Abstract
Objectives
New-onset diabetes mellitus (DM) may herald pancreatic cancer (PaC). We determined if changes in body weight distinguished PaC-associated DM (PaCDM) from type 2 DM.
Methods
Amongst Olmsted County residents, we identified 29 PaCDM and 43 type 2 DM subjects who had serial fasting blood glucose (FBG) measurements, new-onset DM and no cancer-specific symptoms at DM onset. We compared body weight (kg) and FBG (mg/dl) at DM onset, 1–2 years prior and at index date in the two groups.
Results
FBG values were similar prior to and at the onset of DM. Prior to onset of DM, PaCDM and type 2 DM had similar body weight (P=.80). However, at onset of DM 59% of PaCDM lost weight vs. 30% of type 2 DM (P =.02). At onset of DM, 56% of type 2 DM gained weight vs. 31% of PaCDM (P=.04). By index date, PaCDM lost more weight than type 2 DM (8.3±8.3 vs. 0.8±4.8 kg,P<.01).
Conclusions
While new-onset primary type 2 DM is typically associated with weight gain, weight loss frequently precedes onset of PaCDM. The paradoxical development of diabetes in the face of ongoing weight loss may be an important clue to understanding the pathogenesis of PaCDM.
Keywords: Pancreatic cancer, New-onset diabetes mellitus, zinc alpha-2 glycoprotein
INTRODUCTION
Most patients with pancreatic cancer (PaC) lack disease-specific symptoms until late in the course of the disease, resulting in late presentation and a dismal 5 year survival rate.1 To effectively screen for PaC, the disease must be detected in the asymptomatic state. Since PaC is relatively uncommon, screening would have to be done in populations enriched for PaC, i.e, high risk groups. There is accumulating evidence suggesting that patients with new-onset DM have an increased likelihood of having PaC. In a population-based study from Olmsted County we showed that compared to the general population, subjects with new-onset DM (≤36 months) have an 8 times higher likelihood of being diagnosed with PaC within 3 years of meeting criteria for DM.2 Others have targeted selected subjects with recently diagnosed DM for screening and found an even higher prevalence of PaC (5.2 to 13.6%).3, 4 In a recent study we found that at the onset of DM the cancer is often resectable.5 Thus, the collective weight of evidence from these and other studies suggests that recognition of new-onset DM as an early manifestation of PaC could lead to diagnosis of asymptomatic (presumably early) sporadic PaC.
However, type 2 DM is common and PaC-associated DM (PaCDM) relatively rare. The strategy to use new-onset DM to diagnose early PaC will succeed only if we can clearly distinguish new-onset PaCDM from new-onset type 2 DM. Non-invasive imaging techniques, such as abdominal CT scans, are unlikely to have sufficient sensitivity to detect PaC in asymptomatic subjects with new-onset DM. This is based on the results of two studies in which PaC was typically not detected on CT scans performed 6 months or more prior to formal diagnosis.5, 6 Therefore, detection and confirmation of the presence of PaC in the screening setting (i.e., in asymptomatic subjects) will require invasive tests such as endoscopic ultrasonography (EUS). Screening all individuals with new-onset diabetes for PaC using invasive tests is unlikely to be cost-effective as the prevalence of PaC in this population is <1%.2
Since there are as yet no serum biomarkers that can distinguish these two forms of DM, we studied their clinical profiles to identify clinical clues that can help distinguish them. It has been suggested that absence of traditional factors for DM (family history of DM or obesity) are clinical clues to the presence of PaC in new-onset DM. however, in previous studies of all subjects with DM (long-standing and new-onset) we found that PaCDM and type 2 DM could not be distinguished on family history of DM or pre-morbid BMI.7–9
We had previously noted that PaCDM subjects lost more weight than PaC patients without DM.8 In another recent study we showed greater weight loss in PaC versus controls; however, the relationship of weight loss to onset of DM could not be made as most patients in the cohort did not have serial weights or FBGs.10 In these studies it was unclear if the weight loss occurs with other cancer-related symptoms (i.e., late onset) or precedes abdominal pain and symptoms of cachexia, such as fatigue, muscle wasting and anorexia.
From our large database of cases and controls from previous and ongoing studies in Olmsted County we identified PaCDM and type 2 DM subjects who had confirmed new-onset DM (DM duration ≤36 months) and in whom we had detailed information including serial weights and fasting blood glucoses. Based on this cohort we performed a detailed comparison of patterns of weight loss in new-onset type 2 DM and PaCDM.
PaC patients are almost always symptomatic at diagnosis. Cancer-specific symptoms of PaC include anorexia, fatigue, abdominal pain, back pain, and jaundice. Weight loss immediately prior to cancer diagnosis, which is common in PaC, is easily attributable to cancer-induced cachexia. To minimize confounding from cancer-induced cachexia in this study, final analyses were limited to patients without cancer-specific symptoms at DM onset. Thus, the PaC cases studied had no clinical suspicion of PaC at onset of DM and the mean interval between onset of DM and diagnosis of PaC was ~13 months.
METHODS
The study was approved by the Mayo Foundation Institutional Review Board.
Ascertainment of Cases and Controls
For a previous study we had identified all patients with PaC living within a 120-mile radius of Rochester, MN, (the county seat of Olmsted County) from January 1981 to July 2004.9 In that study we also identified gender (same) and age (± one year) matched Olmsted County residents without PaC as controls. For the present study we selected from this list all Olmsted County residents who met DM criteria noted below. In addition, new cases of PaC in Olmsted County residents through June 2007 were identified by searching the Mayo Diagnostic Index for International Classification of Disease, Ninth Revision, Clinical Modification codes for PaC (157.0–157.9, excluding 157.4 [malignant neoplasm of islets of Langerhans]).
Diabetes definitions and identification of new-onset DM
Subjects were defined as having DM if at least one fasting blood glucose (FBG) value was ≥126 mg/dl or they were on prescription anti-diabetic medications. The first date that FBG was ≥126 mg/dl (with previous values being <126 mg/dl) was defined as date of onset of DM.
We manually abstracted all outpatient FBG values in the Mayo records for the 48 months prior to the index date (defined as date of PaC diagnosis in cases) and identified all patients who (a) had FBG measurements in at least 3 of 4 of the following time intervals: (+1–12, 12–24, 24–36, 36–48 months before index date); (b) met criteria for DM up to 36 months prior to index date and had an FBG value of <126 mg/dl in the preceding 12 month time period and (c) had body weight measured at least one year before first meeting criteria for DM, at the onset of DM, and at the index date.
Medical records were reviewed to abstract the following data: age, sex, parental history of DM, FBG value at index date, height, serial body weights, and DM treatment including drug used and dose of drug. The reason for physician visit at onset of DM was also noted.
Duration of cancer-specific symptoms in PaC Cases
Clinic notes were carefully reviewed to identify cancer-specific symptoms (anorexia, fatigue, abdominal pain, back pain, and jaundice) and their duration prior to diagnosis of PaC. Subjects with cancer-specific symptoms at onset of DM were excluded from final analyses.
Comparison of PaCDM and type 2 DM
The two groups were compared in regards to age, gender distribution, parental history of diabetes and mean FBG value at onset of DM.
Trends in body weight
In PaCDM and type 2 DM, we compared body weight and body mass index (BMI) (calculated by weight (kg)/height2 (m2)) at onset of DM with corresponding parameters 1–2 years prior to onset of DM and at index date. We compared the proportion of PaCDM and type 2 DM subjects losing and gaining weight at onset of DM (compared with weight one year prior to onset of DM) and index date (compared with weight at onset of DM).
Antidiabetic drug usage
Patients’ medical records were reviewed to determine antidiabetic drug regimens being administered at the time of meeting the criteria for DM and at the index date.
Statistical analysis
All statistical analyses were carried out using Microsoft Excel 2003 software (Redmond, WA). Univariate analyses were conducted with the chi-square test for comparisons of discrete variables, and the t test for continuous variables. A P value of <0.05 was considered statistically significant.
RESULTS
Subjects with PaCDM and Type 2 DM
We identified 83 Olmsted County subjects with new-onset DM (40 with PaC and 43 controls without PaC (primary type 2 DM)) who met our specified inclusion criteria. Eleven patients with PaC were excluded because cancer–specific symptoms (abdominal pain, back pain, anorexia, or jaundice) were present at the time of DM onset. For the final analyses we studied 29 PaCDM and 43 type 2 DM subjects with new-onset DM.
Indication for clinic visit at onset of DM in Cases
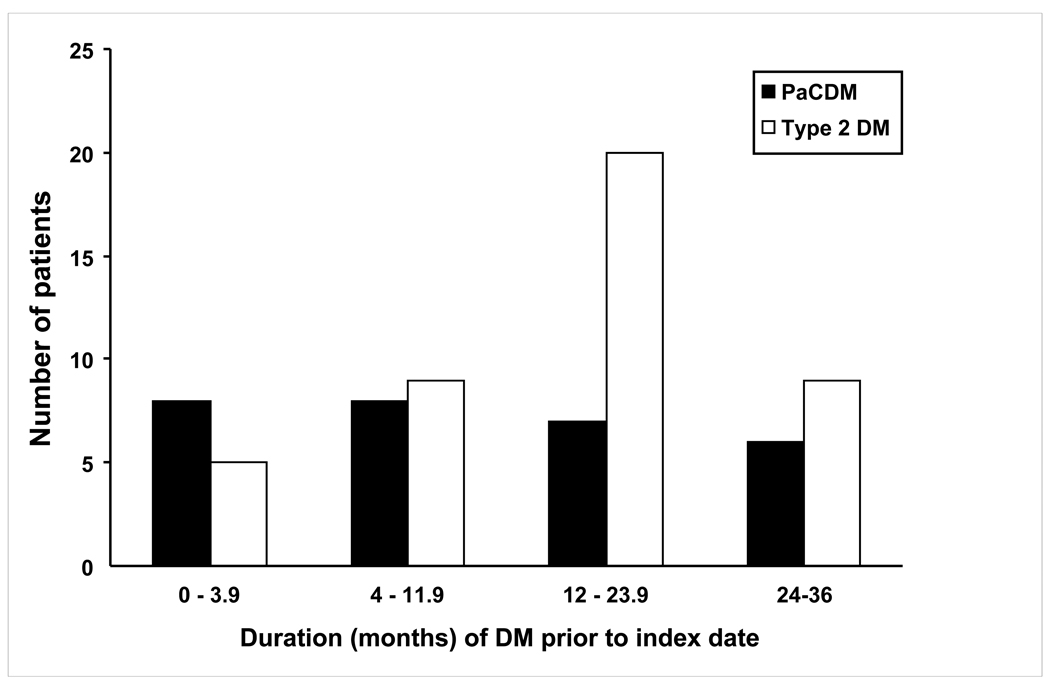
In two cases PaC was incidentally diagnosed at the same time as their DM onset while being investigated for small bowel obstruction and right rib pain. Other indications for the clinic visit at the time of DM onset in cases include cardiopulmonary symptoms (n=8), health maintenance (n=6), musculoskeletal complaints (n=4), GI complaints (n=3; GERD, bloating), neurologic symptoms (n=3), renal/urologic issues (n=2), unrelated oncologic followup (n=2; breast and ovarian), and fatigue (n=2). All PaCDM eventually developed cancer-specific symptoms within 4 months of cancer diagnosis. The mean interval between onset of DM and index date in PaCDM and type 2 DM was (13.0 vs. 15.7 months, P = 0.26) (Figure 1).
Figure 1.
Duration of DM onset prior to cancer diagnosis and index date in PaCDM and type 2 DM, respectively.
Comparison of PaC cases and control with new-onset DM
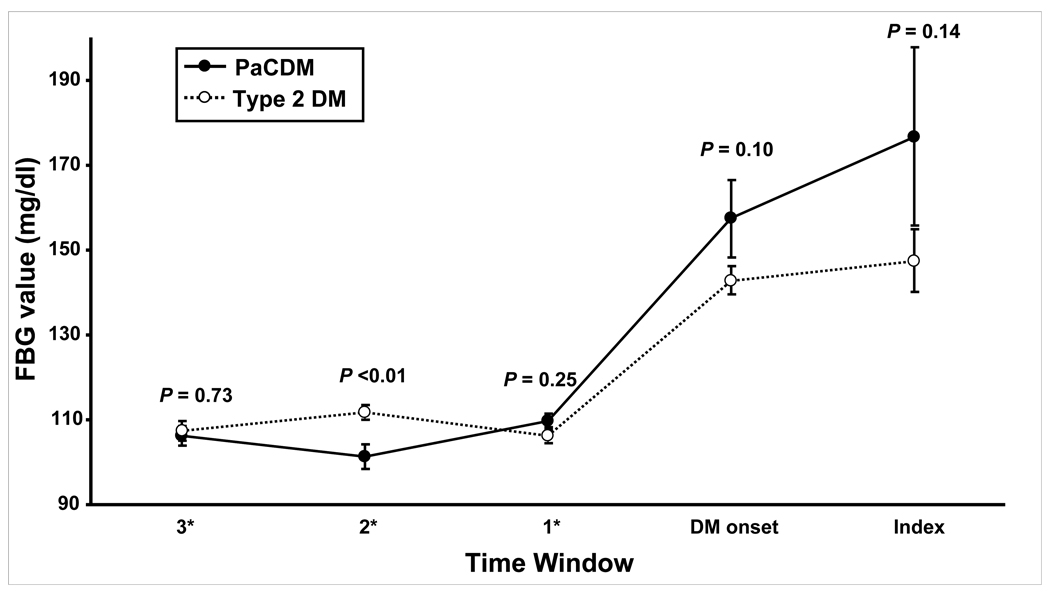
Type 2 DM subjects were younger than PaCDM cases, but similar in gender distribution and prevalence of parental history of DM (Table 1). The time interval from the baseline collection of data (body weight and FBG values) to the onset of DM was similar in PaCDM vs type 2 DM (15.2 ± 7.2 vs. 17.5 ± 6.6 months, P = 0.17). FBG values at baseline (most recent value prior to DM onset) and onset of DM were similar (Table 1). Serial FBG values are shown for the PaCDM and type 2 DM subjects in Figure 2. The increase of FBG from baseline was also similar in PaCDM vs. type 2 DM.
Table 1.
Characteristics of the study population.
| Feature | Type 2 DM (n=43) |
PaCDM (n=29) |
P-Value |
|---|---|---|---|
| Age (yr) | 71.7 ± 9.7 | 76.4 ± 6.8 | 0.03 |
| Gender (%Male) | 55.8 | 37.9 | 0.14 |
| Parental hx of DM (%) | 25.6 | 17.9 | 0.45 |
| FBG at baseline (mg/dl) | 106.5±11.4 | 107.7±10.1 | 0.64 |
| FBG at DM diagnosis (mg/dl) | 142.8 ± 22.3 | 157.5 ± 49.5 | 0.10 |
| FBG increase from baseline (mg/dl) | 35.4 ± 26.7 | 49.9 ± 49.0 | 0.12 |
FBG: fasting blood glucose
Figure 2.
Serial mean FBG values in PaCDM and type 2 DM subjects. Bars denote +/− one standard error of the mean (SEM). *Time windows 3, 2, and 1 indicate the number of years prior to DM onset.
Comparison of body weight at different time points in PaCDM and type 2 DM
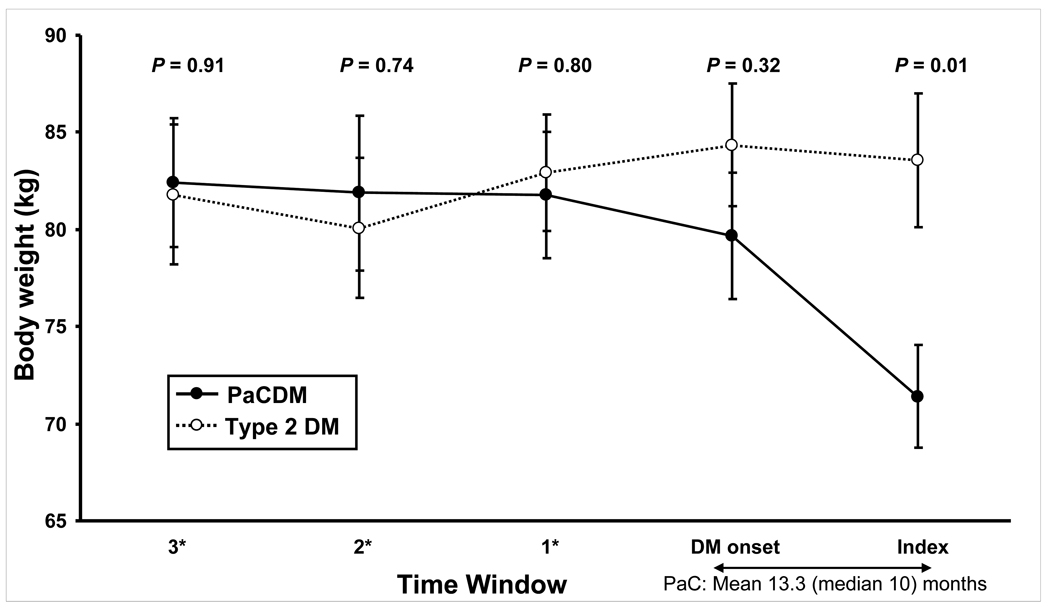
There were no differences in body weight in PaCDM and type 2 DM before onset of DM (81.8 ± 17.5 vs. 82.9 ± 19.5 kg, respectively, P = 0.80), or at the time of onset of DM (79.7 ± 17.4 vs. 84.3 ± 20.7 kg, respectively, P = 0.32). However, the average change in body weight at the onset of DM was a loss of 2.1 ± 3.8 kg in PaCDM vs. gain of 1.4 ± 4.7 kg in type 2 DM (P < 0.01). At index date (date of cancer diagnosis in PaCDM) PaCDM subjects had a significantly lower mean body weight than type 2 DM subjects (71.4 ± 14.3 vs. 83.6 ± 22.7 kg, respectively, P = 0.01). The average change in body weight at the index date was −8.3 ± 8.3 kg in PaCDM vs. −0.8 ± 4.8 kg in type 2 DM (P < 0.01). Similar trends and levels of statistical significance were seen when the BMI of patients were compared over these time intervals (data not shown).
Comparison of PaCDM and type 2 DM stratified by changes in weight at onset of DM
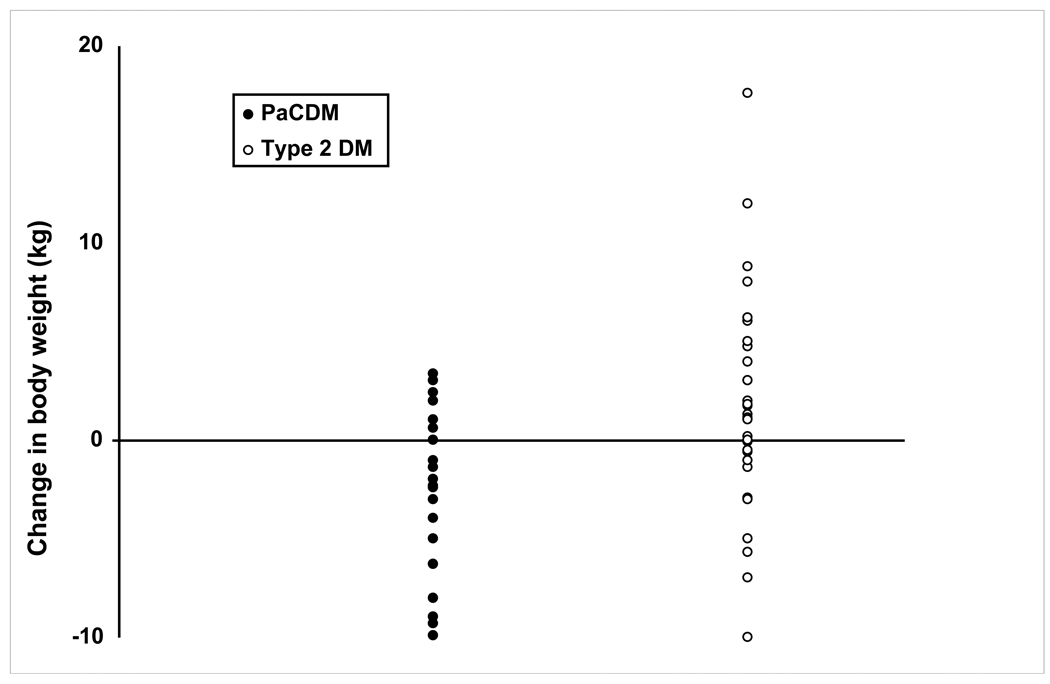
At the onset of DM 17/29 (59%) PaC patients lost weight vs. 13/43 (30%) with type 2 DM (P = 0.02) (Figure 3). Serial body weights are displayed in Figure 4. Of those patients losing weight, PaCDM subjects lost 4.6 ± 2.9 kg vs. 3.0 ± 3.0 kg in type 2 DM (P = 0.16). Mean body weights were similar at each time interval in these patients. In the subjects gaining weight at DM diagnosis, type 2 DM subjects gained 4.2 ± 4.1 kg compared to 1.9 ± 1.1 kg in PaCDM subjects (P = 0.11). In this subset of patients, PaCDM subjects weighed less than type 2 DM subjects at each time interval, however this did not reach statistical significance until the index date. Only two patients with PaC gained weight (2.0 and 0.1 kg) during the interval from DM onset to cancer diagnosis, while weight increased in 33% of type 2 DM (P = 0.01). PaCDM patients had similar intervals from DM onset to cancer diagnosis irrespective of whether they were losing or gaining weight at DM onset (10.1 ± 9.6 vs. 17.8 ± 13.4 months, respectively, P = 0.10), and whether or not they underwent surgical resection (6.4 ± 8.5 vs. 14.1 ± 11.6 months, respectively, P = 0.22).
Figure 3.
Scatterplot of change in body weight at onset of DM in type 2 DM and PaCDM.
Figure 4.
Serial mean body weights in PaCDM and type 2 DM subjects. Bars denote +/− one standard error of the mean (SEM). *Time windows 3, 2, and 1 indicate the number of years prior to DM onset.
Proportion of patients receiving antidiabetic drugs
At the onset of DM more type 2 DM subjects were placed on antidiabetic therapy than PaCDM subjects (37% vs. 14%, respectively, P = 0.03). In the interval between onset of DM and index date, antidiabetic treatment was withdrawn in 8/14 type 2 DM subjects, compared to 0/4 initially treated PaCDM subjects (P = 0.04). Additionally, 2/4 initially treated PaCDM subjects needed escalation in treatment (increase dose or addition of second anti-diabetic agent), and an additional seven patients were started on therapy in the interval between onset of diabetes and index date. Due to these changes in treatment, at the index date there was greater usage of antidiabetic medications in PaCDM vs. type 2 DM (38% vs 10% p <0.01) (Table 2).
Table 2.
Antidiabetic therapy among study subjects.
| Onset of DM | Index Date | |||
|---|---|---|---|---|
| Treatment group | Type 2 DM n (%) |
PaCDM n (%) |
Type 2 DM* n (%) |
PaCDM n (%) |
| Insulin secretagogues | 11 (25.6) | 3 (10.3) | 4 (9.8) | 8 (27.6) |
| Metformin | 4 (9.3) | 1 (3.4) | 0 (0) | 2 (6.9) |
| Exogenous insulin | 0 (0) | 0 (0) | 0 (0) | 2 (6.9) |
| TZD | 1 (2.3) | 0 (0) | 0 (0) | 0 (0) |
| Any treatment | 16 (37.2) | 4 (13.8) | 4 (9.8) | 11 (37.9) |
| No treatment | 27 (62.8) | 25 (86.2) | 37 (90.2) | 18 (62.1) |
Medication data were not available for two of the type 2 DM subjects at the index date.
DISCUSSION
In this study carefully comparing asymptomatic subjects with new-onset PaCDM and matched subjects with new-onset type 2 DM we show that PaCDM patients lose weight at the onset of DM even before they have any other cancer-specific symptoms such as anorexia, fatigue, abdominal/back pain or jaundice. Notably, they continue to lose weight until cancer diagnosis. Despite the weight loss, PaCDM subjects frequently require escalation in their antidiabetic therapy. This is in contrast to type 2 DM subjects who gain weight at the onset of DM and either stay the same weight or gain further weight after onset of DM.
PaCDM and type 2 DM were indistinguishable based on traditional risk factors for DM, such as BMI and family history of DM. These observations are similar to our previously reported findings in other cohorts.7–9 We also noted that the two groups could not easily be distinguished by the FBG level, or increase from baseline, at the onset of DM. Thus, there are few clinical clues to suspect PaC in subjects with asymptomatic new-onset DM.
Weight loss is well known to accompany PaC. This has traditionally been thought to be due to the profound cachexia that accompanies PaC. Therefore, we limited our study to those in whom the DM started before onset of other symptoms of cachexia (anorexia, fatigue) or other cancer-specific symptoms (abdominal/back pain and jaundice). Despite exclusion of these patients, we found that 59% of PaCDM lost weight by the time of onset of DM. Among PaCDM subjects who lost weight, the mean amount of weight lost over the 12–15 months prior to DM onset was an appreciable 4.6 kg (approximately 10 pounds). This could not be explained by more severe DM in PaC, as mean FBG in the two groups at onset of DM was similar and they started with similar weights one year prior. The weight loss continued until cancer diagnosis.
One limitation in this retrospective analysis is the inability to completely limit ascertainment bias. It could be suggested that PaCDM patients losing weight were more likely to seek medical attention, which increased the likelihood of their enrollment in the current study. However, review of the clinic documentation showed that there were no instances in which patients sought evaluation for workup of weight loss at the time of onset of DM. Another concern with our study is that the small sample size prevents drawing any definite conclusions from these data. Our inclusion criteria requiring serial FBG levels and body weights in three out of four years prior to the index dates were stringent and restricted the number of patients who qualified for the study. Nevertheless we found 40 with PaC and 43 controls without PaC. Of these we only included patients without PaC symptoms at onset of DM. We hope these data will be lead to larger studies to examine our observation.
Additionally, one could suggest that PaCDM patients losing weight represent a subset of patients with more aggressive cancer who are more likely to demonstrate early cancer-associated cachexia. However our observations do not support this assertion. While there is no direct measure of PaC aggressiveness, the duration from DM onset to cancer diagnosis is one clinical surrogate. In our study, PaCDM patients losing weight at DM onset had a similar interval from DM onset to cancer diagnosis as those who gained weight at DM onset. Additionally, there was no difference in this time interval in PaCDM patients who underwent surgical resection compared to those who did not. Finally, those who lost weight at DM onset were not at significantly increased chances of undergoing surgical resection than those with no change or increased body weight; however this study was not designed or powered to address this issue. These data suggest that weight loss in PaCDM cannot be explained by a more aggressive course of the cancer in these patients. On the contrary, it is our clinical experience that, in retrospect, PaCDM patients losing weight at DM onset often feel well despite the weight loss.
Our findings raise many questions: Why does weight loss occur in PaCDM? How is it linked to the onset and progression of diabetes? Can it provide a clue to the presence of PaC in new-onset DM?
Obesity is thought to play a major role in the pathogenesis of type 2 DM through the effects of adipocyte products and adipokines, which cause abnormalities in regulating body weight, appetite, energy expenditure, and ultimately increased insulin resistance. By decreasing overall adipocyte mass weight loss is expected to produce reversal of these abnormalities and improved insulin sensitivity. In PaC, however, a paradoxical need for intensification of antidiabetic therapy is often required despite weight loss. When questioned, patients often state that they were feeling well at the time of onset of diabetes (i.e. several months prior to the clinical symptoms or radiologic evidence of PaC) and in fact are pleasantly surprised at the ease with which they had lost weight. What is pathophysiological explanation for this apparent paradox?
One possible explanation for this profound weight loss would be the increased utilization of fatty acids as the preferred energy source leading to fat mobilization and weight loss. It has been proposed that weight loss in pancreatic and other cancers is due to production of a “lipid mobilizing factor”.11 Zinc α2-glycoprotein (ZAG) is one such potential “lipid mobilizing factor” that is under investigation. Overexpression of ZAG has been demonstrated in several types of malignant tumors including prostate, colon, and breast cancer.12–14 ZAG is a plasma protein that has been shown to modulate lipolysis in white adipose tissue and affect the production of adipokines, such as adiponectin, in murine models.15, 16 A more recent study by Tisdale et al. showed that ZAG infusions in a diabetic mouse model led to weight loss without altering their food or water intake. Interestingly, the authors also noted an increase in body temperature suggesting an increase in resting energy expenditure that might explain the enhanced lipolysis and weight loss.17 If ZAG were to be implicated in PaCDM induced weight loss, it would certainly explain the profound weight loss in PaCDM. However, the same study showed a 25% decrease in plasma glucose levels after ZAG administration, which would suggest an alternative mechanism for worsening hyperglycemia in the context of weight loss in PaCDM. These observations warrant further investigations into the mechanisms of fat loss and hyperglycemia in PaC.
Finally, do our observations suggest that subjects with new-onset DM and weight loss should be screened for PaC? While our results should be interpreted with caution due to small sample size, they are consistent with previous observations from our group. We have previously demonstrated in a population-based study that approximately 1 in 125 (or 8 in 1,000) patients with new-onset DM develop PaC.2 Extrapolating the data herein, we would estimate the prevalence of PaC per 1,000 patients with new-onset DM in those with preceding weight loss, no significant change, or weight gain to be 16, 6, and 4 per 1,000 patients with new-onset DM, respectively. The fact that a third of type 2 DM subjects lose weight demonstrates that this finding is not specific for PaCDM, however the presence of weight loss does further enrich the screening population of new-onset DM for PaC. Screening for PaC would require endoscopic ultrasound (EUS) as non-invasive imaging (i.e. abdominal CT scanning) may show no radiographic abnormalities in asymptomatic subjects.5, 6 Endoscopic ultrasonography (EUS) has been shown to be superior to CT for detecting small pancreatic cancers; however EUS has not been evaluated in asymptomatic patients.19 We believe that it would be appropriate to study the role of EUS to detect PaC in asymptomatic new-onset DM subjects with weight loss, however further filters to further enrich the screening population would be ideal.
In conclusion, we report that patients with new-onset DM associated with PaC could potentially be distinguishable from those with new-onset type 2 DM in that they were more likely to lose weight at DM onset. Weight loss over the 12–15 month interval prior to a recent DM diagnosis may prove to be a useful clinical clue to identify those who may have underlying PaC. However, since this finding is neither sensitive, nor specific it is likely that additional biomarkers, whether clinical, biochemical or radiologic will be needed to most accurately make this distinction. Conversely, DM is less likely to be attributable to PaC in those who continue to gain weight after onset of DM. The paradoxical development and worsening of DM in the face of weight loss in PaCDM is intriguing and may hold the clue to understanding its pathogenesis and identifying its mediator. Further studies should be directed at prospectively validating our observations, and describing other clinical phenotypes (ex. serum biomarkers) to facilitate targeted screening for PaC, with the hopes of early diagnosis and improved survival related to PaC.
Acknowledgments
Grant Support: Dr Chari’s research was funded by grants from NIH (R01 CA 100685) and the Mayo Clinic Pancreas Cancer SPORE (P50 CA 102701)
Abbreviations
- BMI
body mass index
- DM
diabetes mellitus
- EUS
endoscopic ultrasound
- FBG
fasting blood glucose
- PaC
pancreatic cancer
- PaCDM
new-onset diabetes mellitus associated with pancreatic cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest/disclosures: No conflicts of interest exist.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008 Mar–Apr;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Chari S, Leibson CL, de Andrade M, et al. Probability of pancreatic cancer following diabetes: A population-based study. Gastroenterology. 2005;129(2):504–511. doi: 10.1053/j.gastro.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogawa Y, Tanaka M, Inoue K, et al. A prospective pancreatographic study of the prevalence of pancreatic carcinoma in patients with diabetes mellitus. Cancer. 2002 May 1;94(9):2344–2349. doi: 10.1002/cncr.10493. [DOI] [PubMed] [Google Scholar]
- 4.Damiano J, Bordier L, Le Berre JP, et al. Should pancreas imaging be recommanded in patients over 50 years when diabetes is discovered because of acute symptoms? Diabetes Metab. 2004 Apr;30(2):203–207. doi: 10.1016/s1262-3636(07)70111-8. [DOI] [PubMed] [Google Scholar]
- 5.Pelaez-Luna M, Takahashi N, Fletcher JG, et al. Resectability of presymptomatic pancreatic cancer and its relationship to onset of diabetes: a retrospective review of CT scans and fasting glucose values prior to diagnosis. Am J Gastroenterol. 2007 Oct;102(10):2157–2163. doi: 10.1111/j.1572-0241.2007.01480.x. [DOI] [PubMed] [Google Scholar]
- 6.Gangi S, Fletcher JG, Nathan MA, et al. Time interval between abnormalities seen on CT and the clinical diagnosis of pancreatic cancer: retrospective review of CT scans obtained before diagnosis. AJR Am J Roentgenol. 2004 Apr;182(4):897–903. doi: 10.2214/ajr.182.4.1820897. [DOI] [PubMed] [Google Scholar]
- 7.Chari ST, Klee GG, Miller LJ, et al. Islet amyloid polypeptide is not a satisfactory marker for detecting pancreatic cancer. Gastroenterology. 2001 Sep;121(3):640–645. doi: 10.1053/gast.2001.27210. [DOI] [PubMed] [Google Scholar]
- 8.Pannala R, Leirness JB, Bamlet WR, et al. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008 Apr;134(4):981–987. doi: 10.1053/j.gastro.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008 Jan;134(1):95–101. doi: 10.1053/j.gastro.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pannala R, Leibson CL, Rabe KG, et al. Temporal association of changes in fasting blood glucose and body mass index with diagnosis of pancreatic cancer. Am J Gastroenterol. 2009 Sep;104(9):2318–2325. doi: 10.1038/ajg.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell ST, Tisdale MJ. Effect of a tumour-derived lipid-mobilising factor on glucose and lipid metabolism in vivo. Br J Cancer. 2002 Aug 27;87(5):580–584. doi: 10.1038/sj.bjc.6600493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hale LP, Price DT, Sanchez LM, et al. Zinc alpha-2-glycoprotein is expressed by malignant prostatic epithelium and may serve as a potential serum marker for prostate cancer. Clin Cancer Res. 2001 Apr;7(4):846–853. [PubMed] [Google Scholar]
- 13.Diez-Itza I, Sanchez LM, Allende MT, et al. Zn-alpha 2-glycoprotein levels in breast cancer cytosols and correlation with clinical, histological and biochemical parameters. Eur J Cancer. 1993;29A(9):1256–1260. doi: 10.1016/0959-8049(93)90068-q. [DOI] [PubMed] [Google Scholar]
- 14.Todorov PT, McDevitt TM, Meyer DJ, et al. Purification and characterization of a tumor lipid-mobilizing factor. Cancer Res. 1998 Jun 1;58(11):2353–2358. [PubMed] [Google Scholar]
- 15.Gohda T, Makita Y, Shike T, et al. Identification of epistatic interaction involved in obesity using the KK/Ta mouse as a Type 2 diabetes model: is Zn-alpha2 glycoprotein-1 a candidate gene for obesity? Diabetes. 2003 Aug;52(8):2175–2181. doi: 10.2337/diabetes.52.8.2175. [DOI] [PubMed] [Google Scholar]
- 16.Bing C, Bao Y, Jenkins J, et al. Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed in adipocytes and is up-regulated in mice with cancer cachexia. Proc Natl Acad Sci U S A. 2004 Feb 24;101(8):2500–2505. doi: 10.1073/pnas.0308647100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell ST, Tisdale MJ. Antidiabetic properties of zinc-alpha2-glycoprotein in ob/ob mice. Endocrinology. Mar;151(3):948–957. doi: 10.1210/en.2009-0827. [DOI] [PubMed] [Google Scholar]
- 18.DeWitt J, Devereaux B, Chriswell M, et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med. 2004 Nov 16;141(10):753–763. doi: 10.7326/0003-4819-141-10-200411160-00006. [DOI] [PubMed] [Google Scholar]