Abstract
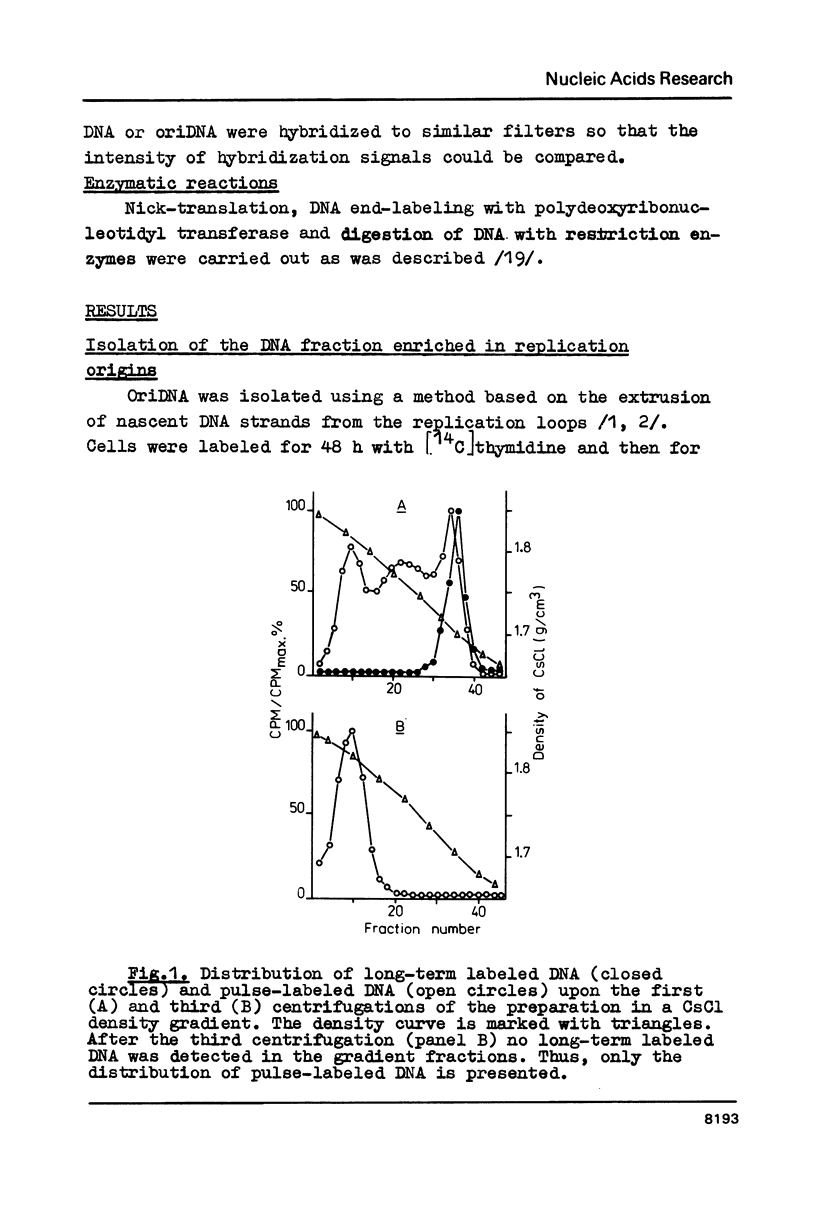
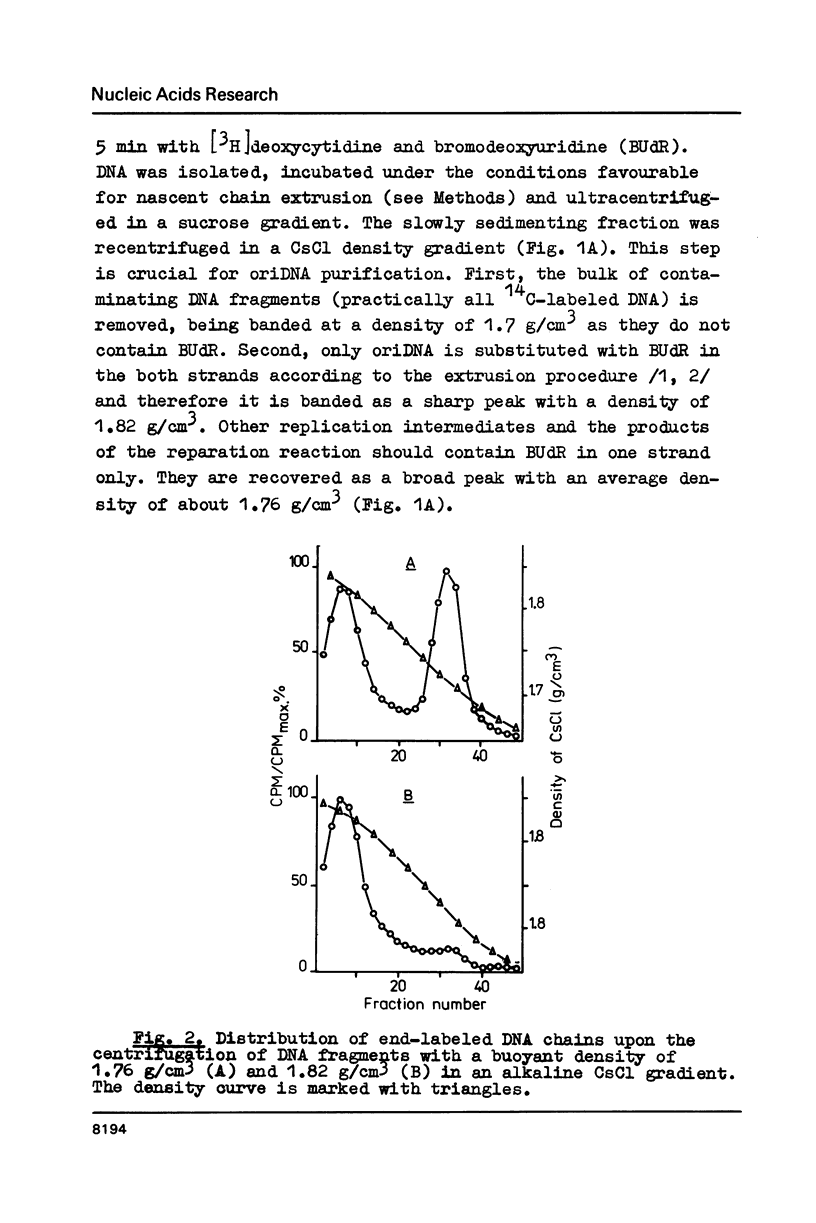
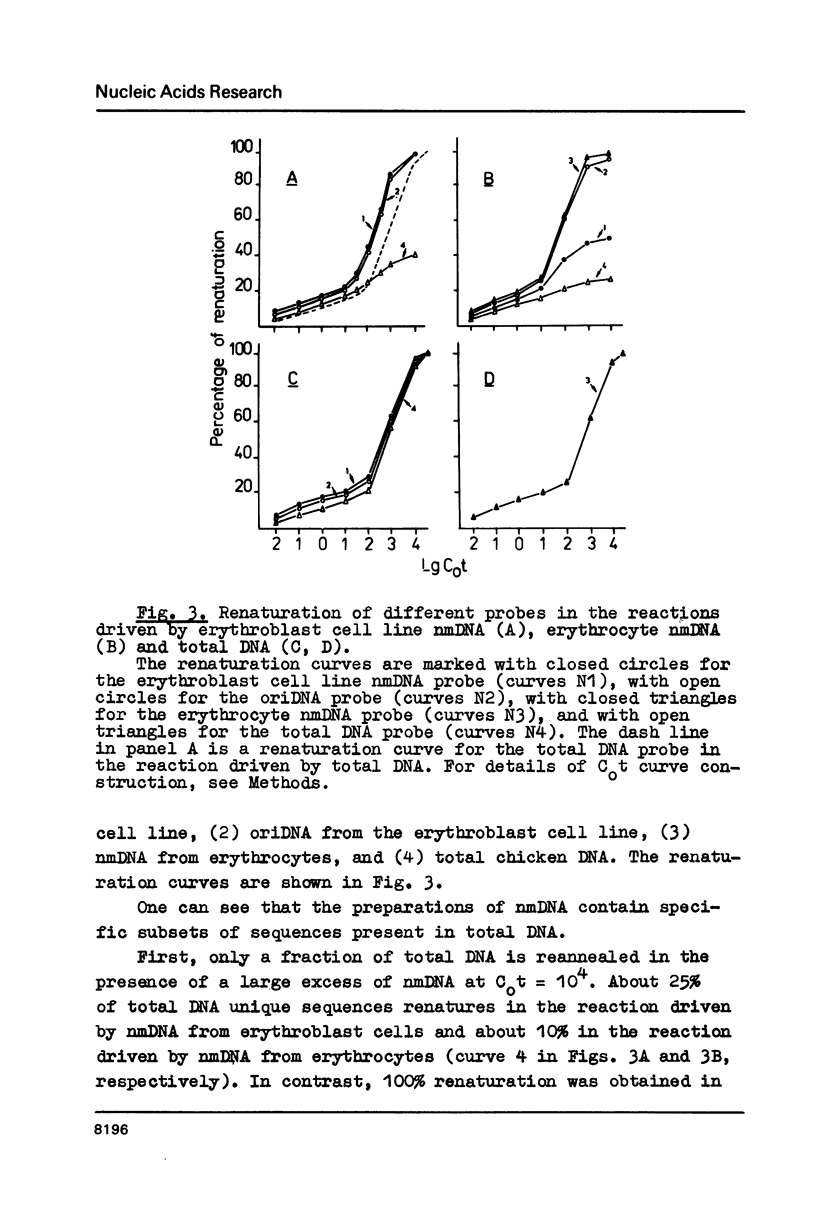
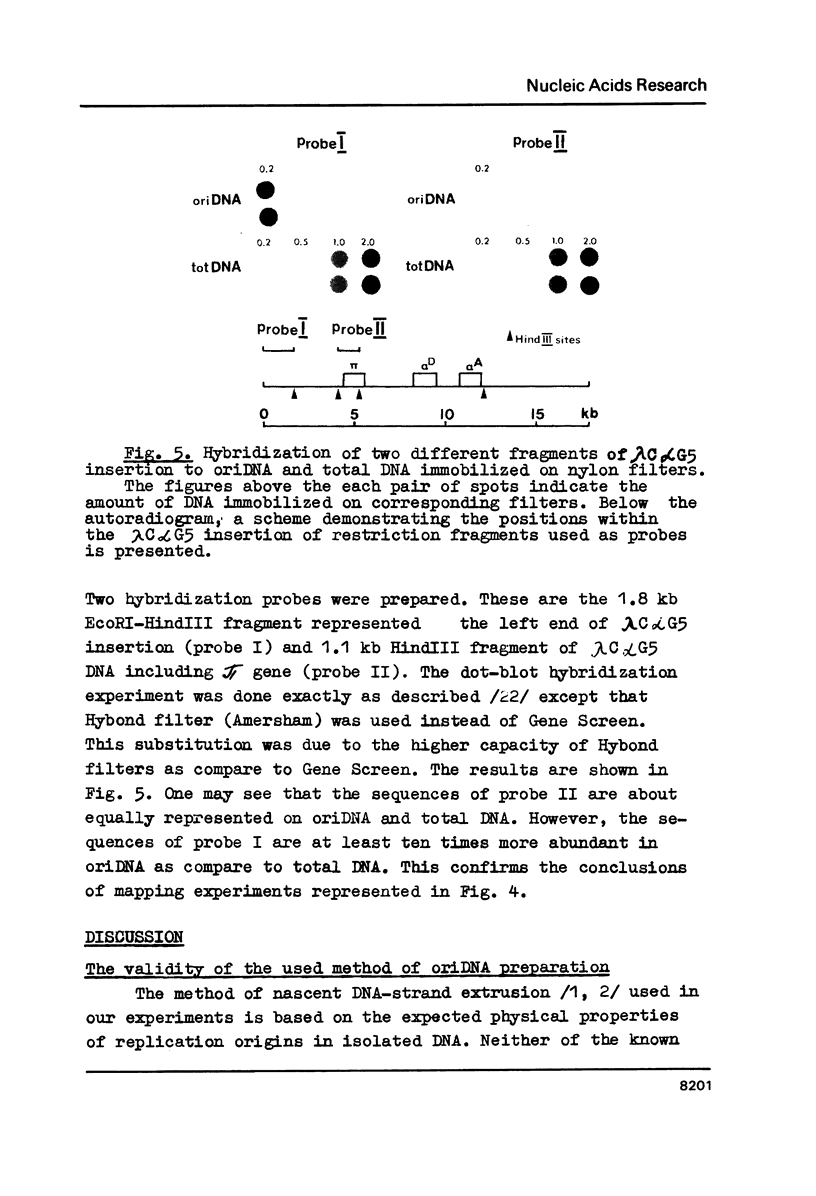
DNA fragments containing replication origins (oriDNA) were isolated from a chicken erythroblast cell line by a modified procedure of Zannis-Hadjopoulos et al. and studied in the renaturation reaction driven by either total or nuclear matrix DNA (nmDNA) from the same cells or from mature erythrocytes. We found that the unique sequences of nmDNA from erythroblasts (5 kb long) represented a specific subset of sequences constituting about a quarter of total DNA unique sequences, while the erythrocyte nmDNA 5 kb fragments constitute only about one tenth of total unique DNA and all are recovered among erythroblast nmDNA. Virtually all oriDNA sequences are present in the fraction of erythrocyte nmDNA. Thereafter, the putative positions of replication origins within the alpha-globine gene domain have been mapped by hybridization experiments. They were found to coincide with the previously established positions of permanent sites of DNA attachment to the nuclear matrix.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aelen J. M., Opstelten R. J., Wanka F. Organization of DNA replication in Physarum polycephalum. Attachment of origins of replicons and replication forks to the nuclear matrix. Nucleic Acids Res. 1983 Feb 25;11(4):1181–1195. doi: 10.1093/nar/11.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aelen J. M., Wanka F. Newly-initiated DNA isolated from Physarum in early S phase consists of nascent-nascent duplexes. Exp Cell Res. 1984 Oct;154(2):394–401. doi: 10.1016/0014-4827(84)90163-0. [DOI] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Identification of a nuclear protein matrix. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1410–1417. doi: 10.1016/0006-291x(74)90355-6. [DOI] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Nuclear matrix. Isolation and characterization of a framework structure from rat liver nuclei. J Cell Biol. 1977 Jun;73(3):616–637. doi: 10.1083/jcb.73.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P. R., Lang J. The spatial organization of sequences involved in initiation and termination of eukaryotic DNA replication. Nucleic Acids Res. 1984 Jan 25;12(2):1069–1075. doi: 10.1093/nar/12.2.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. D., Dodgson J. B. Analysis of the closely linked adult chicken alpha-globin genes in recombinant DNAs. Proc Natl Acad Sci U S A. 1980 May;77(5):2596–2600. doi: 10.1073/pnas.77.5.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D., Davies N. D., Bryant J. A., Hughes S. G., Sibson D. R., Fitchett P. N. Effects of psoralen on replicon size and mean rate of DNA synthesis in partially synchronized cells of Pisum sativum L. Exp Cell Res. 1985 Jun;158(2):500–508. doi: 10.1016/0014-4827(85)90473-2. [DOI] [PubMed] [Google Scholar]
- Gasser S. M., Laemmli U. K. The organisation of chromatin loops: characterization of a scaffold attachment site. EMBO J. 1986 Mar;5(3):511–518. doi: 10.1002/j.1460-2075.1986.tb04240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. A., McCready S. J., Cook P. R. RNA is synthesized at the nuclear cage. Nature. 1981 Aug 6;292(5823):552–555. doi: 10.1038/292552a0. [DOI] [PubMed] [Google Scholar]
- McCready S. J., Cook P. R. Lesions induced in DNA by ultraviolet light are repaired at the nuclear cage. J Cell Sci. 1984 Aug;70:189–196. doi: 10.1242/jcs.70.1.189. [DOI] [PubMed] [Google Scholar]
- Mirkovitch J., Mirault M. E., Laemmli U. K. Organization of the higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell. 1984 Nov;39(1):223–232. doi: 10.1016/0092-8674(84)90208-3. [DOI] [PubMed] [Google Scholar]
- Razin S. V., Mantieva V. L., Georgiev G. P. The similarity of DNA sequences remaining bound to scaffold upon nuclease treatment of interphase nuclei and metaphase chromosomes. Nucleic Acids Res. 1979 Nov 24;7(6):1713–1735. doi: 10.1093/nar/7.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. V., Rzeszowska-Wolny J., Moreau J., Scherrer K. Lokalizatsiia uchastkov prikrepleniia DNK k iadernomu skeletu v ramkakh domena alpha-globinovykh genov kur v funktsional'no aktivnykh i funktsional'no neaktivnykh iadrakh. Mol Biol (Mosk) 1985 Mar-Apr;19(2):456–466. [PubMed] [Google Scholar]
- Razin S. V., Yarovaya O. V., Georgiev G. P. Low ionic strength extraction of nuclease-treated nuclei destroys the attachment of transcriptionally active DNA to the nuclear skeleton. Nucleic Acids Res. 1985 Oct 25;13(20):7427–7444. doi: 10.1093/nar/13.20.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russev G., Vassilev L. Isolation of a DNA fraction from Ehrlich ascites tumour cells containing the putative origin of replication. J Mol Biol. 1982 Oct 15;161(1):77–87. doi: 10.1016/0022-2836(82)90279-0. [DOI] [PubMed] [Google Scholar]
- Small D., Nelkin B., Vogelstein B. The association of transcribed genes with the nuclear matrix of Drosophila cells during heat shock. Nucleic Acids Res. 1985 Apr 11;13(7):2413–2431. doi: 10.1093/nar/13.7.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren A. C., Cook P. R. Supercoiling of DNA and nuclear conformation during the cell-cycle. J Cell Sci. 1978 Apr;30:211–226. doi: 10.1242/jcs.30.1.211. [DOI] [PubMed] [Google Scholar]
- Zannis-Hadjopoulos M., Chepelinsky A. B., Martin R. G. Mapping of the 3'-end positions of simian virus 40 nascent strands. J Mol Biol. 1983 Apr 25;165(4):599–607. doi: 10.1016/s0022-2836(83)80269-1. [DOI] [PubMed] [Google Scholar]
- Zannis-Hadjopoulos M., Persico M., Martin R. G. The remarkable instability of replication loops provides a general method for the isolation of origins of DNA replication. Cell. 1981 Nov;27(1 Pt 2):155–163. doi: 10.1016/0092-8674(81)90369-x. [DOI] [PubMed] [Google Scholar]
- van der Velden H. M., van Willigen G., Wetzels R. H., Wanka F. Attachment of origins of replication to the nuclear matrix and the chromosomal scaffold. FEBS Lett. 1984 Jun 4;171(1):13–16. doi: 10.1016/0014-5793(84)80451-2. [DOI] [PubMed] [Google Scholar]