doi: 10.1177/0269881106061514. [DOI] [PubMed] [Google Scholar]
- 45.Tops M, van Peer JM, Korf J, Wijers AA, Tucker DM. Anxiety, cortisol, and attachment predict plasma oxytocin. Psychophysiology. 2007;44(3):444–9. doi: 10.1111/j.1469-8986.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 46.Cyranowski JM, Hofkens TL, Frank E, Seltman H, Cai HM, Amico JA. Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosom Med. 2008;70(9):967–75. doi: 10.1097/PSY.0b013e318188ade4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35(1):83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 48.Parker KJ, Kenna HA, Zeitzer JM, Keller J, Blasey CM, Amico JA, Schatzberg AF. Preliminary evidence that plasma oxytocin levels are elevated in major depression. Psychiatry Res. 2010;178(2):359–62. doi: 10.1016/j.psychres.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris M, STEVENS SW, Adams MR. Plasma Oxytocin During Pregnancy and Lactation in the Cynomolgus Monkey. Biol Reprod. 1980;23(4):782–7. doi: 10.1095/biolreprod23.4.782. [DOI] [PubMed] [Google Scholar]
- 50.Hopster H, Bruckmaier RM, Van der Werf JTN, Korte SM, Macuhova J, Korte-Bouws G, van Reenen CG. Stress Responses during Milking; Comparing Conventional and Automatic Milking in Primiparous Dairy Cows. J Dairy Sci. 2002;85(12):3206–16. doi: 10.3168/jds.S0022-0302(02)74409-3. [DOI] [PubMed] [Google Scholar]
- 51.Paredes J, Szeto A, Levine JE, Zaias J, Gonzales JA, Mendez AJ, Llabre MM, Schneiderman N, McCabe PM. Social experience influences hypothalamic oxytocin in the WHHL rabbit. Psychoneuroendocrinology. 2006;31(9):1062–75. doi: 10.1016/j.psyneuen.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 52.Morris M. Neurohypophyseal response to dehydration in the spontaneously hypertensive rat. Hypertension. 1982;4(1):161–6. doi: 10.1161/01.hyp.4.1.161. [DOI] [PubMed] [Google Scholar]
- 53.Windle RJ, Forsling ML. Variations in oxytocin secretion during the 4-day oestrous cycle of the rat. J Endocrinol. 1993;136(2):305–11. doi: 10.1677/joe.0.1360305. [DOI] [PubMed] [Google Scholar]
- 54.Engelmann M, Ebner K, Landgraf R, Holsboer F, Wotjak CT. Emotional stress triggers intrahypothalamic but not peripheral release of oxytocin in male rats. J Neuroendocrinol. 1999;11(11):867–72. doi: 10.1046/j.1365-2826.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- 55.Petersson M, Eklund M, Uvnas-Moberg K. Oxytocin decreases corticosterone and nociception and increases motor activity in OVX rats. Maturitas. 2005;51(4):426–33. doi: 10.1016/j.maturitas.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 56.O’Byrne KT, Ring JP, Summerlee AJ. Plasma oxytocin and oxytocin neurone activity during delivery in rabbits. J Physiol. 1986;370:501–13. doi: 10.1113/jphysiol.1986.sp015947. [DOI] [PMC free article] [PubMed] [Google Scholar]