1. INTRODUCTION
Caloric restriction (CR) generally extends the lifespan of invertebrates and rodents. Surprisingly, recent studies have demonstrated that some strains of mice have reduced lifespan when consuming fewer calories (Liao et al., 2010). It appears that mice exhibiting the greatest metabolic efficiency exhibit the greatest enhancement of life span (Rikke et al., 2010). While short-term CR consistently reduces energy expenditure in rodents, the evidence generally suggests that this suppression in metabolic rate is not sustained during extended periods of CR; i.e., months or years (Masoro et al., 1982; McCarter et al., 1985; McCarter and Palmer, 1992). Since CR rats are lighter than ad libitum controls (AL), there is considerable debate and discussion concerning the best approach to compare metabolic rates in control and CR rats (Greenberg and Boozer, 2000; MacLean et al., 2004; McCarter and Palmer, 1992; Selman et al., 2005). It is an important issue as some recent reports indicate the CR lowers energy expenditure in humans even after adjustments for reduced lean body mass (Heilbronn et al., 2006; Redman et al., 2009). The primary purpose of the current study was to examine the long-term metabolic and cardiovascular responses to CR. To examine this relationship, we used home cage indirect calorimetry to estimate energy expenditure and regressed these values on metabolic organ mass (sum of heart, liver, kidney, and brain mass) (Greenberg and Boozer, 2000). In addition, the metabolic and cardiovascular responses to long-term CR were examined in rats with low and high baseline metabolic rate and heart rate due to differences in home cage ambient temperature (Ta). We hypothesized that long-term CR would be associated with a sustained suppression of metabolic rate and heart rate irrespective of the baseline state imposed by variations in Ta.
2. METHODS
2.1 Animals and housing
Male FBNF1 rats (n = 96; age = 4 mo; Harlan, Indianapolis, IN) were acclimated to either cool (COOL; Ta = 15.0 ± 1.0°C) or thermoneutral (TMN; Ta = 30.0 ± 1.0°C) temperatures for 2 months. This rat strain was selected because upon reaching adulthood, the FBNF1 rat maintains a relatively stable plateau of body weight when fed ad libitum unlike Sprague Dawley or Wistar rats which display continuous body weight gain (Altun et al., 2007; Glendinning and Smith, 1994; Story et al., 1981). Animals were housed individually in standard polycarbonate cages containing wood chip bedding maintained on 12:12-h light-dark cycle with ad libitum (AL) access to powdered chow (Purina 5001) and deionized water. After acclimation, rats at each Ta were assigned to either continuous AL feeding or were subjected to 25% (COOL) or 40% (TMN) caloric restriction (CR). We have previously found that TMN and COOL-housed rats both under 40% CR diverge in body weight due to the increased energy demand in COOL conditions (Evans et al., 2005b). Therefore, we placed COOL rats on a milder CR regimen (25%), which compensated for the decreased metabolic demand of TMN-CR rats. Thus, the goal was to induce comparable decreases in energy balance at both Ta. To prevent any complications of juvenile growth and maturation on the adaptive responses, CR began at 6 months of age. Food amounts for CR rats were not adjusted throughout the study because despite a gradual increase in body weight, food intake remains fairly constant in AL FBNF1 rats. The final hour of the light phase (during which daily chamber maintenance procedures were performed) was excluded from analysis, resulting in 12-h averages for the dark phase and 11-h averages for the light phase. All animals were fed once daily at the onset of the dark phase at which time metabolic, cardiovascular and behavioral data collection was resumed in sealed, undisturbed calorimeters.
2.2 Metabolic chambers for calorimetry and activity monitoring
Measurements of gas exchange and monitoring of animal behavior were performed using previously-published approaches (Overton et al., 2001a; Rashotte et al., 1995). At designated time points, rats were transferred from housing rooms to calorimeters inside environmental chambers to provide precise control of cage Ta (±0.1°C). The calorimeters were constructed from standard shoebox cages fitted with a custom-made polycarbonate lid providing a near airtight seal for continuous determination of oxygen consumption (VO2; ml/min) and carbon dioxide production (VCO2; ml/min). Food and water were provided and animals remained on AL or CR feeding schedules to obtain 23 hour metabolic data for two days. The first day was considered an acclimation day and the second day was used for data analysis. Total energy expenditure was estimated using the Weir equation: energy expenditure (kcal/min) = 3.91(VO2) + 1.1(CO2)/1,000. Energy expenditure was estimated separately for the dark phase and the light phase. Daily energy balance was estimated as the difference between caloric intake and caloric expenditure.
Each cage was positioned on a custom-designed force platform to obtain precise quantification of locomotor activity in meters. Stiff strain-gauge load-beam transducers are attached under two adjacent corners of the platform. The transducers measure changes in the center of gravity allowing localization of the animal’s position in two dimensions. Changes in X and Y coordinates are combined using the Pythagorean Theorem to compute distance moved. Movements must exceed one cm distance traveled without direction reversal to be considered as locomotor activity.
2.3 Cardiovascular Measurements by Telemetry
Prior to study, all rats (n=96) were anesthetized with halothane and instrumented with a catheter in the descending aorta coupled with a sensor and transmitter (model TA11PA-C40, Data Sciences, St. Paul, MN) for telemetric monitoring of blood pressure and determination of cardiovascular variables (Williams et al., 2000). A group of 32 young animals were instrumented before the onset of 10 week CR studies and were followed for this 10 week period prior to sacrifice. Another group of 64 animals were instrumented after 11 months of treatment, in order to obtain one year and re-feeding measurements. All animals were allowed a minimum of 14 days to recover from surgery before being transferred to calorimeters for data collection. Animal recovery was assessed by recovery of food intake following the procedure. The telemetry receiver that collects cardiovascular data from the implanted telemetry device is located beneath the activity platform in the calorimeter (Overton et al., 2001a).
2.4 Metabolic Mass and Body Fat Percentage
Metabolic mass is defined as the sum mass of the major metabolic organs of the body which includes the heart, liver, kidneys, and brain. This approach has been used by others as an approximate yet reliable independent predictor of metabolic rate (Greenberg, 1999; Greenberg and Boozer, 2000). Metabolic mass and body fat percentage were determined post mortem in groups of rats after 10 weeks CR and 1 year of CR. Rats were anesthetized (sodium pentobarbital, 60 mg/kg, ip) and decapitated late in the light phase. Metabolic mass was defined as the collective weight of the blot-dry heart, liver, kidneys, and brain. All organs were returned to the carcass. The GI tract was cleaned and returned to the carcass, which was weighed and frozen. Total body fat percentage was estimated using chloroform extraction from homogenates of rat carcass (Feely et al., 2000).
2.5 Serum leptin analysis
During carcass dissection, trunk blood was obtained and stored temporarily at 4°C prior to centrifugation (3,000 g for 15 minutes at 4°C). Supernatant serum was removed and stored at −20°C for hormone concentrations. Leptin hormone analysis was performed using a radioimmunoassay kit for rat leptin (RL-83K Linco, St Charles, MO).
2.6 Protocols
At 6 months of age, baseline metabolic and cardiovascular data were obtained for 32 rats (n=16 for TMN and COOL). Half of the animals in TMN and COOL groups were then placed on CR as described above and the other half remained under AL conditions (n=8 per diet at each Ta). For clarification, the distribution of rat assignments for each group is summarized in Table 1. Data were collected for one day every other week for a total five bi-weekly increments up to 10 weeks of CR prior to sacrifice and determination of metabolic mass and body fat.
TABLE 1.
Number of rats in each group
| TMN (30°C) | Cool (15°C) | |||
|---|---|---|---|---|
| AL | CR | AL | CR | |
| 10 weeks CR | 8 | 8 | 8 | 8 |
| 1 year CR | 16 | 12 | 16 | 12 |
| Re-feed | - | 4 | - | 4 |
| Group Total | 24 | 24 | 24 | 24 |
The remaining 64 rats were kept under TMN or COOL (n=32) conditions in standard polycarbonate cages for one year of CR or AL feeding until they reached 18 months of age (n=16 per diet at each Ta). Several animals in each group were implanted with telemetry devices and allowed to recover for two full weeks. At this point, these animals were acclimated to the metabolic chambers (3 days) and data were collected for four days. After obtaining data, all AL rats and half of each CR group were sacrificed for organ weights and body fat determination. The remaining half of each CR group (n=4 for each Ta) were re-fed and monitored in the metabolic chambers for four days and then sacrificed.
2.7 Data collection and analysis
During the last hour of the light phase, data were saved for offline analysis, diagnostics were performed on the equipment, and body weight and food and water intake were recorded. Light-phase and dark-phase data averages were obtained for blood pressure, heart rate, VO2, VCO2, and locomotor activity. Oxygen consumption was also normalized to body weight using the Kleiber coefficient as an estimate of metabolic tissue. Respiratory quotient (RQ) was calculated by VCO2/VO2. All results are reported as means ± SEM.
2.8 Statistical Analysis
Measurements taken over multiple weeks or days were compared by two-way repeated measures ANOVA (SPSS v.13.0), [Time (days, weeks) × Diet (AL vs CR)]. One year CR measurements taken for a single day and metabolic mass and body fat % were compared by one-way ANOVA [Diet (AL vs CR)]. Post-hoc tests were used to distinguish differences between AL and CR groups within a particular temperature treatment. Interactions were probed using temperature as a co-variate of the response to CR. Statistical significance was set at the p < 0.05 level.
3. RESULTS
3.1 Body Weight and Energy Balance
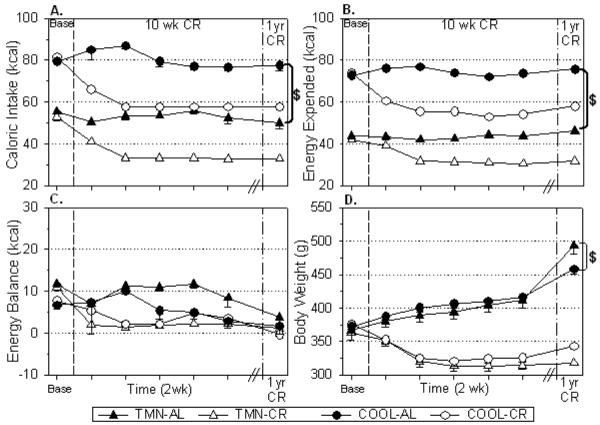
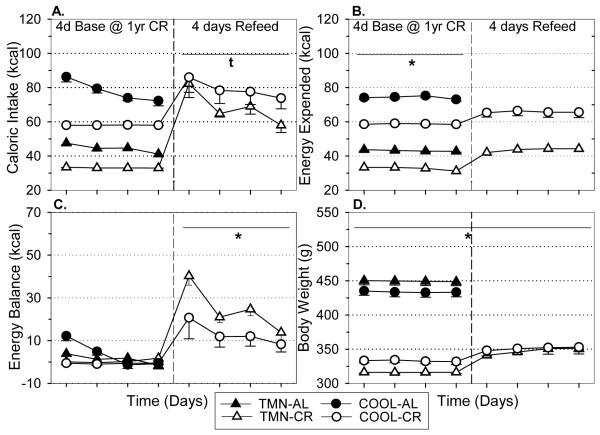
In AL rats, the COOL environment increased food intake (F=316.67, p<.001; Fig. 1A) and energy expenditure (F=517.52, p<.001; Fig. 1B) compared to TMN, and these effects persisted for one year. Throughout the study, both groups of AL rats were in a state of positive energy balance that decreased in magnitude over time (F=442.48, p<.001; Fig. 1C). TMN-CR and COOL-CR rats maintained similar energy balance values across the study indicating that the 25% and 40% CR regimens contributed comparably to energy homeostasis. Energy balance was significantly greater at baseline and for the first 10 weeks of study in TMN-AL rats compared to COOL-AL rats, which led to a greater body weight (F=21.46, p<.001; Fig. 1D), body fat, and hyperleptinemia (Table 2) after one year. Caloric restriction (CR) decreased energy expenditure (p<.001), energy balance (p<.001), and body weight (p<.001) at both Ta compared to controls (Fig. 1B-D), and these effects on energy expenditure (F=44.30, p<.001) and body weight (F=157.22, p<.001) were consistently maintained long-term. Similarly, CR decreased body fat and leptin levels (F=17.76; p<.001) at both Ta (Table 2). Interestingly, leptin levels in long-term CR rats remained below the normal levels in young, AL fed rats (F=95.45; p<.001), likely due to the maintenance of a reduced body weight (F=157.92; p<.001) and body fat (F=77.74; p<.001).
FIGURE 1.
Effect of 10 wks and one year of CR on (A) caloric intake, (B) energy expenditure, (C) energy balance, and (D) body weight in rats housed under thermoneutral (TMN; Ta = 30°C) or cool (COOL; Ta=15°C) conditions. Baseline values (Base) are shown prior to first vertical break. Responses to 10 wks are shown in 2 wk intervals prior to second break. Responses to one year of CR in separate groups of animals are shown after second vertical break. *p<.01 AL vs CR at both Ta $p<.01 COOL AL vs TMN AL.
TABLE 2.
Effect of Caloric Restriction on Metabolic Mass and % Body Fat.
| TMN (30°C) | Cool (15°C) | |||
|---|---|---|---|---|
| AL | CR | AL | CR | |
| MetMass (g) | ||||
| 10 weeks CR | 13.67 ± 0.55*+ | 9.07 ± 0.12$ | 12.04 ± 0.23*+ | 16.03 ± 0.26 |
| 1 year CR | 18.61 ± 0.61*$ | 10.78 ± 0.13$ | 21.32 ± 0.27* | 15.44 ± 0.21 |
| Leptin (ng/ml) | ||||
| 10 weeks CR | 5.6 ± 0.9*+ | 1.3 ± 0.3+ | 4.9 ± 0.5*+ | 1.3 ± 0.2+ |
| 1 year CR | 18.8 ± 1.0*$ | 3.1 ± 0.5 | 13.6 ± 0.9* | 3.3 ± 0.7 |
| Body Fat (%) | ||||
| 1 year CR | 22.7 ± 0.8*$ | 9.6 ± 0.4 | 17.4 ± 0.5* | 11.2 ± 0.9 |
Data are expressed as mean ± S.E.M. n=8 for 10 week groups; n =16 for one year groups.
p<.01 AL vs CR at same Ta
p<.01 vs 1 year at same Ta
p<.01 vs COOL on same diet
3.2 Oxygen Consumption and Locomotor Activity
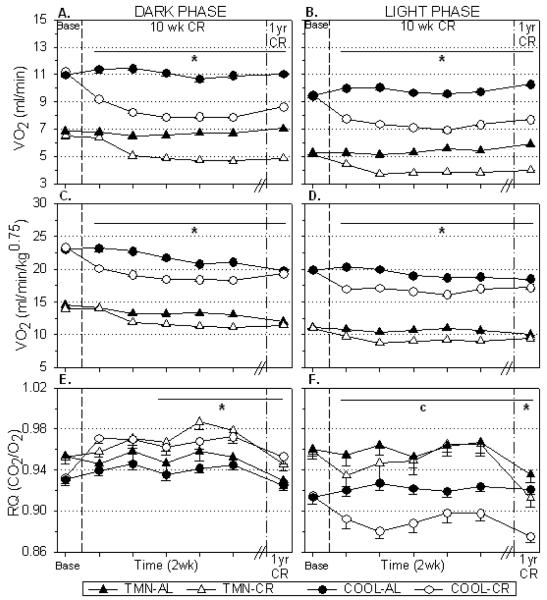
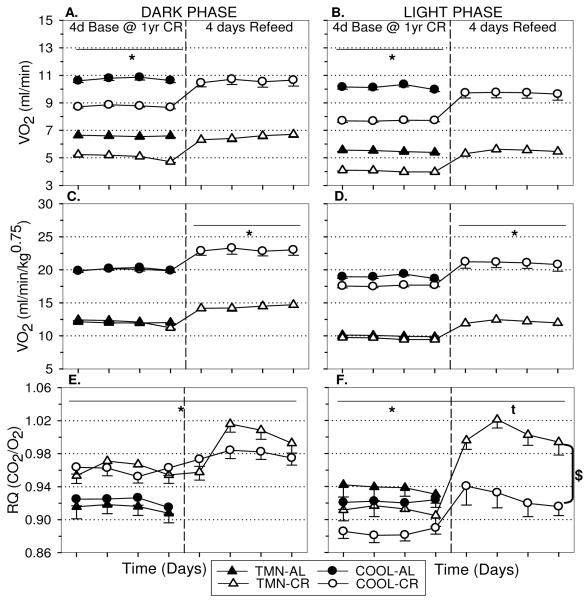
As expected, the COOL environment increased both absolute (Dk:F=341.46, p<.001; Lt:F=574.55, p<.001) and normalized VO2 (Dk:F=810.46, p<.001; Lt:F=1340.58, p<.001) compared to TMN in AL rats (Fig. 2A,B). Caloric restriction reduced dark (p<.001; Fig. 2A,C) and light phase (p<.001; Fig. 2B,D) VO2 in both TMN and COOL rats compared to controls; this effect remained significant for both absolute VO2 (Fig. 2A,B) and when VO2 was normalized to body weight (Fig. 2C,D) using the Kleiber coefficient. It should be noted that in COOL-CR rats, normalized VO2 was not significantly suppressed (F=1.60, p=.065) at one year in the dark phase but remained suppressed in the light phase throughout (F=2.68, p<.01). There was no significant main effect of CR on dark or light locomotor activity (data not shown). Housing in COOL conditions significantly decreased respiratory quotient (RQ) compared to TMN conditions regardless of dietary treatment. During the dark phase, CR rats consume all of their daily food supply inducing an increase in RQ compared to AL controls at both Ta (Fig. 2E). During the light phase, CR reduced RQ in COOL animals and to lesser extent in TMN animals after one year (Fig. 2F).
FIGURE 2.
Effect of 10 wks and one year CR on (A,C) dark and (B,D) light phase oxygen consumption (VO2) and (E,F) respiratory quotient (RQ) in rats housed under thermoneutral (TMN; Ta = 30°C) or cool (COOL; Ta=15°C) conditions.. Graphs depict total VO2 (A,B) and normalized VO2 (C,D) using the Kleiber method. Baseline values (Base) are shown prior to first vertical break. Responses to 10 wks are shown in 2 wk intervals prior to second break. Responses to one year of CR in separate groups of animals are shown after second vertical break. *p<.01 AL vs CR at both Ta; cp<.05 COOL:AL vs CR only
3.3 Metabolic Mass versus Energy Expenditure: Regression Analysis
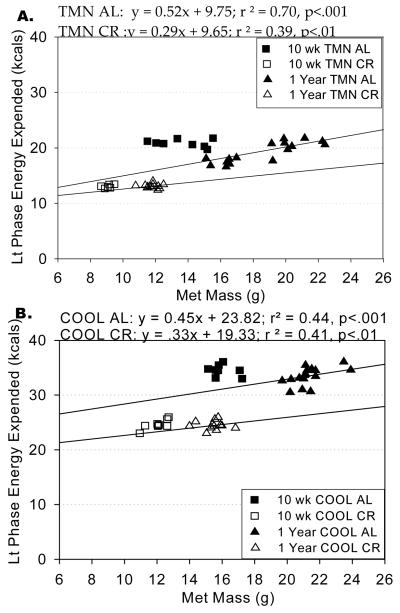
Short-term CR significantly reduced metabolic mass in TMN rats, but increased metabolic mass in COOL rats (F=78.166, p<.001; Table 2). However, long-term CR significantly reduced metabolic mass in both TMN and COOL rats (F=162.11, p<.001). For all animals, there was a highly significant correlation between metabolic mass and light phase energy expenditure (r=.72, p <.001, n=96). Regression analysis (Fig. 3) of individual animal metabolic masses to their light phase energy expenditure reveals a clear shift towards lower energy expenditure per unit of metabolic mass for CR rats. These regression analyses revealed the following equations for the trend line of each group over time:
The “slope” of these regression trend lines can be further analyzed by expressing light phase energy expenditure/metabolic mass as a ratio for each animal. Analysis of variance for this ratio indicated that indeed CR reduced energy expenditure further than predicted by reductions in metabolic mass (F=170.22; p<.001) from these dietary conditions (TMN-AL: 0.40 TMN-CR: 0.36, p<.01) (COOL-AL: 0.28 COOL-CR: 0.26, p<.05).
FIGURE 3.
Linear regression of metabolic mass on light phase energy expenditure for groups of rats housed in thermoneutral (TMN; Ta = 30°C) or cool (COOL; Ta=15°C) conditions and studied during ad libitum (AL) or caloric restriction (CR) feeding conditions. Graph points indicate individual animal values for metabolic mass and corresponding light phase energy expenditure after 10 wks or one year of CR. Best-fit lines are included for each dietary treatment. The equation for each best-fit line is displayed above the graph.
3.4 Blood Pressure and Heart Rate
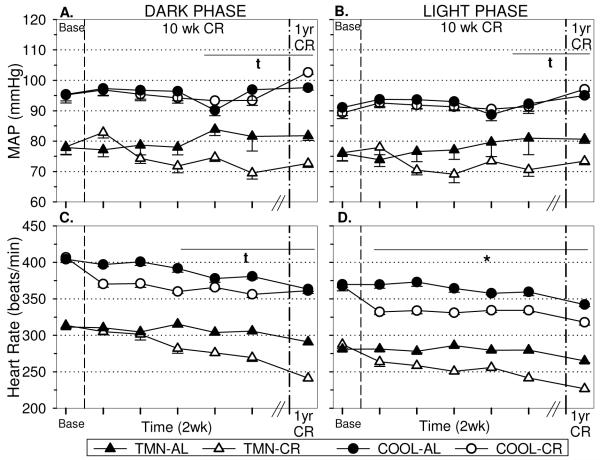
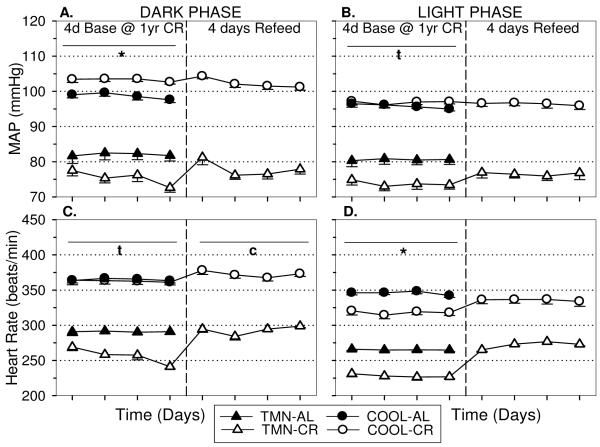
TMN-AL rats exhibit reduced dark (Fig. 4A) and light (Fig.4B) mean arterial pressure (MAP) and heart rate (HR) compared to COOL-AL rats. Caloric restriction had no effect on MAP in the short-term; however, longer duration of CR decreased dark and light MAP (Fig. 4AB) in TMN rats only (Dk:F=5.58, p<.001; Lt:F=3.94, p<.01). Interestingly, COOL-CR rats had elevated MAP compared to controls in the dark phase but not the light phase after one year CR (Dk:F=10.42, p<.001 Lt:F=0.50, p=.62). CR quickly reduced HR in both the dark (Fig. 4C) and light (Fig. 4D) phases irrespective of Ta (Dk:F=8.32, p<.05; Lt:F=28.64, p<.001). However, in the long term, COOL-CR rats did not exhibit bradycardia in the dark phase but maintained significant bradycardia in the light phase.
FIGURE 4.
Effect of 10 wks and one year CR on (A) dark and (B) light phase mean arterial pressure (MAP), and (C,D) heart rate (HR) in rats housed under thermoneutral (TMN; Ta = 30°C) or cool (COOL; Ta=15°C) conditions. Baseline values (Base) are shown prior to first vertical break. Responses to 10 wks are shown in 2 wk intervals prior to second break. Responses to one year of CR in separate groups of animals are shown after second vertical break. *p<.01 AL vs CR at both Ta tp<.05 TMN:ALvsCR only cp<.05 COOL:ALvsCR only
3.5 Re-feeding
After one year CR, animals that were fed AL for four days exhibited hyperphagia (F=231.04, p<.001; Fig. 5A) that was more prominent and sustained at TMN than COOL conditions. Additionally, total energy expenditure was rapidly restored (F=99.93, p<.001; Fig. 5B) to the level of control animals fed AL despite the reduced body weight of the re-fed animals. The duration of positive energy balance was brief and the magnitude of positive energy balance rapidly declines towards control levels that are at or near zero within the four day observation period (Fig. 5C). The rate of body weight regain was very slow over the four days of re-feeding remaining far below controls (F=270.54, p<.001; Fig. 5D). Re-feeding doubled serum leptin to 6.0 ± 0.4 in TMN-CR rats and 6.4 ± 0.2 in COOL-CR rats, but this was well below serum levels in the 18 month old AL rats (see Table 2).
FIGURE 5.
Effect of four days of re-feeding after one year CR on (A) caloric intake, (B) energy expenditure, (C) energy balance, and (D) body weight in rats housed under thermoneutral (TMN; Ta = 30°C) or cool (COOL; Ta=15°C) conditions. Data are mean baseline values obtained daily at one year of CR or AL ± S.E.M. up to the break. After the break, four days of re-feeding data are shown. *p<.05 vs AL at both Ta tp<.05 TMN:ALvsRF only
The reductions in absolute VO2 induced by CR were completely reversed by re-feeding in both the dark and light phases at both Ta within 1-2 days (Dk:p=.73 Lt:p=.15; Fig. 6A,B). Additionally, re-feeding induced an immediate and sustained increase in normalized VO2 above controls at both temperatures (Dk:p<.001 Lt:p<.01)Fig. 6C,D) suggesting that CR continued to suppress metabolism up to one year. Further, RQ increased in response to re-feeding in the dark phase at both Ta (Fig. 6E). However, while TMN-CR rats exhibited elevated light phase RQ compared to TMN-AL rats, RQ returned to COOL-AL levels in COOL-CR rats (Fig. 6F).
FIGURE 6.
Effect of four days re-feeding after one year CR on (A,C,E) dark and (B,D,F) light phase oxygen consumption (VO2) and respiratory quotient (RQ) in rats housed under thermoneutral (TMN; Ta = 30°C) or cool (COOL; Ta=15°C) conditions. Graphs depict total oxygen consumption (A,B) and normalized oxygen consumption (C,D) using the Kleiber method. Data are mean baseline values obtained daily at one year of CR ± S.E.M. up to the break. After the break, four days of re-feeding data are shown. *p<.05 vs AL at both Ta.
Long-term CR induced a mild reduction in blood pressure in TMN rats, but increased blood pressure in COOL rats. These effects were still evident after one year of CR. Re-feeding after this long-term CR had no effect on blood pressure in COOL rats (Dk:p<.05 Lt:p=.97) but restores blood pressure in TMN rats to control levels (Dk:p=.27; Lt:p=.21 Fig. 7A,B) despite lower body weight (See Fig. 5D). Re-feeding immediately induced an increase in heart rate correcting the bradycardia seen in TMN-CR rats and in the light phase in COOL-CR rats (Fig 7C,D).
FIGURE 7.
Effect of four days re-feeding after one year CR on (A) Dark and (B) Light phase mean arterial pressure (MAP), and (C,D) heart rate (HR) in rats housed under thermoneutral (TMN; Ta = 30°C) or cool (COOL; Ta=15°C) conditions. Data are mean baseline values obtained daily at one year of CR ± S.E.M. up to the break. After the break, four days of re-feeding data are shown. *p<.01 vs AL Ta; tp<.05 TMN:ALvsRF only; cp<.05 COOL:ALvsRF only
4. DISCUSSION
4.1 Significance
A major component of the homeostatic response to decreased energy intake is reduced energy expenditure. However, the duration of this adaptation remains poorly understood. We sought to address this issue by performing long-term CR studies in rats with low and high metabolic rates. We discovered that irrespective of background energy expenditure, the reduction in metabolic rate resulting from CR was sustained for 1 year. CR lowered heart rate and oxygen consumption in both the awake and sleeping phases of the circadian cycle. Further, we determined that CR clearly shifted the regression relationship relating metabolic mass to energy expenditure. This approach has been used by others (Greenberg, 1999; Greenberg and Boozer, 2000) and the findings suggest that CR enhances the metabolic efficiency of these tissues. This finding was observed in CR animals maintained in both cool and thermoneutral conditions. Interestingly, 10 wk-old TMN rats fed ad libitum did not display a strong relationship between metabolic mass and energy expenditure as evidenced by a reduced slope of the regression line. This may be due to reduced sympathetic drive and lipolysis required to maintain body temperature at thermoneutrality (Guerra et al., 1998a; Guerra et al., 1998b). Indeed, we consistently observe higher respiratory quotient during indirect calorimetry studies in rats studied at thermoneutrality. Therefore, the utilization of lipid in these animals is likely lower than in the COOL animals consistent with a “lipid sparing” effect (Iossa et al., 2002; Samec et al., 1998, 2000).
It is important to note that we chose to perform this study using mature, adult FBNF1 rats. This particular strain exhibits slow growth and development of mild obesity with aging (Altun et al., 2007; Scarpace and Tumer, 2001; Story et al., 1981). Therefore, the effects of CR reported herein are unlikely to involve a stunting of growth that occurs if CR is imposed on growing animals shortly after weaning (Rollo, 2002; Straus, 1994). Another consideration in the interpretation of these findings is the magnitude of CR used in this study. We have observed previously that imposing the same relative magnitude of CR (as a % of ad lib caloric intake) produces a greater metabolic stress on animals housed in a COOL environment (Evans et al., 2005b). This is not surprising since this represents a greater absolute caloric deficit and the animals are in less positive energy balance than animals housed in TMN. Therefore, we chose to impose similar absolute caloric deficits on the two groups of animals.
It is also important to note that the study of caloric restriction under COOL and TMN conditions allows us to compare the physiological consequences of caloric restriction and of re-feeding on mechanistically-unique metabolic backgrounds. Adaptive thermogenesis such as metabolic heat production by brown adipose tissue is a significant component of energy expenditure under COOL conditions but these processes are relatively dormant at TMN (Champigny and Ricquier, 1990; Melnyk et al., 1997; Wiesinger et al., 1990). Although there is no direct evidence in this study, CR decreases BAT expression of uncoupling proteins at temperatures below TMN (Mookerjee et al., 2010; Sivitz et al., 1999a; Sivitz et al., 1999b; Valle et al., 2005; Valle et al., 2007). Therefore, the physiological comparison between these two paradigms provides indirect evidence that suppression of BAT metabolism is not a critical component of the metabolic response to caloric restriction.
4.2 Oxygen consumption is suppressed during long-term caloric restriction
An important finding from these studies is that when VO2 is corrected for decreased body weight or metabolic mass, an overall suppression of metabolism is still observed over 10 weeks and after one year of CR. Additionally, regression analysis revealed lower energy expenditure per gram of metabolic organ mass in CR animals compared to controls under both COOL and TMN conditions. Further, re-feeding induced a significant increase in both absolute and normalized oxygen consumption suggesting that caloric restriction suppressed metabolism that was restored within 1-2 days after access to AL food. Conflicting reports have indicated that CR does reduce metabolism (Blanc et al., 2003; Dulloo and Girardier, 1993a; Greenberg, 1999; Heilbronn et al., 2006; MacLean et al., 2004) and that CR does not reduce metabolism (Masoro et al., 1982; McCarter et al., 1985; McCarter and Palmer, 1992; Selman et al., 2005). Importantly, our finding is also consistent with evidence indicating sustained CR-induced suppression of metabolism after adjusting for body composition (Heilbronn et al., 2006; Redman et al., 2009). Herein, we find that, irrespective of background metabolic rates established by variations in Ta, that CR reduces energy expenditure when analyzed on the basis of metabolic mass.
The notion that CR extends lifespan in part by reducing metabolic rate (Conti et al., 2006) and generation of reactive oxygen species (Colom et al., 2007; Csiszar et al., 2009) is an attractive hypothesis consistent with Pearl’s rate of living theory (Speakman et al., 2002). However, there is a growing body of evidence that directly and effectively disputes the rate of living concept. We utilized cool exposure in the current study to elevate metabolic rate, which should, according to Pearl’s ideas, shorten lifespan. However, daily intermittent cold-exposure stress in rats (Holloszy and Smith, 1986), and chronic cold exposure in voles does not alter lifespan (Selman et al., 2008). An analysis of metabolic rate of various breeds of dogs suggests that those with higher levels of energy expenditure live longer (Speakman et al., 2003). Finally, within a strain of mice, individuals with elevated metabolic rates live longer (Speakman et al., 2004). It is interesting to note that recent evidence indicates mice with the lowest body temperature and the greater metabolic efficiency exhibited the greatest CR-induced lifespan extension (Conti et al., 2006). CR reduces body temperature (Rikke and Johnson, 2007), but it is not yet known if CR-induced reductions in body temperature are a requisite response to observe lifespan extension.
Furthermore, it is now known that CR induces autophagy both in lower organisms (Jia and Levine, 2007) and mammals (Bergamini et al., 2003). Autophagy leads to targeting and degradation of organelles (Bergamini, 2006) and maintains healthy cells by replacing components such as mitochondria and peroxisomes, which have become damaged due to oxidative stress (Donati et al., 2006). This damage repair mechanism to oxidative stress preventing apoptosis (Cecconi and Levine, 2008). Failure of autophagy to repair damaged tissues at the cellular level is also implicated in aging-related cell damage (Bergamini, 2006; Vellai, 2009). Thus, it is likely that autophagy represents another important component of the generally observed life extension produced by CR. In this context, it is very intriguing to acknowledge that a recent report that CR-reduces lifespan in some strains of mice (Rikke et al., 2010). Taken together, the findings clearly indicate that much remains to be understood regarding the mechanisms linking energy balance, metabolism and lifespan.
4.3 Bradycardia persists during long-term caloric restriction
Heart rate is very sensitive to both energetic and thermal stimuli. Short term CR consistently produces bradycardia (Evans et al., 2005a; Evans et al., 2005b). In this report, we observe a remarkable stability of CR-induced bradycardia for an entire year of CR regardless of ambient temperature. The sustained bradycardia suggests that suppression of the sympathetic nervous system during negative energy balance may be maintained to an extent of that observed in acute studies (Young and Landsberg, 1977). Spectral analysis suggests that CR-induced bradycardia may be due to increased vagal tone (Mager et al., 2006). Several studies of caloric restriction in mice (Hunt et al., 2004; Swoap, 2001; Williams et al., 2002) and acute fasting in rats (Overton et al., 2001b; Williams et al., 2000; Young and Landsberg, 1977) observed a decrease in both heart rate and blood pressure. However, we have observed the bradycardia without hypotension during short-term CR in several rodent strains (Evans et al., 2005a; Evans et al., 2005b). CR-induced hypotension can be observed in normotensive rats studied at standard Ta, but is delayed several weeks (Mager et al., 2006; Sharifi et al., 2008). In conjunction with this, TMN rats exhibited a delayed decrease in blood pressure from after 6 weeks to one year of CR. Interestingly, this response was not observed in rats housed in COOL conditions. It is well known that exposure to cooler environments increases HR, MAP and sympathetic activity in rats (Sun et al., 1997) and it may be that the chronic mechanisms associated with elevated MAP in COOL prevent the physiologic expression of the hypotensive actions of CR.
4.4 Rapid recovery of heart rate and metabolic rate during re-feeding following long-term CR
The homeostatic responses to one year of CR were generally absent after just 4 days of AL re-feeding even though body weight, body fat and leptin levels were substantially below levels of AL controls. Considering a body weight deficit greater than 100 grams compared to age-matched controls (Figure 5B) and the drastic differences in serum leptin, it was expected that re-feeding would be associated with sustained hyperphagia and reduced metabolic rate would be observed in order to favor the catch-up growth seen in previous studies observing rapid weight and adipose tissue restoration after short-term restriction (Crescenzo et al., 2003; Dulloo and Girardier, 1993a; MacLean et al., 2004; Summermatter et al., 2007). The findings suggest the possibility that long-term CR may indeed produce a modest “re-setting” of the regulated levels of body fat. However, the cessation of growth due to age in these rats alters energy expenditure due to the normal energy cost of anabolic growth in younger controls compared to weight-matched re-fed animals, as others have reported (Dulloo and Girardier, 1993b). Therefore, the hypothesis that body fat “set point” is reset is clearly speculative and in need of experimental evaluation as we have planned in the future.
It is well-established that short term food restriction reduces respiratory quotient suggesting increased utilization of fat stores (Duffy et al., 1989; Evans et al., 2005a). Careful analysis of our continuous indirect calorimetry data reveals that the situation is actually quite complex. Animals placed on a CR regimen quickly consolidate their eating behavior into one large continuous meal whenever the food is presented. In these studies, we presented the food at the onset of the dark cycle. Thus, RQ is generally much higher in the dark phase, when ingested food is available for utilization. However, during the light phase, it is clear that RQ is consistently decreased, indicating that these animals may be relying more on stored lipid during the light phase. Furthermore, this response was clearly more evident in rats housed in COOL conditions with elevated energy requirements. After one year of CR, animals housed in the thermoneutral environment exhibited the greatest hyperphagia and likely explaining a higher RQ during re-feeding. COOL animals exhibit blunted increases in RQ during re-feeding hyperphagia, perhaps reflecting altered substrate flux associated with altered expression of UCPs. We acknowledge that additional information on the metabolic alterations in muscle and fat associated with long term CR and re-feeding would be of interest. Furthermore, while the study suggests that some of the systemic physiologic responses to long-term CR are quickly restored with re-feeding, it is clear that other adaptations at the cellular and molecular level, including adaptations associate with autophagy, are not likely influenced in a similar way. Further study is needed to clarify the role of physiological processes such as autophagy, oxidative stress, and cellular adaptations to re-feeding after long-term caloric restriction.
4.5 Conclusion
Our findings provide support for the hypothesis that an important physiological response to CR is suppression of metabolism and bradycardia and that these responses are maintained across one year of restriction. In addition, re-feeding induces a rapid restoration of energy expenditure after a long period of restriction. Lastly, the mechanistic role of neurobiological signals in these dynamic changes to restriction and re-feeding warrant further study as duration of restriction and re-feeding may play a differential role in these neuronal and hormonal responses.
5. ACKNOWLEDGMENTS
We would like to thank the contributions of E. Mariano, J. Jaramillo and A. Chandler to the completion of this work. Supported by NIA AG023837.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. REFERENCES
- Altun M, Bergman E, Edstrom E, Johnson H, Ulfhake B. Behavioral impairments of the aging rat. Physiology & behavior. 2007;92:911–923. doi: 10.1016/j.physbeh.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Bergamini E. Autophagy: a cell repair mechanism that retards ageing and age-associated diseases and can be intensified pharmacologically. Mol Aspects Med. 2006;27:403–410. doi: 10.1016/j.mam.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Bergamini E, Cavallini G, Donati A, Gori Z. The anti-ageing effects of caloric restriction may involve stimulation of macroautophagy and lysosomal degradation, and can be intensified pharmacologically. Biomed Pharmacother. 2003;57:203–208. doi: 10.1016/s0753-3322(03)00048-9. [DOI] [PubMed] [Google Scholar]
- Blanc S, Schoeller D, Kemnitz J, Weindruch R, Colman R, Newton W, Wink K, Baum S, Ramsey J. Energy Expenditure of Rhesus Monkeys Subjected to 11 Years of Dietary Restriction. J Clin Endocrinol Metab. 2003;88:16–23. doi: 10.1210/jc.2002-020405. [DOI] [PubMed] [Google Scholar]
- Cecconi F, Levine B. The Role of Autophagy in Mammalian Development: Cell Makeover Rather than Cell Death. Developmental Cell. 2008;15:344–357. doi: 10.1016/j.devcel.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champigny O, Ricquier D. Effects of fasting and refeeding on the level of uncoupling protein mRNA in rat brown adipose tissue: evidence for diet-induced and cold-induced responses. J Nutr. 1990;120:1730–1736. doi: 10.1093/jn/120.12.1730. [DOI] [PubMed] [Google Scholar]
- Colom B, Oliver J, Roca P, Garcia-Palmer FJ. Caloric restriction and gender modulate cardiac muscle mitochondrial H2O2 production and oxidative damage. Cardiovascular Research. 2007;74:456–465. doi: 10.1016/j.cardiores.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, Fabre V, Huitron-Resendiz S, Henriksen S, Zorrilla EP, de Lecea L, Bartfai T. Transgenic mice with a reduced core body temperature have an increased life span. Science (New York, N.Y. 2006;314:825–828. doi: 10.1126/science.1132191. [DOI] [PubMed] [Google Scholar]
- Crescenzo R, Samec S, Antic V, Rohner-Jeanrenaud F, Seydoux J, Montani JP, Dulloo AG. A role for suppressed thermogenesis favoring catch-up fat in the pathophysiology of catch-up growth. Diabetes. 2003;52:1090–1097. doi: 10.2337/diabetes.52.5.1090. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: Role of circulating factors and SIRT1. Mechanisms of Ageing and Development. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati A, Taddei M, Cavallini G, Bergamini E. Stimulation of macroautophagy can rescue older cells from 8-OHdG mtDNA accumulation: a safe and easy way to meet goals in the SENS agenda. Rejuvenation Res. 2006;9:408–412. doi: 10.1089/rej.2006.9.408. [DOI] [PubMed] [Google Scholar]
- Duffy PH, Feuers RJ, Leakey JA, Nakamura K, Turturro A, Hart RW. Effect of chronic caloric restriction on physiological variables related to energy metabolism in the male Fischer 344 rat. Mech Ageing Dev. 1989;48:117–133. doi: 10.1016/0047-6374(89)90044-4. [DOI] [PubMed] [Google Scholar]
- Dulloo AG, Girardier L. 24 hour energy expenditure several months after weight loss in the underfed rat: evidence for a chronic increase in whole-body metabolic efficiency. Int J Obes Relat Metab Disord. 1993a;17:115–123. [PubMed] [Google Scholar]
- Dulloo AG, Girardier L. Adaptive role of energy expenditure in modulating body fat and protein deposition during catch-up growth after early undernutrition. Am J Clin Nutr. 1993b;58:614–621. doi: 10.1093/ajcn/58.5.614. [DOI] [PubMed] [Google Scholar]
- Dulloo AG, Jacquet J. An adipose-specific control of thermogenesis in body weight regulation. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S22–29. doi: 10.1038/sj.ijo.0801907. [DOI] [PubMed] [Google Scholar]
- Evans SA, Messina MM, Knight WD, Parsons AD, Overton JM. Long-Evans and Sprague-Dawley rats exhibit divergent responses to refeeding after caloric restriction. Am J Physiol Regul Integr Comp Physiol. 2005a;288:R1468–1476. doi: 10.1152/ajpregu.00602.2004. [DOI] [PubMed] [Google Scholar]
- Evans SA, Parsons AD, Overton JM. Homeostatic responses to caloric restriction: influence of background metabolic rate. J Appl Physiol. 2005b;99:1336–1342. doi: 10.1152/japplphysiol.01380.2004. [DOI] [PubMed] [Google Scholar]
- Feely RS, Larkin LM, Halter JB, Dengel DR. Chemical versus dual energy x-ray absorptiometry for detecting age-associated body compositional changes in male rats. Experimental gerontology. 2000;35:417–427. doi: 10.1016/s0531-5565(00)00095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning JI, Smith JC. Consistency of meal patterns in laboratory rats. Physiology & behavior. 1994;56:7–16. doi: 10.1016/0031-9384(94)90255-0. [DOI] [PubMed] [Google Scholar]
- Greenberg JA. Organ metabolic rates and aging: two hypotheses. Med Hypotheses. 1999;52:15–22. doi: 10.1054/mehy.1997.0619. [DOI] [PubMed] [Google Scholar]
- Greenberg JA, Boozer CN. Metabolic mass, metabolic rate, caloric restriction, and aging in male Fischer 344 rats. Mech Ageing Dev. 2000;113:37–48. doi: 10.1016/s0047-6374(99)00094-9. [DOI] [PubMed] [Google Scholar]
- Guerra C, Koza RA, Walsh K, Kurtz DM, Wood PA, Kozak LP. Abnormal nonshivering thermogenesis in mice with inherited defects of fatty acid oxidation. J Clin Invest. 1998a;102:1724–1731. doi: 10.1172/JCI4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998b;102:412–420. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-Month Calorie Restriction on Biomarkers of Longevity, Metabolic Adaptation, and Oxidative Stress in Overweight Individuals: A Randomized Controlled Trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. for the Pennington, C.T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy JO, Smith EK. Longevity of cold-exposed rats: a reevaluation of the “rate-of-living theory”. J Appl Physiol. 1986;61:1656–1660. doi: 10.1152/jappl.1986.61.5.1656. [DOI] [PubMed] [Google Scholar]
- Hunt LM, Hogeland EW, Henry MK, Swoap SJ. Hypotension and bradycardia during caloric restriction in mice are independent of salt balance and do not require ANP receptor. Am J Physiol Heart Circ Physiol. 2004;287:H1446–1451. doi: 10.1152/ajpheart.00353.2004. [DOI] [PubMed] [Google Scholar]
- Iossa S, Mollica M, Lionetti L, Crescenzo R, Botta M, Samec S, Solinas G, Mainieri D, Dulloo A, Liverini G. Skeletal muscle mitochondrial efficiency and uncoupling protein 3 in overeating rats with increased thermogenesis. Pflügers Archiv European Journal of Physiology. 2002;445:431–436. doi: 10.1007/s00424-002-0964-0. [DOI] [PubMed] [Google Scholar]
- Jia K, Levine B. Autophagy is required for dietary restriction-mediated life span extension in C. elegans. Autophagy. 2007;3:597–599. doi: 10.4161/auto.4989. [DOI] [PubMed] [Google Scholar]
- Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9:92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Peters JC, Hill JO. Metabolic adjustments with the development, treatment, and recurrence of obesity in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R288–297. doi: 10.1152/ajpregu.00010.2004. [DOI] [PubMed] [Google Scholar]
- Mager DE, Wan R, Brown M, Cheng A, Wareski P, Abernethy DR, Mattson MP. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J. 2006;20:631–637. doi: 10.1096/fj.05-5263com. [DOI] [PubMed] [Google Scholar]
- Masoro EJ, Yu BP, Bertrand HA. Action of food restriction in delaying the aging process. Proc Natl Acad Sci U S A. 1982;79:4239–4241. doi: 10.1073/pnas.79.13.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter R, Masoro EJ, Yu BP. Does food restriction retard aging by reducing the metabolic rate? Am J Physiol Endocrinol Metab. 1985;248:E488–490. doi: 10.1152/ajpendo.1985.248.4.E488. [DOI] [PubMed] [Google Scholar]
- McCarter RJ, Palmer J. Energy metabolism and aging: a lifelong study of Fischer 344 rats. Am J Physiol Endocrinol Metab. 1992;263:E448–452. doi: 10.1152/ajpendo.1992.263.3.E448. [DOI] [PubMed] [Google Scholar]
- Melnyk A, Harper ME, Himms-Hagen J. Raising at thermoneutrality prevents obesity and hyperphagia in BAT-ablated transgenic mice. Am J Physiol. 1997;272:R1088–1093. doi: 10.1152/ajpregu.1997.272.4.R1088. [DOI] [PubMed] [Google Scholar]
- Mookerjee SA, Divakaruni AS, Jastroch M, Brand MD. Mitochondrial uncoupling and lifespan. Mech Ageing Dev. 2010;131:463–472. doi: 10.1016/j.mad.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton JM, Williams TD, Chambers JB, Rashotte ME. Cardiovascular and metabolic responses to fasting and thermoneutrality are conserved in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2001a;280:R1007–1015. doi: 10.1152/ajpregu.2001.280.4.R1007. [DOI] [PubMed] [Google Scholar]
- Overton JM, Williams TD, Chambers JB, Rashotte ME. Central Leptin Infusion Attenuates the Cardiovascular and Metabolic Effects of Fasting in Rats. Hypertension. 2001b;37:663–669. doi: 10.1161/01.hyp.37.2.663. [DOI] [PubMed] [Google Scholar]
- Rashotte ME, Basco PS, Henderson RP. Daily cycles in body temperature, metabolic rate, and substrate utilization in pigeons: influence of amount and timing of food consumption. Physiology & behavior. 1995;57:731–746. doi: 10.1016/0031-9384(94)00315-7. [DOI] [PubMed] [Google Scholar]
- Redman LM, Heilbronn LK, Martin CK, de Jonge L, Williamson DA, Delany JP, Ravussin E. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One. 2009;4:e4377. doi: 10.1371/journal.pone.0004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikke BA, Johnson TE. The Physiological Genetics of Dietary Restriction: Uncoupling the Body Temperature and Body Weight Responses. Am J Physiol Regul Integr Comp Physiol. 2007 doi: 10.1152/ajpregu.00215.2007. [DOI] [PubMed] [Google Scholar]
- Rikke BA, Liao CY, McQueen MB, Nelson JF, Johnson TE. Genetic dissection of dietary restriction in mice supports the metabolic efficiency model of life extension. Experimental gerontology. 2010 doi: 10.1016/j.exger.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollo CD. Growth negatively impacts the life span of mammals. Evol Dev. 2002;4:55–61. doi: 10.1046/j.1525-142x.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- Samec S, Seydoux J, Dulloo AG. Role of UCP homologues in skeletal muscles and brown adipose tissue: mediators of thermogenesis or regulators of lipids as fuel substrate? FASEB J. 1998;12:715–724. doi: 10.1096/fasebj.12.9.715. [DOI] [PubMed] [Google Scholar]
- Samec S, Seydoux J, Dulloo AG. Downregulation of skeletal muscle UCP-3 gene expression during refeeding is prevented by cold exposure. Pflugers Arch. 2000;439:723–729. doi: 10.1007/s004249900237. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Tumer N. Peripheral and hypothalamic leptin resistance with age-related obesity. Physiology & behavior. 2001;74:721–727. doi: 10.1016/s0031-9384(01)00616-3. [DOI] [PubMed] [Google Scholar]
- Selman C, McLaren JS, Collins AR, Duthie GG, Speakman JR. The impact of experimentally elevated energy expenditure on oxidative stress and lifespan in the short-tailed field vole Microtus agrestis. Proceedings. 2008 doi: 10.1098/rspb.2008.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, Phillips T, Staib JL, Duncan JS, Leeuwenburgh C, Speakman JR. Energy expenditure of calorically restricted rats is higher than predicted from their altered body composition. Mechanisms of Ageing and Development. 2005;126:783. doi: 10.1016/j.mad.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Sharifi A, Mohseni S, Nekoparvar S, Larijani B, Fakhrzadeh H, Oryan S. Effect of caloric restriction on nitric oxide production, ACE activity, and blood pressure regulation in rats. Acta Physiologica Hungarica. 2008;95:55–63. doi: 10.1556/APhysiol.95.2008.1.3. [DOI] [PubMed] [Google Scholar]
- Sivitz WI, Fink BD, Donohoue PA. Fasting and leptin modulate adipose and muscle uncoupling protein: divergent effects between messenger ribonucleic acid and protein expression. Endocrinology. 1999a;140:1511–1519. doi: 10.1210/endo.140.4.6668. [DOI] [PubMed] [Google Scholar]
- Sivitz WI, Fink BD, Morgan DA, Fox JM, Donohoue PA, Haynes WG. Sympathetic inhibition, leptin, and uncoupling protein subtype expression in normal fasting rats. Am J Physiol. 1999b;277:E668–677. doi: 10.1152/ajpendo.1999.277.4.E668. [DOI] [PubMed] [Google Scholar]
- Speakman JR, Selman C, McLaren JS, Harper EJ. Living fast, dying when? The link between aging and energetics. J Nutr. 2002;132:1583S–1597S. doi: 10.1093/jn/132.6.1583S. [DOI] [PubMed] [Google Scholar]
- Speakman JR, Talbot DA, Selman C, Snart S, McLaren JS, Redman P, Krol E, Jackson DM, Johnson MS, Brand MD. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell. 2004;3:87–95. doi: 10.1111/j.1474-9728.2004.00097.x. [DOI] [PubMed] [Google Scholar]
- Speakman JR, van Acker A, Harper EJ. Age-related changes in the metabolism and body composition of three dog breeds and their relationship to life expectancy. Aging Cell. 2003;2:265–275. doi: 10.1046/j.1474-9728.2003.00061.x. [DOI] [PubMed] [Google Scholar]
- Story JA, Gomolinski E, Czarnecki SK, Tepper SA, Kritchevsky D. Age-strain interrelations in lipid metabolism of rats. Lipids. 1981;16:87–92. doi: 10.1007/BF02535679. [DOI] [PubMed] [Google Scholar]
- Straus DS. Nutritional regulation of hormones and growth factors that control mammalian growth. FASEB J. 1994;8:6–12. doi: 10.1096/fasebj.8.1.8299891. [DOI] [PubMed] [Google Scholar]
- Summermatter S, Mainieri D, Russell AP, Seydoux J, Montani JP, Buchala A, Solinas G, Dulloo AG. Thrifty metabolism that favors fat storage after caloric restriction: a role for skeletal muscle phosphatidylinositol-3-kinase activity and AMP-activated protein kinase. Faseb J. 2007 doi: 10.1096/fj.07-8972com. [DOI] [PubMed] [Google Scholar]
- Sun Z, Cade JR, Fregly MJ, Rowland NE. Effect of chronic treatment with propranolol on the cardiovascular responses to chronic cold exposure. Physiology & behavior. 1997;62:379–384. doi: 10.1016/s0031-9384(97)00033-4. [DOI] [PubMed] [Google Scholar]
- Swoap SJ. Altered leptin signaling is sufficient, but not required, for hypotension associated with caloric restriction. Am J Physiol Heart Circ Physiol. 2001;281:H2473–2479. doi: 10.1152/ajpheart.2001.281.6.H2473. [DOI] [PubMed] [Google Scholar]
- Valle A, Catala-Niell A, Colom B, Garcia-Palmer FJ, Oliver J, Roca P. Sex-related differences in energy balance in response to caloric restriction. Am J Physiol Endocrinol Metab. 2005;289:E15–22. doi: 10.1152/ajpendo.00553.2004. [DOI] [PubMed] [Google Scholar]
- Valle A, Garcia-Palmer FJ, Oliver J, Roca P. Sex differences in brown adipose tissue thermogenic features during caloric restriction. Cell Physiol Biochem. 2007;19:195–204. doi: 10.1159/000099207. [DOI] [PubMed] [Google Scholar]
- Vellai T. Autophagy genes and ageing. Cell Death Differ. 2009;16:94–102. doi: 10.1038/cdd.2008.126. [DOI] [PubMed] [Google Scholar]
- Wiesinger H, Klaus S, Heldmaier G, Champigny O, Ricquier D. Increased nonshivering thermogenesis, brown fat cytochrome-c oxidase activity, GDP binding, and uncoupling protein mRNA levels after short daily cold exposure of Phodopus sungorus. Can J Physiol Pharmacol. 1990;68:195–200. doi: 10.1139/y90-030. [DOI] [PubMed] [Google Scholar]
- Williams TD, Chambers JB, Henderson RP, Rashotte ME, Overton JM. Cardiovascular responses to caloric restriction and thermoneutrality in C57BL/6J mice. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1459–1467. doi: 10.1152/ajpregu.00612.2001. [DOI] [PubMed] [Google Scholar]
- Williams TD, Chambers JB, May OL, Henderson RP, Rashotte ME, Overton JM. Concurrent reductions in blood pressure and metabolic rate during fasting in the unrestrained SHR. Am J Physiol Regul Integr Comp Physiol. 2000;278:R255–262. doi: 10.1152/ajpregu.2000.278.1.R255. [DOI] [PubMed] [Google Scholar]
- Young JB, Landsberg L. Suppression of sympathetic nervous system during fasting. Science (New York, N.Y. 1977;196:1473–1475. doi: 10.1126/science.867049. [DOI] [PubMed] [Google Scholar]