Abstract
Insulin-stimulated glucose uptake through GLUT4 plays a pivotal role in maintaining normal blood glucose levels. Glucose transport through GLUT4 requires both GLUT4 translocation to the plasma membrane and GLUT4 activation at the plasma membrane. Here we report that a cell-permeable phosphoinositide-binding peptide, which induces GLUT4 translocation without activation, sequestered PI 4,5-P2 in the plasma membrane from its binding partners. Restoring PI 4,5-P2 to the plasma membrane after the peptide treatment increased glucose uptake. No additional glucose transporters were recruited to the plasma membrane, suggesting that the increased glucose uptake was attributable to GLUT4 activation. Cells overexpressing phosphatidylinositol-4-phosphate 5-kinase treated with the peptide followed by its removal exhibited a higher level of glucose transport than cells stimulated with a submaximal level of insulin. However, only cells treated with submaximal insulin exhibited translocation of the PH-domains of the general receptor for phosphoinositides (GRP1) to the plasma membrane. Thus, PI 4,5-P2, but not PI 3,4,5-P3 converted from PI 4,5-P2, induced GLUT4 activation. Inhibiting F-actin remodeling after the peptide treatment significantly impaired GLUT4 activation induced either by PI 4,5-P2 or by insulin. These results suggest that PI 4,5-P2 in the plasma membrane acts as a second messenger to activate GLUT4, possibly through F-actin remodeling.
Keywords: GLUT4; Glucose transport; PI 4,5-P2; F-actin
1. Introduction
Insulin stimulates glucose uptake into adipose tissues and skeletal muscle through the glucose transporter GLUT4. In type 2 diabetes, this process is frequently impaired, resulting in abnormal glucose homeostasis [1,2]. Thus, identifying the molecular basis of GLUT4-mediated glucose uptake stimulated by insulin is crucial in finding a countermeasure to type 2 diabetes.
Multiple steps that are regulated by insulin lead to transport of glucose through GLUT4 [3–6]. First, insulin stimulation induces translocation of GLUT4-containing vesicles from the cytoplasm to the plasma membrane by a process that requires an intact cytoskeleton [7–9]. Next, insulin stimulation provokes membrane fusion of GLUT4-containing vesicles with the plasma membrane, inserting GLUT4 in the plasma membrane so that GLUT4 faces both the inside and outside of the cells [10,11]. Coincident with membrane insertion, insulin stimulates SNARE complex formation to provoke the membrane fusion step, which is inhibited by wortmannin suggesting a role for inositol lipids produced by the activity of PI 3-kinase [12,13]. Translocation and proper insertion of GLUT4 into the plasma membrane is necessary for insulin-stimulated glucose uptake, but an inconsistency between GLUT4 translocation/insertion and glucose uptake has been observed [14–27], suggesting that an additional step of GLUT4 activation within the plasma membrane may also be required. For instance, when insulin-stimulated glucose uptake was completely inhibited by wortmannin, GLUT4 insertion into the plasma membrane stimulated by insulin was only partially blocked [13,26]. On the other hand, the increased binding of impermeant glucose analogues, such as ATB-BMPA, to the plasma membrane after insulin stimulation was reported to match the increased glucose uptake [28,29], supporting a model that GLUT4 translocation/insertion is necessary and sufficient for glucose uptake. However, ATB-BMPA binds to the exofacial glucose binding site of GLUT4 that would engage during glucose transport. Therefore, the increased amount of GLUT4 exposed at the cell surface detected by this method reflects not only a recruitment of GLUT4 to the cell surface but also an increase in the fraction of GLUT4 in the plasma membrane that has changed from a ‘closed’ form to an ‘open’ form required to transport glucose. Thus, the discrepancy between GLUT4 translocation/insertion and glucose uptake implicates an additional step, activation of the glucose transport function of GLUT4, to regulate glucose transport by insulin.
Activating the glucose transport function of GLUT4 after translocation and insertion into the plasma membrane is also consistent with studies showing insulin-independent pathways to insert GLUT4 in the membrane without allowing glucose uptake. In one recent observation, a cell-permeable phosphoinositide-binding peptide (PBP10) induced GLUT4 translocation to the plasma membrane, thereby properly inserting GLUT4 into the plasma membrane. However, no glucose uptake was observed without additional insulin stimulation [30]. Treating adipocytes with cytochalasin B after insulin stimulation decreases insulin-stimulated glucose uptake, since cytochalasin B blocks the glucose-binding site of GLUT4 and inhibits the glucose transport ability of GLUT4 [31,32]. On the other hand, PBP10 treatment of 3T3-L1 adipocytes that were pretreated with insulin did not affect insulin-stimulated glucose uptake [30]. Therefore, an inability of PBP10 to increase glucose uptake is not due to an occlusion of GLUT4 by PBP10. Instead, these previous observations indicate that GLUT4 translocation/insertion and GLUT4 activation are separate events. While insulin stimulation induces both, resulting in increased glucose uptake, PBP10 treatment can induce GLUT4 translocation/insertion, but not GLUT4 activation. In our previous study, 3T3-L1 adipocytes that were pretreated with PBP10, which had the inactive form of GLUT4 in the plasma membrane, started to increase glucose uptake after subsequent insulin stimulation more quickly than cells stimulated by insulin without PBP10 pretreatment. Thus, insulin stimulation caused GLUT4 activation at the plasma membrane after completing translocation. However, at later time points after adding insulin, glucose uptake in cells not pretreated with PBP10 caught up with that in cells pretreated with PBP10, and surpassed it thereafter [30]. These observations suggest that PBP10 treatment not only lacks the ability to activate GLUT4, but also has an inhibitory effect on insulin-induced GLUT4 activation and prevents GLUT4 from its full activation by insulin.
Although the molecular mechanism of the cellular events caused by PBP10 treatment is still unclear, the available in vitro and cellular data suggest that PBP10 binds to phosphoinositides [33,34]. In an attempt to identify the mechanism of GLUT4 activation, we particularly focused on phosphoinositide signaling at the plasma membrane where, in our previous study, GLUT4 was activated [30].
Mammalian cells have multiple types of phosphoinositides that are phosphorylated at the 3′, 4′, or 5′ positions of the inositol ring, singly or in combinations. Depending on which position(s) in the inositol ring is (are) phosphorylated, each phosphoinositide exhibits specific cellular functions and binds specific ligands at its specific subcellular location [35–37]. PI 4,5-P2 is found abundantly in the plasma membrane and has been reported to be involved in regulating the actin cytoskeleton, membrane trafficking, and ion channel activity at the plasma membrane [38], which led us to investigate an involvement of PI 4,5-P2 in GLUT4 activation at the plasma membrane.
Here we report that PI 4,5-P2 in the plasma membrane is sequestered from its binding partners in the presence of PBP10. PI 4,5-P2 replenished after PBP10 treatment, without being phosphorylated into PI 3,4,5-P3, induced GLUT4 activation, which was significantly impaired by inhibiting F-actin remodeling. Thus, these results suggest that PI 4,5-P2 functions as a second messenger in GLUT4 activation, presumably by regulating F-actin remodeling.
2. Materials and methods
2.1. Materials
PBP10 and its control peptide were synthesized as described previously [34]. Recombinant adenovirus for expressing the PH-domain of the general receptor for phosphoinositides (GRP1) tagged with EGFP, anti-GLUT1 antibody and anti-GLUT4 antibody were generous gifts from Dr. Morris J. Birnbaum. Plasmids for expressing the PH-domain of PLCδ1 tagged with EGFP and recombinant adenovirus for expressing Myc-tagged human type 1α phosphatidylinositol 4-phosphate 5-kinase were generous gifts from Dr. Helen L. Yin. Anti-Myc antibodies were purchased from Cell Signaling (Beverly, MA). Control IgG, Alexa Fluor-labeled secondary antibodies, latrunculin A and jasplakinolide were purchased from Molecular Probes (Eugene, OR). 2-deoxy-D-[3H]glucose and 3-O-methy-D-[3H] glucose were purchased from Amersham Biosciences (Piscataway, NJ). BODIPY TR-X C6 PI 3-P and BODIPY TR-X C6 PI 4,5-P2 were purchase from Echelon (Salt Lake City, UT). All other reagents from commercial sources were of analytical grade.
2.2. Cell culture
3T3-L1 fibroblasts were maintained and induced to differentiate into adipocytes as described previously [30]. The experiments were conducted 7–9 days after inducing differentiation, when > 90% of cells expressed the adipocyte phenotype to accumulate lipid droplets. 3T3-L1 adipocytes were serum starved overnight before each experiment. To express the PH-domain of PLCδ1, cells were electroporated as described previously [39]. Carrier-mediated phosphoinositide delivery was conducted according to the manufacturer’s instructions.
2.3. Immunostaining and microscopy
Cells were fixed and immunostained as described previously [30]. Images were acquired by a Delta Vision system (Applied Precision, Issaquah, WA) and deconvolved by SoftWoRx (Applied Precision). Membrane sheets were prepared and immunostained for GLUT4. The membrane sheets were identified by staining them with fluorescently-labeled concanavalin A. Images of GLUT4 in membrane sheets were obtained by a Leica DM-IRBE2 microscope and quantified by Openlab (Improvision, Lexington, MA) as described previously [30]. To quantify the amount of the HA-tag exposed to the extracellular surface, cells expressing HA-GLUT4-EGFP were immunostained for the HA-tag without permeation as described previously [40]. Fluorescence intensity for both the HA-tag and EGFP was counted. To measure the background fluorescence, control IgG was used instead of anti-HA antibodies. The expression level of HA-GLUT4-EGFP in each cell was calibrated by dividing the fluorescence intensity of the HA-tag by the fluorescence intensity of EGFP.
2.4. Glucose transport assay
3T3-L1 adipocytes plated in 24-well culture dishes were serum-starved as indicated above, and incubated in KRP buffer (128 mM NaCl, 4.7 mM KCl, 1.25 mM CaCl2, 1.25 mM MgSO4, 5 mM sodium phosphate, pH 7.4) for an additional 1 h prior to each treatment. The assay was initiated by adding 2-deoxy-D[3H]glucose or 3-O-methyl-D-[3H ]glucose (0.5 μCi/sample, 0.1 mmol), incubated at 37 °C for 4 min or for 1 min, respectively, and was terminated by washing the cells once with ice-cold KRP buffer containing 0.3 mM phloretin and then twice with ice-cold KRP buffer. The cells were then solubilized in 0.1% SDS, and the incorporated radioactivity was determined by scintillation counting.
3. Results
3.1. PBP10 sequesters PI 4,5-P2 from its binding partners
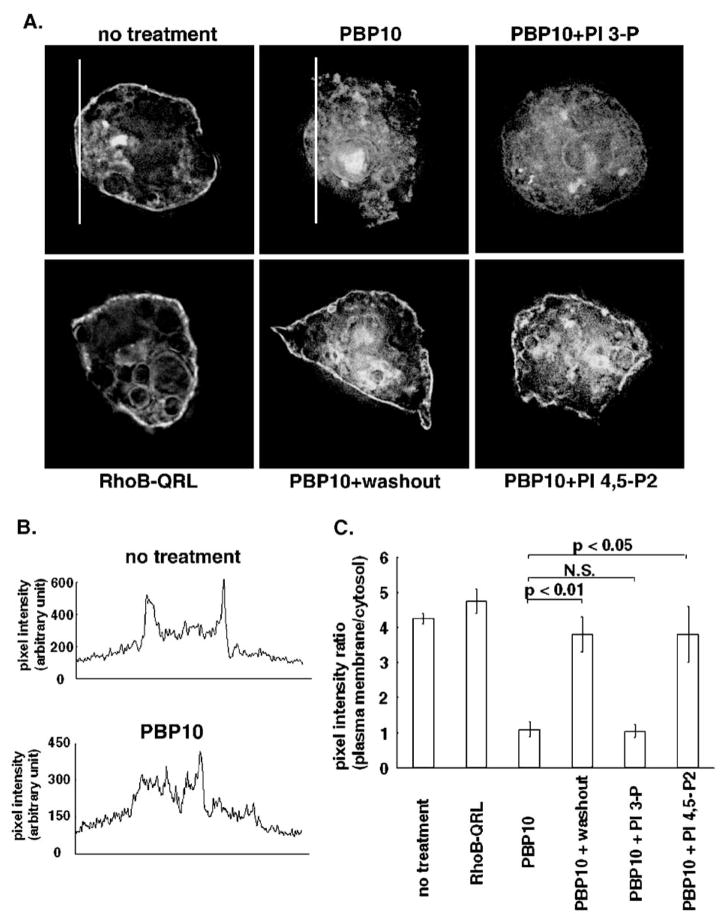
In our previous study, a cell-permeable phosphoinositide-binding peptide, PBP10, induced GLUT4 translocation to the plasma membrane and insertion into the plasma membrane without activaing the glucose transport activity of GLUT4 in 3T3-L1 adpocytes [30]. To elucidate the molecular mechanism of the cellular events caused by PBP10, changes in the availability of PI 4,5-P2 for its binding partners were monitored. To this end, we expressed EGFP-tagged PH-domains of PLCδ1, known to bind PI 4,5-P2 [41,42] in 3T3-L1 adipocytes. As shown in Fig. 1, in cells treated with a control rhodamine B-labeled peptide (RhoB-QRL), which is cell-permeable but does not bind phosphoinositide [34], the PH-domain was targeted to the plasma membrane. When cells were treated with PBP10, a rhodamine B-labeled cell-permeable phosphoinositide-binding peptide derived from a phosphoinositide-binding region in gelsolin [34], the PH-domain was dissociated from the plasma membrane. However, a plasma membrane localization of the PH-domain was significantly restored either by removing PBP10 or by addition of exogenous PI 4,5-P2, but not PI 3-P (Fig. 1C). These results suggest that PBP10 can sequester PI 4,5-P2 from its binding partners, but either a removal of PBP10 or a replenishment of PI 4,5-P2 makes PI 4,5-P2 available for PH-domains again.
Fig. 1.
PBP10 sequesters PI 4,5-P2 from its binding partners. The PH-domain of PLCδ1 tagged with EGFP was expressed in 3T3-L1 adipocytes by electroporation as described in Materials and methods. Cells were left untreated or treated either with 40 μM control peptide (RhoB-QRL) for 15 min, or with 40 μM PBP10 for 15 min. For washing out PBP10, cells were washed with a medium without PBP10 and incubated for 20 min with the medium. For replenishing exogenous phosphoinositides after PBP10 treatment, the medium was replaced with the one supplemented with either BODIPY TR-X C6 PI 3-P or BODIPY TR-X C6 PI 4,5-P2 along with its shuttle carriers. Cells were incubated in the presence of exogenous phosphoinositide for 5 min. (A) Each Panel shows the subcellular distribution of EGFP signal. Images were taken by deconvolution microscopy in sections through the middle of the cells. Representative images are shown from one experiment. Similar results were obtained from two other independent experiments. (B) For cells untreated and treated with PBP10 shown in A, the fluorescent intensity across the white lines was plotted as a line profile. (C) The fluorescence intensity ratio of the plasma membrane relative to the cytosol was measured from the line profile data of fluorescence intensity across cells as described previously [41,65]. Line profile data were obtained from each cell, as shown in B. For the plasma membrane, the highest intensity in the plasma membrane was adopted each time a line crosses the plasma membrane, obtaining two values from each cell. These two values were averaged for each cell. For the cytosol, the intensity of each pixel across a line in the cytosol of each cell was averaged. The average values±S.E. obtained from three independent experiments, in which 10 cells were counted for each treatment, are shown. N.S., not significant.
3.2. Combining PBP10 treatment and replenishing PI 4,5-P2 increase glucose uptake
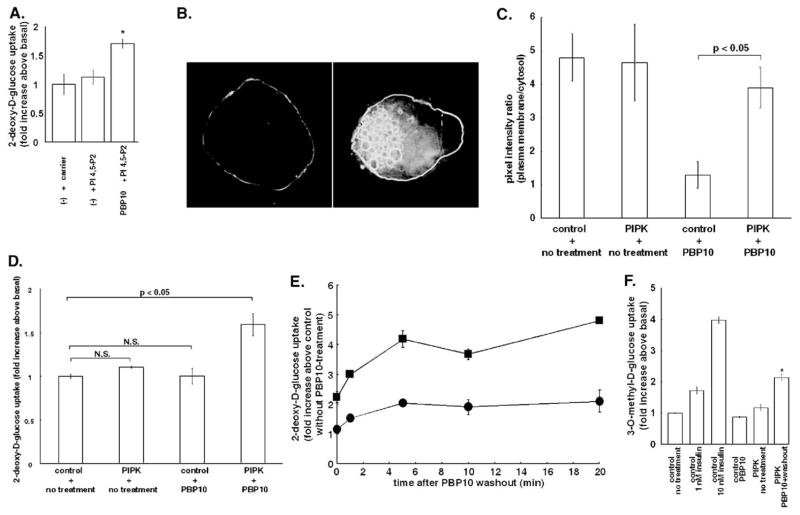
Since PBP10 pretreatment partially inhibited GLUT4 activation by insulin in our previous study [30], and PBP10 sequestered PI 4,5-P2 from its binding partners, by a mechanism that was reversed either by washing out PBP10 or by replenishing PI 4,5-P2 (Fig. 1), we investigated the effect of replenishing PI 4,5-P2 after PBP10 pretreatment on glucose uptake. For this purpose, we first measured 2-deoxy-D-glucose uptake in 3T3-L1 adipocytes treated with PBP10 followed by a delivery of exogenous PI 4,5-P2. As shown in Fig. 2A, delivering PI 4,5-P2 itself did not affect 2-deoxy-D-glucose uptake. However, when cells were pretreated with PBP10 to have inactive GLUT4 in the plasma membrane, exogenous PI 4,5-P2 significantly increased 2-deoxy-D-glucose uptake.
Fig. 2.
Replenishing PI 4,5-P2 after PBP10 treatment increases glucose uptake. (A) 3T3-L1 adipocytes were either left untreated(−) or treated with 40 μM PBP10 for 15 min. Then the medium was replaced with the one supplemented either with shuttle carrier only or with a mixture of shuttle carrier and BODIPY TR-X C6 PI 4,5-P2, and cells were incubated for 5 min. 2-deoxy-D-glucose uptake was measured. Data are mean±S.E., and are expressed as fold increases of glucose uptake in control cells without exogenous PI 4,5-P2 or PBP10. *p<0.05 versus control cells. (B) 3T3-L1 adipocytes were infected with a recombinant adenovirus that expresses myc-tagged human type 1α PIP-kinase at a level similar to its endogenous homologue (left panel) or at a level 10 times higher than the endogenous homologue (right panel). Cells were stained for the Myc-tag. Images were taken by deconvolution microscopy in sections through the middle of the cells. A representative image for each expression is presented. (C and D) 3T3-L1 adipocytes expressing the PH-domain of PLCδ1 tagged with EGFP (C) or 3T3-L1 adipocytes (D) were infected either with control LacZ adenovirus or with recombinant adenovirus for expressing human type 1α PIP-kinase (PIPK). Cells were then either left untreated or treated with 40 μM PBP10 for 15 min. In C, the fluorescence intensity ratio of the PH-domain in the plasma membrane relative to that in the cytosol was measured, as described in Fig. 1C. The average value±S.E. obtained from three independent experiments are shown. In D, 2-deoxy-D-glucose uptake was measured. Data are mean±S.E., and are expressed as fold increases of glucose uptake in control cells infected by control adenovirus and left untreated. N.S., not significant. (E) 3T3-L1 adipocytes were infected either with control LacZ adenovirus (circle) or with recombinant adenovirus for expressing human type 1α PIP-kinase (square). Cells were treated with 40 μM PBP10 for 15 min, washed with the medium and incubated in the medium for the indicated times. 2-deoxy-D-glucose uptake was measured. Data are mean±S.E., and are expressed as fold increases of glucose uptake in control cells infected by control adenovirus and left untreated with PBP10. (F) 3T3-L1 adipocytes were infected either with control LacZ adenovirus or with recombinant adenovirus for expressing human type 1α PIP-kinase (PIPK). Cells were then either left untreated or treated with 1 nM insulin, 10 nM insulin or with 40 μM PBP10 for 15 min. For washing out PBP10, cells were washed with the medium and incubated in the medium for additional 20 min. 3-O-methyl-D-glucose uptake was measured. Data are mean±S.E., and are expressed as fold increases of glucose uptake in control cells infected by control adenovirus and left untreated. *p<0.05 versus cells stimulated with 1 nM insulin. For panels A, D–F, similar results were obtained from two other independent experiments.
The effect of replenishing PI 4,5-P2 was also tested by overexpressing human type Iα phosphatidylinositol 4-phosphate 5-kinase (PIP-kinase), a lipid kinase that synthesizes PI 4,5-P2 from PI 4-P, by adenovirus-mediated gene transfer. Kanzaki et al. have reported that type I PIP-kinase overexpression in 3T3-L1 adipocytes led to formation of enlarged vacuoles coated with exogenous PIP-kinase and an accumulation of GLUT4 in the plasma membrane due to inhibition of GLUT4 endocytosis [43]. However, when human type Iα PIP-kinase was expressed at a low level, similar to that of endogenous mouse type Iβ PIP-kinase (a homologue of human type Iα PIP-kinase), forced expression of PIP-kinase did not induce vacuole formation or accumulate GLUT4 in the plasma membrane (Fig. 2B, left panel, and Fig. 3A). In these cells, the exogenous PIP-kinase was mainly targeted to the plasma membrane (Fig. 2B), where GLUT4 activation was observed in our previous study [30]. On the other hand, when human type Iα PIP-kinase was over-expressed at a level approximately 10 times higher than that of endogenous PIP-kinase, the exogenous PIP-kinase not only localized in the plasma membrane but also coated large vacuoles as reported previously (Fig. 2B, right panel) [43]. Thus, a low level expression of exogenous PIP-kinase was adopted in the experiments thereafter. A low level overexpression of PIP-kinase, which was targeted to the plasma membrane, significantly restored the plasma membrane localization of the PH-domain of PLCδ1 even in the presence of PBP10 (Fig. 2C). Thus, exogenous PIP-kinase replenished PI 4,5-P2 in the plasma membrane.
Fig. 3.
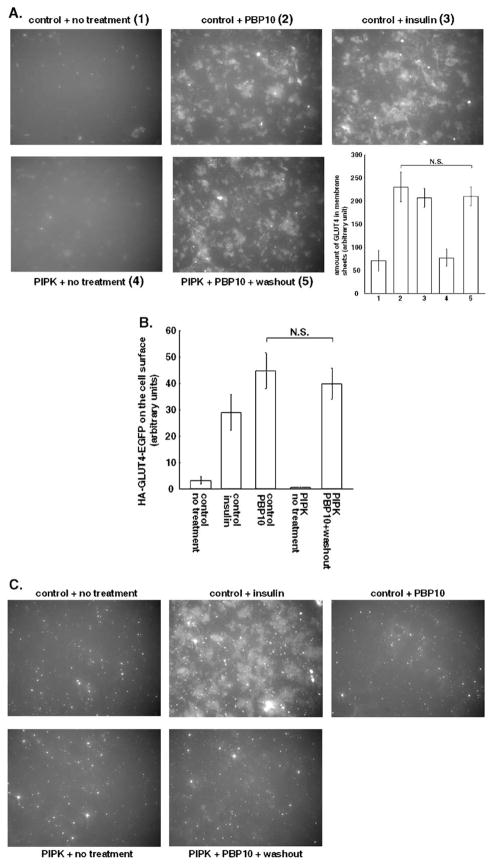
Increased glucose uptake by PI 4,5-P2 replenishment after PBP10 treatment is not attributable to recruitment of GLUT4 or GLUT1. 3T3-L1 adipocytes (A, C) or 3T3-L1 adipocytes expressing HA-GLUT4-EGFP (B) were infected either with control LacZ adenovirus (control) or with recombinant adenovirus for expressing human type 1α PIP-kinase (PIPK). Cells were then either left untreated or treated with 100 nM insulin or with 40 μM PBP10 for 15 min. For washing out PBP10, cells were, then, washed with the medium and incubated in the medium for 20 min. (A) Membrane sheets were prepared as described in Materials and methods. A representative image from three independent experiments is shown. For quantifying the amount of GLUT4 in the membrane sheets, the average values±S.E. obtained from three independent experiments are shown. N.S., not significant. (B) Cells were immunostained for the HA-tag without permeation and the amount of the HA-tag exposed at the cell surface was quantified as described in Materials and methods. The amount of the HA-tag on the cell surface per the amount of EGFP expressed in each cell was measured. The average value±S.E. obtained from three independent experiments, in which at least 20 cells were counted, are shown. N.S., not significant. (C) Membrane sheets were prepared and stained for GLUT1. A representative image out of three independent experiments is shown.
We next looked at the effect of combining PIP-kinase overexpression and PBP10 treatment on glucose uptake. Neither PBP10 treatment nor human type Iα PIP-kinase-overexpression alone increased 2-deoxy-D-glucose uptake (Fig. 2D). In contrast, 3T3-L1 adipocytes that overexpressed PIP-kinase significantly increased 2-deoxy-D-glucose uptake after PBP10 treatment. Consistent with the hypothesis that PI 4,5-P2 may need to be resynthesized or liberated at the plasma membrane in order to activate GLUT4, glucose uptake could also be restored by washing out PBP10 after GLUT4 was delivered to the plasma membrane. As shown in Fig. 2E, in adipocytes transfected with control adenovirus, washing out PBP10 had a tendency to increase 2-deoxy-D-glucose uptake, but the increase was not statistically significant. However, PIP-kinase-overexpressing cells exhibited a significantly enhanced 2-deoxy-D-glucose uptake at all time points over control adenovirus-transfected cells. In PIP-kinase-overexpressing cells, washing out PBP10 increased 2-deoxy-D-glucose uptake up to 4.8 fold over control cells infected by control adenovirus and left untreated.
Increased glucose uptake by PBP10 treatment followed by its removal in PIP-kinase overexpressing cells was also observed by measuring 3-O-methyl-D-glucose uptake, instead of measuring 2-deoxy-D-glucose uptake, confirming an increased glucose transport activity in these cells (Fig. 2F). The increase in glucose uptake in these cells was lower than that shown in Fig. 2E, presumably due to multiple passages, but still significantly higher than that in cells stimulated with a submaximal level of insulin.
Thus, PBP10 treatment followed by a replenishment of PI 4,5-P2 leads to an increased glucose uptake.
3.3. Increased glucose uptake by PI 4,5-P2 replenishment is not attributable to recruitment of GLUT4 or GLUT1
The increased glucose uptake in 3T3-L1 adipocytes treated with PBP10 and replenished with PI 4,5-P2 can be achieved by newly recruiting and activating GLUT4, instead of activating GLUT4 that is already distributed in the plasma membrane by PBP10 treatment. This could also be achieved by recruiting to the plasma membrane another type of glucose transporter, GLUT1, which is abundantly expressed in 3T3-L1 adipocytes.
To examine these possibilities, we quantified the amount of GLUT4 and GLUT1 in the plasma membrane. As shown in Fig. 3A, both insulin stimulation and PBP10 treatment increased the amount of GLUT4 in plasma membrane sheets by inducing GLUT4 translocation. PIP-kinase overexpression did not cause GLUT4 translocation. Treating PIP-kinase-overexpressing cells with PBP10 followed by its removal did not increase GLUT4 content in the membrane sheets compared to addition of PBP10 to control cells, but did significantly increase glucose uptake (Fig. 2E). The amount of GLUT4 in membrane sheets made from PBP10-treated cells was not changed when PBP10 was washed out from either control or PIP-kinase-overexpressing cells (data not shown).
A direct relation between GLUT4 present in membrane sheets and glucose uptake rate is complicated by evidence that GLUT4-containing vesicles tethered to but not inserted in the plasma membrane stay connected during membrane sheet preparation [44,45]. To evaluate the effect of PI 4,5-P2 replenishment on recruiting GLUT4 that is capable of glucose transport, we expressed HA-GLUT4-EGFP in 3T3-L1 adipocytes and quantified the amount of the HA-tag exposed at the cell surface. This epitope-tagged GLUT4 has been reported to translocate identically to endogenous GLUT4 when cells are stimulated with insulin [46]. In accordance with data for endogenous GLUT4 detected in membrane sheets, HA-GLUT4-EGFP was detected on the cell surface after PBP10 treatment, indicating GLUT4 translocation and insertion to the plasma membrane (Fig. 3B). PIP-kinase-overexpression itself did not expose HA-GLUT4-EGFP at the cell surface. Treating PIP-kinase-overexpressing cells with PBP10 followed by its removal, which exhibited a significant increase in glucose uptake, induced a comparable level of the HA-tag exposed at the cell surface as PBP10 treatment only. Thus, the increased glucose uptake was not due to a de novo recruitment of GLUT4. As reported previously [30], PBP10 treatment failed to translocate GLUT1 (Fig. 3C). Neither PIP-kinase overexpression nor a combination of PIP-kinase overexpression and PBP10 treatment followed by its removal caused GLUT1 translocation.
These results suggest that the increased glucose uptake observed in PIP-kinase overexpressing cells treated with PBP10 followed by its removal is attributable to an activation of GLUT4 but not GLUT1 in the plasma membrane.
3.4. PI 4,5-P2 functions as a second messenger to activate GLUT4
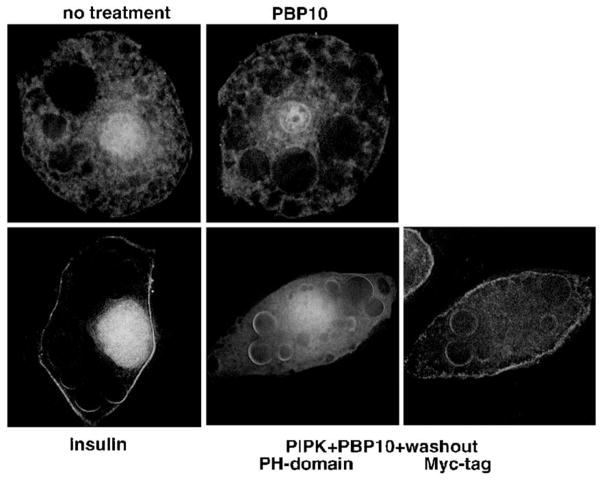
3T3-L1 adipocytes overexpressing PIP-kinase exhibited GLUT4 activation after PBP10 treatment and its removal, presumably by supplying more PI 4,5-P2. Since GLUT4 activation, as well as GLUT4 translocation, downstream of insulin stimulation requires PI 3-kinase activation [30], GLUT4 activation in PIP-kinase overexpressing cells may have been due to a conversion of PI 4,5-P2 supplied by the overexpressed PIP-kinase into PI 3,4,5-P3. To test this possibility, we looked at the subcellular distribution of the PH-domain of GRP1, whose translocation to the plasma membrane indicates PI 3,4,5-P3 production [47]. As shown in Fig. 4, insulin stimulation even at a submaximal level (1 nM) translocated the PH-domain of GRP1 to the plasma membrane. On the contrary, PIP-kinase-overexpressing cells treated with PBP10 followed by its removal failed to translocate the PH-domains of GRP1 to the plasma membrane, which exhibited a significantly higher level of glucose uptake than cells stimulated with 1 nM insulin (Fig. 2F).
Fig. 4.
PBP10 treatment followed by its removal in PIP-kinase-overexpressing cells does not increase PI 3,4,5-P3. 3T3-L1 adipocytes were infected with a recombinant adenovirus that expresses the PH-domains of GRP1 tagged with EGFP. For overexpressing PIP-kinase, cells were co-infected with a recombinant adenovirus that expresses Myc-tagged human type 1α PIP-kinase (PIPK). Cells were left untreated or treated either with 1 nM insulin or 40 μM PBP10 for 15 min. Cells overexpressing PIP-kinase were washed with the medium after PBP10 treatment, and incubated in the medium for additional 20 min. The subcellular localization of the PH-domains of GRP1 is shown. Cells overexpressing PIP-kinase were identified by staining for the Myc-tag. Images were taken by deconvolution microscopy in sections taken through the middle of the cells. Similar results were obtained from two other independent experiments.
These results suggest that PI 4,5-P2 acts directly to activate GLUT4 and not as a source of PI 3,4,5-P3.
3.5. Intact F-actin is required for PI 4,5-P2-dependent and insulin-dependent GLUT4 activation
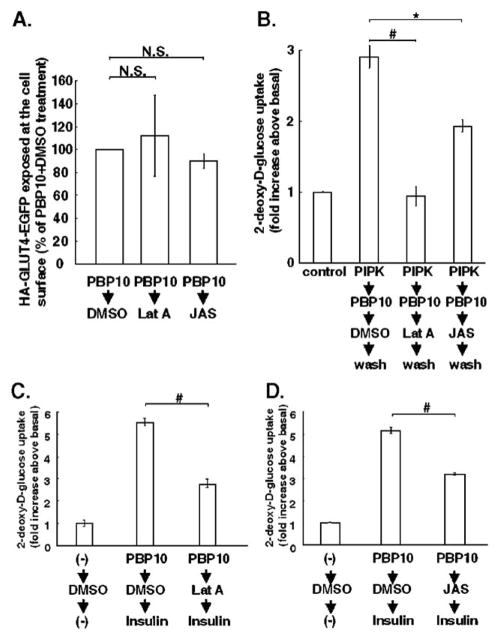
One of the cellular effects that are regulated by PI 4,5-P2 is F-actin remodeling [48]. Thus, to reveal the signal transduction pathway downstream of PI 4,5-P2 in activating GLUT4, we next investigated an involvement of F-actin in GLUT4 activation. Previous studies indicate a requirement of F-actin for GLUT4 translocation [7,49–52]. Thus, we first treated 3T3-L1 adipocytes with PBP10 to recruit GLUT4 to the plasma membrane in an inactive form. Further treatment of cells either with latrunculin A to depolymerize F-actin or with jasplakinolide to stabilize F-actin, in the presence of PBP10, did not affect the amount of GLUT4 exposed at the cell surface (Fig. 5A). Therefore, a comparison of glucose uptake between cells treated with the carrier DMSO and cells treated either with latrunculin A or with jasplakinolide after PBP10-pretreatment reflects the effect of inhibiting F-actin remodeling on GLUT4 activation at the plasma membrane.
Fig. 5.
GLUT4 activation requires intact F-actin remodeling. (A–B) 3T3-L1 adipocytes were electroporated with HA-GLUT4-EGFP plasmids (A) or were infected either with control adenovirus or with recombinant adenovirus for expressing human type 1α PIP-kinase (PIPK) (B). Cells were first treated with 40 μM PBP10 for 15 min to bring GLUT4 in an inactive form to the plasma membrane. Then, in the presence of PBP10, cells were treated for 30 min either with vehicle (DMSO), 20 μM latrunculin A (Lat A) to depolymerize F-actin, or 20 μM jasplakinolide (JAS) to stabilize F-actin. (A) Cells were immunostained for the HA-tag without permeation and the amount of the HA-tag exposed at the cell surface was quantified as described in Materials and methods. The amount of the HA-tag on the cell surface per the amount of EGFP expressed in each cell was counted, and the average value for cells treated with PBP10 and DMSO was set as 100%. The average value±S.E. obtained from three independent experiments, in which at least 20 cells were counted, are shown. N.S., not significant. (B) After the treatment described above, cells were washed to remove PBP10, but kept incubated either with DMSO, latrunculin A, or jasplakinolide for 20 min. 2-deoxy-D-glucose uptake was measured. Data are mean±S.E., and are expressed as fold increases of glucose uptake in control cells infected by control LacZ adenovirus and left untreated. #p<0.01; *p<0.05 C–D. 3T3-L1 adipocytes were first left untreated (−) or treated with 40 μM PBP10 for 15 min to bring GLUT4 in an inactive form to the plasma membrane. Then, in the presence of PBP10, cells were treated either with DMSO, 20 μM latrunculin A (Lat A) or 20 μM jasplakinolide (JAS) for 30 min, followed by insulin stimulation for 15 min in the presence of PBP10 and either DMSO, latrunculin A or jasplakinolide. 2-deoxy-D-glucose uptake was measured. Data are mean±S.E., and are expressed as fold increases of glucose uptake in control cells treated with DMSO only. #p<0.01. Similar results were obtained from two other independent experiments for B–D, respectively.
As shown in Fig. 5B, the increased glucose uptake induced by PBP10 treatment followed by its removal in PIP-kinase-overexpressing cells was completely abolished by latrunculin A, and partially but significantly inhibited by jasplakinolide. Thus, GLUT4 activation induced by PI 4,5-P2 replenishment requires intact F-actin remodeling.
We also investigated an involvement of F-actin remodeling in GLUT4 activation downstream of insulin stimulation. In our previous observation, insulin-stimulated GLUT1 translocation was almost undetectable in PBP10-pretreated cells [30]. Thus, the increased glucose uptake by insulin stimulation in PBP10-pretreated cells is due to an increased glucose transport through GLUT4. Both latrunculin A and jasplakinolide significantly attenuated glucose uptake through GLUT4 that was activated by insulin (Fig. 5C and D). These results suggest that GLUT4 activation downstream of insulin stimulation also depends on F-actin remodeling.
4. Discussion
4.1. PI 4,5-P2-dependent increase in the intrinsic glucose transport activity of GLUT4
Evidence for GLUT4 activation as a separate step from GLUT4 translocation/insertion to the plasma membrane during stimulation of glucose transport through GLUT4 has been suggested by previous studies (reviewed in [53,54]). However, attempts to identify the molecular mechanism of GLUT4 activation have been less successful than those to identify the mechanism of GLUT4 translocation/insertion. SB203580, an inhibitor of p38 MAPK, was reported to inhibit glucose transport without affecting insulin-induced GLUT4 recruitment to the plasma membrane [27]. However, a recent finding demonstrates that SB203580 inhibits the glucose transport activity of GLUT4 independent of inhibiting signal transduction by p38 MAPK [55]. In our previous report, we induced GLUT4 translocation by a phosphoinositide-binding peptide (PBP10), placing the extracellular domain of GLUT4 at the cell surface [30]. This GLUT4, despite its proper insertion into the plasma membrane, did not transport glucose. PBP10 did not influence the glucose transport activity of GLUT4, once GLUT4 was recruited to the plasma membrane and was fully activated by insulin before challenging with PBP10 [30]. Thus, the inability of PBP10 to increase glucose uptake is not due to its direct inhibition of GLUT4, and PBP10 provides a suitable condition to analyze the signal transduction to activate GLUT4 by completely separating it from that of GLUT4 recruitment.
Although once GLUT4 is fully activated by insulin stimulation, PBP10 does not influence the glucose transport activity of GLUT4, pretreatment of cells with PBP10 before insulin stimulation caused an attenuated glucose transport [30]. One possible explanation is the inhibitory effect of PBP10 on insulin-induced GLUT1 translocation [30]. However, this is unlikely to account for the attenuated glucose uptake, since we failed to detect GLUT1 translocation that could explain the increased, although attenuated, glucose transport in cells pretreated with PBP10 followed by insulin stimulation [30]. Thus, we came to hypothesize that PBP10 not only lacks an ability to activate GLUT4, but also has an inhibitory effect on insulin signal transduction to activate GLUT4, presumably due to the ability of PBP10 to bind phosphoinositide. As we expected, PBP10 displaced the PH-domain of PLCδ1 from the plasma membrane, which requires binding to PI 4,5-P2 for its plasma membrane localization (Fig. 1), demonstrating the affinity of PBP10 toward PI 4,5-P2 in the plasma membrane is high enough to affect at least some PI 4,5-P2-dependent signaling events. Based on this observation, we replenished PI 4,5-P2 in 3T3-L1 adipocytes that were pretreated with PBP10, and demonstrated that the glucose transport activity of GLUT4 was increased (Figs. 2, 3). Although our previous data suggest an involvement of PI 3-kinase in GLUT4 activation [30], we demonstrated that GLUT4 activation by PI 4,5-P2 replenishment was not accompanied by translocation of the PI 3,4,5-P3-specific PH-domains of GRP1 (Figs. 2F, 4). Thus, production of PI 3,4,5-P3, locally or at a whole cell level, from the replenished PI 4,5-P2 is unlikely to explain the mechanism of GLUT4 activation. Instead, our data suggest that PI 4,5-P2 in the plasma membrane functions as a second messenger in GLUT4 activation.
Since PI 4,5-P2-dependent signaling events downstream of PI 3-kinase activation have been suggested in other systems [56–60], future studies should be directed to investigate whether or not PI 4,5-P2 transmits a GLUT4 activation signal downstream of PI 3-kinase.
4.2. The source of PI 4,5-P2 to regulate glucose transport through GLUT4
In order to increase glucose uptake through GLUT4, GLUT4 has to go through multiple steps that need to be coordinated; translocation to the plasma membrane, insertion into the plasma membrane, and activation at the plasma membrane. Although the molecular mechanism of GLUT4 translocation and insertion by PBP10 treatment is still not known, PI 4,5-P2 is masked from its binding partners in the presence of PBP10 (Fig. 1), when PBP10 causes GLUT4 translocation and insertion. Thus, one possible mechanism of GLUT4 translocation and insertion is a loss of PI 4,5-P2-binding from some signaling molecule(s), whereas our data indicate PI 4,5-P2 is required for GLUT4 activation. Even if this is the case, the apparently contradictory signal transduction is still plausible, since recent reports implicate compartmentalization of PI 4,5-P2. PI 4,5-P2 is reported to distribute unevenly in the plasma membrane [61]. Some growth factor signaling is found to utilize PI 4,5-P2 selectively from a detergent-insoluble membrane rafts [62,63]. Thus, it is possible for agonists to coordinately induce GLUT4 translocation, insertion and activation by selectively signaling through compartmentalized PI 4,5-P2. Since GLUT4 translocation and insertion into the plasma membrane are followed by GLUT4 activation in the plasma membrane [30], it is also possible that a biphasic change in the cellular amount of PI 4,5-P2 coordinates these cellular events. Namely, a transient reduction in the cellular amount of PI 4,5-P2 may cause GLUT4 translocation and insertion, while increased amount of PI 4,5-P2 later may induce GLUT4 activation. Platelets stimulated with thrombin have been known to exhibit a biphasic change in the cellular amount of PI 4,5-P2 [64], although this has not been reported in insulin stimulation as yet. Further investigations are required to address these questions.
4.3. The molecular mechanism of GLUT4 activation downstream of PI 4,5-P2
The increased glucose uptake through GLUT4 by replenishing PI 4,5-P2 was significantly impaired by inhibiting F-actin remodeling (Fig. 5B). Although F-actin remodeling has been shown to be involved in GLUT4 translocation [50], the impaired glucose uptake is due to an impaired GLUT4 activation, since both latrunculin A and jasplakinolide were added to the medium after GLUT4 was inserted into the plasma membrane in an inactive form by PBP10, and latrunculin A and jasplakinolide did not affect the amount of GLUT4 exposed at the cell surface (Fig. 5A). Thus, an intact F-actin remodeling is required for PI 4,5-P2-dependent GLUT4 activation. At this moment, the mechanism of F-actin-dependent GLUT4 activation is unclear. PI 4,5-P2-dependent F-actin remodeling may recruit GLUT4-activating molecules to the plasma membrane just as it recruits GLUT4-containing vesicles. Alternatively, PI 4,5-P2-dependent F-actin remodeling may modify the microenvironment of GLUT4 in the plasma membrane so that GLUT4 has better access to GLUT4-activating molecules. Further studies are required on the mechanism of GLUT4 activation downstream of PI 4,5-P2-dependent F-actin remodeling.
Insulin-induced GLUT4 activation in the plasma membrane requires an intact F-actin remodeling (Fig. 5C, D). Since PI 4,5-P2 is an important regulator of F-actin remodeling [48], it is possible that insulin stimulation activates GLUT4 by way of PI 4,5-P2 and F-actin remodeling. Future studies should be directed to address whether or not PI 4,5-P2 is playing a role in GLUT4 activation by insulin stimulation.
In conclusion, we provide evidence that GLUT4 that has translocated to and inserted into the plasma membrane in an inactive form can be activated to initiate glucose transport by replenishing PI 4,5-P2, which causes F-actin remodeling. These data suggest that PI 4,5-P2 functions as a second messenger in addition to serving as a substrate for PI 3,4,5-P3 production in GLUT4 activation.
Acknowledgments
This work was supported by junior faculty award 1-05-JF-16 from American Diabetes Association (MF) and by research grant AR38910 from the National Institutes of Health (PJ).
We thank Dr. Morris J. Birnbaum for helpful advice and generous gifts of the recombinant adenovirus for the PH-domain of GRP1 and the antibodies. We thank Dr. Helen L. Yin for generous gifts of recombinant adenovirus for human type Iα PIP-kinase and plasmids for the PH-domains of PLCδ1. We thank Dr. Robert A. Bucki for a valuable discussion. We also thank Ms. Margaret McCormick, Mr. Chris Mullin, Ms. Audrey Jean-Jacques, Ms. Mayra Lujan Suarez and Mr. Brian Anderson for an excellent technical assistance.
Abbreviations
- PI-4,5-P2
phosphatidylinositol 4,5-bisphosphate
- PI 3,4,5-P3
phosphatidylinositol 3,4,5-trisphosphate
- GLUT
glucose transporter
- PIP-kinase
phosphatidylinositol phosphate-kinase
- PH-domains
pleckstrin homology domains
- DMSO
dimethyl sulfoxide
References
- 1.Wallberg-Henriksson H, Zierath JR. GLUT4: a key player regulating glucose homeostasis? Insights from transgenic and knockout mice. Mol Membr Biol. 2001;18:205–211. doi: 10.1080/09687680110072131. [DOI] [PubMed] [Google Scholar]
- 2.Smith U. Impaired (‘diabetic’) insulin signaling and action occur in fat cells long before glucose intolerance-is insulin resistance initiated in the adipose tissue? Int J Obes Relat Metab Disord. 2002;26:897–904. doi: 10.1038/sj.ijo.0802028. [DOI] [PubMed] [Google Scholar]
- 3.Saltiel AR, Pessin JE. Insulin signaling pathways in time and space. Trends Cell Biol. 2002;12:65–71. doi: 10.1016/s0962-8924(01)02207-3. [DOI] [PubMed] [Google Scholar]
- 4.Whiteman EL, Cho H, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab. 2002;13:444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- 5.James DE. MUNC-ing around with insulin action. J Clin Invest. 2005;115:219–221. doi: 10.1172/JCI24158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thong FS, Dugani CB, Klip A. Turning signals on and off: GLUT4 traffic in the insulin-signaling highway. Physiology (Bethesda) 2005;20:271–284. doi: 10.1152/physiol.00017.2005. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, Bilan PJ, Tsakiridis T, Hinek A, Klip A. Actin filaments participate in the relocalization of phosphatidylinositol3-kinase to glucose transporter-containing compartments and in the stimulation of glucose uptake in 3T3-L1 adipocytes. Biochem J. 1998;331:917–928. doi: 10.1042/bj3310917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanzaki M, Watson RT, Khan AH, Pessin JE. Insulin stimulates actin comet tails on intracellular GLUT4-containing compartments in differentiated 3T3L1 adipocytes. J Biol Chem. 2001;276:49331–49336. doi: 10.1074/jbc.M109657200. [DOI] [PubMed] [Google Scholar]
- 9.Semiz S, Park JG, Nicoloro SMC, Furcinitti P, Zhang C, Chawla A, Leszyk J, Czech MP. Conventional kinesin KIF5B mediates insulin-stimulated GLUT4 movements on microtubules. EMBO J. 2003;22:2387–2399. doi: 10.1093/emboj/cdg237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue M, Chang L, Hwang J, Chiang SH, Saltiel AR. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature. 2003;422:629–633. doi: 10.1038/nature01533. [DOI] [PubMed] [Google Scholar]
- 11.Bose A, Robida S, Furcinitti PS, Chawla A, Fogarty K, Corvera S, Czech MP. Unconventional myosin Myo1c promotes membrane fusion in a regulated exocytic pathway. Mol Cell Biol. 2004;24:5447–5458. doi: 10.1128/MCB.24.12.5447-5458.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Randhawa VK, Bilan PJ, Khayat ZA, Daneman N, Liu Z, Ramlal T, Volchuk A, Peng XR, Coppola T, Regazzi R, Trimble WS, Klip A. VAMP2, but not VAMP3/cellubrevin, mediates insulin-dependent incorporation of GLUT4 into the plasma membrane of L6 myoblasts. Mol Biol Cell. 2000;11:2403–2417. doi: 10.1091/mbc.11.7.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanda H, Tamori Y, Shinoda H, Yoshikawa M, Sakaue M, Udagawa J, Otani H, Tashiro F, Miyazaki J, Kasuga M. Adipocytes from Munc18c-null mice show increased sensitivity to insulin-stimulated GLUT4 externalization. J Clin Invest. 2005;115:291–301. doi: 10.1172/JCI22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joost HG, Weber TM, Cushman SW, Simpson IA. Activity and phosphorylation state of glucose transporters in plasma membranes from insulin-, isoproterenol-, and phorbol ester-treated rat adipose cells. J Biol Chem. 1987;262:11261–11267. [PubMed] [Google Scholar]
- 15.Holman GD, Kozka IJ, Clark AE, Flower CJ, Saltis J, Habberfield AD, Simpson IA, Cushman SW. Cell surface labeling of glucose transporter isoform GLUT4 by bis- mannose photolabel. Correlation with stimulation of glucose transport in rat adipose cells by insulin and phorbol ester. J Biol Chem. 1990;265:18172–18179. [PubMed] [Google Scholar]
- 16.Calderhead DM, Kitagawa K, Tanner LI, Holman GD, Lienhard GE. Insulin regulation of the two glucose transporters in 3T3-L1 adipocytes. J Biol Chem. 1990;265:13801–13808. [PubMed] [Google Scholar]
- 17.Clark AE, Holman GD, Kozka IJ. Determination of the rates of appearance and loss of glucose transporters at the cell surface of rat adipose cells. Biochem J. 1991;278:235–241. doi: 10.1042/bj2780235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison SA, Clancy BM, Pessino A, Czech MP. Activation of cell surface glucose transporters measured by photoaffinity labeling of insulin-sensitive 3T3-L1 adipocytes. J Biol Chem. 1992;267:3783–3788. [PubMed] [Google Scholar]
- 19.King PA, Horton ED, Hirshman MF, Horton ES. Insulin resistance in obese Zucker rat (fa/fa) skeletal muscle is associated with a failure of glucose transporter translocation. J Clin Invest. 1992;90:1568–1575. doi: 10.1172/JCI116025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith RM, Tiesinga JJ, Shah N, Smith JA, Jarett L. Genistein inhibits insulin-stimulated glucose transport and decreases immunocytochemical labeling of GLUT4 carboxyl-terminus without affecting translocation of GLUT4 in isolated rat adipocytes: additional evidence of GLUT4 activation by insulin. Arch Biochem Biophys. 1993;300:238–246. doi: 10.1006/abbi.1993.1033. [DOI] [PubMed] [Google Scholar]
- 21.Palfreyman RW, Clark AE, Denton RM, Holman GD, Kozka IJ. Kinetic resolution of the separate GLUT1 and GLUT4 glucose transport activities in 3T3-L1 cells. Biochem J. 1992;284:275–282. doi: 10.1042/bj2840275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karnieli E, Garvey WT, Olefsky JM, Hueckstead TP, Harel C, Maianu L, Armoni M. Potential role for insulin and cycloheximide in regulating the intrinsic activity of glucose transporters in isolated rat adipocytes. Endocrinology. 1993;133:2943–2950. doi: 10.1210/endo.133.6.8243322. [DOI] [PubMed] [Google Scholar]
- 23.Brozinick JT, Jr, Yaspelkis BB, III, Wilson CM, Grant KE, Gibbs EM, Cushman SW, Ivy JL. Glucose transport and GLUT4 protein distribution in skeletal muscle of GLUT4 transgenic mice. Biochem J. 1996;313:133–140. doi: 10.1042/bj3130133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moyers JS, Bilan PJ, Reynet C, Kahn CR. Overexpression of Rad inhibits glucose uptake in cultured muscle and fat cells. J Biol Chem. 1996;271:23111–23116. doi: 10.1074/jbc.271.38.23111. [DOI] [PubMed] [Google Scholar]
- 25.Hansen PA, Wang W, Marshall BA, Holloszy JO, Mueckler M. Dissociation of GLUT4 translocation and insulin-stimulated glucose transport in transgenic mice overexpressing GLUT1 in skeletal muscle. J Biol Chem. 1998;273:18173–18179. doi: 10.1074/jbc.273.29.18173. [DOI] [PubMed] [Google Scholar]
- 26.Hausdorff SF, Fingar DC, Morioka K, Garza LA, Whiteman EL, Summers SA, Birnbaum MJ. Identification of wortmannin-sensitive targets in 3T3-L1 adipocytes. Dissociation of insulin-stimulated glucose uptake and glut4 translocation. J Biol Chem. 1999;274:24677–24684. doi: 10.1074/jbc.274.35.24677. [DOI] [PubMed] [Google Scholar]
- 27.Sweeney G, Somwar R, Ramlal T, Volchuk A, Ueyama A, Klip A. An inhibitor of p38 mitogen-activated protein kinase prevents insulin-stimulated glucose transport but not glucose transporter translocation in 3T3-L1 adipocytes and L6 myotubes. J Biol Chem. 1999;274:10071–10078. doi: 10.1074/jbc.274.15.10071. [DOI] [PubMed] [Google Scholar]
- 28.Lund S, Holman GD, Schmitz O, Pedersen O. Glut 4 content in the plasma membrane of rat skeletal muscle: comparative studies of the subcellular fractionation method and the exofacial photolabelling technique using ATB-BMPA. FEBS Lett. 1993;330:312–318. doi: 10.1016/0014-5793(93)80895-2. [DOI] [PubMed] [Google Scholar]
- 29.Wilson CM, Cushman SW. Insulin stimulation of glucose transport activity in rat skeletal muscle: increase in cell surface GLUT4 as assessed by photolabelling. Biochem J. 1994;299(Pt 3):755–759. doi: 10.1042/bj2990755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Funaki M, Randhawa P, Janmey PA. Separation of insulin signaling into distinct GLUT4 translocation and activation steps. Mol Cell Biol. 2004;24:7567–7577. doi: 10.1128/MCB.24.17.7567-7577.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olefsky JM. Mechanisms of the ability of insulin to activate the glucose-transport system in rat adipocytes. Biochem J. 1978;172:137–145. doi: 10.1042/bj1720137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hellwig B, Joost HG. Differentiation of erythrocyte-(GLUT1), liver-(GLUT2), and adipocyte-type (GLUT4) glucose transporters by binding of the inhibitory ligands cytochalasin B, forskolin, dipyridamole, and isobutylmethylxanthine. Mol Pharmacol. 1991;40:383–389. [PubMed] [Google Scholar]
- 33.Janmey PA, Lamb J, Allen PG, Matsudaira PT. Phosphoinositide-binding peptides derived from the sequences of gelsolin and villin. J Biol Chem. 1992;267:11818–11823. [PubMed] [Google Scholar]
- 34.Cunningham CC, Vegners R, Bucki R, Funaki M, Korde N, Hartwig JH, Stossel TP, Janmey PA. Cell permeant polyphosphoinositide-binding peptides that block cell motility and actin assembly. J Biol Chem. 2001;276:43390–43399. doi: 10.1074/jbc.M105289200. [DOI] [PubMed] [Google Scholar]
- 35.Giudici ML, Hinchliffe KA, Irvine RF. Phosphatidylinositol phosphate kinases. J Endocrinol Invest. 2004;27:137–142. [PubMed] [Google Scholar]
- 36.Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 37.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 38.Doughman RL, Firestone AJ, Anderson RA. Phosphatidylinositol phosphate kinases put PI4,5P(2) in its place. J Membr Biol. 2003;194:77–89. doi: 10.1007/s00232-003-2027-7. [DOI] [PubMed] [Google Scholar]
- 39.Muller G, Wied S, Frick W. Cross talk of pp125(FAK) and pp59(Lyn) non-receptor tyrosine kinases to insulin-mimetic signaling in adipocytes. Mol Cell Biol. 2000;20:4708–4723. doi: 10.1128/mcb.20.13.4708-4723.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eyster CA, Duggins QS, Olson AL. Expression of constitutively active Akt/protein kinase B signals GLUT4 translocation in the absence of an intact actin cytoskeleton. J Biol Chem. 2005;280:17978–17985. doi: 10.1074/jbc.M409806200. [DOI] [PubMed] [Google Scholar]
- 41.Varnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol. 1998;143:501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stauffer TP, Ahn S, Meyer T. Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr Biol. 1998;8:343–346. doi: 10.1016/s0960-9822(98)70135-6. [DOI] [PubMed] [Google Scholar]
- 43.Kanzaki M, Furukawa M, Raab W, Pessin JE. Phosphatidylinositol 4,5-bisphosphate regulates adipocyte actin dynamics and GLUT4 vesicle recycling. J Biol Chem. 2004;279:30622–30633. doi: 10.1074/jbc.M401443200. [DOI] [PubMed] [Google Scholar]
- 44.Ishiki M, Randhawa VK, Poon V, Jebailey L, Klip A. Insulin regulates the membrane arrival, fusion, and C-terminal unmasking of glucose transporter-4 via distinct phosphoinositides. J Biol Chem. 2005;280:28792–28802. doi: 10.1074/jbc.M500501200. [DOI] [PubMed] [Google Scholar]
- 45.Steimle PA, Fulcher FK, Patel YM. A novel role for myosin II in insulin-stimulated glucose uptake in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2005;331:1560–1565. doi: 10.1016/j.bbrc.2005.04.082. [DOI] [PubMed] [Google Scholar]
- 46.Al-Hasani H, Kunamneni RK, Dawson K, Hinck CS, Muller-Wieland D, Cushman SW. Roles of the N- and C-termini of GLUT4 in endocytosis. J Cell Sci. 2002;115:131–140. doi: 10.1242/jcs.115.1.131. [DOI] [PubMed] [Google Scholar]
- 47.Oatey PB, Venkateswarlu K, Williams AG, Fletcher LM, Foulstone EJ, Cullen PJ, Tavare JM. Confocal imaging of the subcellular distribution of phosphatidylinositol 3,4,5-trisphosphate in insulin- and PDGF-stimulated 3T3-L1 adipocytes. Biochem J. 1999;344(Pt 2):511–518. [PMC free article] [PubMed] [Google Scholar]
- 48.Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol. 2003;65:761–789. doi: 10.1146/annurev.physiol.65.092101.142517. [DOI] [PubMed] [Google Scholar]
- 49.Kao AW, Noda Y, Johnson JH, Pessin JE, Saltiel AR. Aldolase mediates the association of F-actin with the insulin-responsive glucose transporter GLUT4. J Biol Chem. 1999;274:17742–17747. doi: 10.1074/jbc.274.25.17742. [DOI] [PubMed] [Google Scholar]
- 50.Kanzaki M, Pessin JE. Insulin-stimulated GLUT4 translocation in adipocytes is dependent upon cortical actin remodeling. J Biol Chem. 2001;276:42436–42444. doi: 10.1074/jbc.M108297200. [DOI] [PubMed] [Google Scholar]
- 51.Bose A, Cherniack AD, Langille SE, Nicoloro SM, Buxton JM, Park JG, Chawla A, Czech MP. G(alpha)11 signaling through ARF6 regulates F-actin mobilization and GLUT4 glucose transporter translocation to the plasma membrane. Mol Cell Biol. 2001;21:5262–5275. doi: 10.1128/MCB.21.15.5262-5275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen G, Raman P, Bhonagiri P, Strawbridge AB, Pattar GR, Elmendorf JS. Protective effect of phosphatidylinositol 4,5-bisphosphate against cortical filamentous actin loss and insulin resistance induced by sustained exposure of 3T3-L1 adipocytes to insulin. J Biol Chem. 2004;279:39705–39709. doi: 10.1074/jbc.C400171200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zierler K. Does insulin-induced increase in the amount of plasma membrane GLUTs quantitatively account for insulin-induced increase in glucose uptake? Diabetologia. 1998;41:724–730. doi: 10.1007/s001250050975. [DOI] [PubMed] [Google Scholar]
- 54.Furtado LM, Somwar R, Sweeney G, Niu W, Klip A. Activation of the glucose transporter GLUT4 by insulin. Biochem Cell Biol. 2002;80:569–578. doi: 10.1139/o02-156. [DOI] [PubMed] [Google Scholar]
- 55.Antonescu CN, Huang C, Niu W, Liu Z, Eyers PA, Heidenreich KA, Bilan PJ, Klip A. Reduction of insulin-stimulated glucose uptake in L6 myotubes by the protein kinase inhibitor SB203580 is independent of p38MAPK activity. Endocrinology. 2005;146:3773–3781. doi: 10.1210/en.2005-0404. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P, Waterfield MD, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 57.He H, Watanabe T, Zhan X, Huang C, Schuuring E, Fukami K, Takenawa T, Kumar CC, Simpson RJ, Maruta H. Role of phosphatidy-linositol 4,5-bisphosphate in Ras/Rac-induced disruption of the cortactin–actomyosin II complex and malignant transformation. Mol Cell Biol. 1998;18:3829–3837. doi: 10.1128/mcb.18.7.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Honda A, Nogami M, Yokozeki T, Yamazaki M, Nakamura H, Watanabe H, Kawamoto K, Nakayama K, Morris AJ, Frohman MA, Kanaho Y. Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;99:521–532. doi: 10.1016/s0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- 59.Venkateswarlu K, Cullen PJ. Signalling via ADP-ribosylation factor 6 lies downstream of phosphatidylinositide 3-kinase. Biochem J. 2000;345(Pt 3):719–724. [PMC free article] [PubMed] [Google Scholar]
- 60.Saci A, Carpenter CL. RhoA GTPase regulates B cell receptor signaling. Mol Cell. 2005;17:205–214. doi: 10.1016/j.molcel.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 61.Huang S, Lifshitz L, Patki-Kamath V, Tuft R, Fogarty K, Czech MP. Phosphatidylinositol-4,5-bisphosphate-rich plasma membrane patches organize active zones of endocytosis and ruffling in cultured adipocytes. Mol Cell Biol. 2004;24:9102–9123. doi: 10.1128/MCB.24.20.9102-9123.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hope HR, Pike LJ. Phosphoinositides and phosphoinositide-utilizing enzymes in detergent-insoluble lipid domains. Mol Biol Cell. 1996;7:843–851. doi: 10.1091/mbc.7.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pike LJ, Casey L. Localization and turnover of phosphatidylinositol 4,5-bisphosphate in caveolin-enriched membrane domains. J Biol Chem. 1996;271:26453–26456. doi: 10.1074/jbc.271.43.26453. [DOI] [PubMed] [Google Scholar]
- 64.Holmsen H, Dangelmaier CA, Rongved S. Tight coupling of thrombin-induced acid hydrolase secretion and phosphatidate synthesis to receptor occupancy in human platelets. Biochem J. 1984;222:157–167. doi: 10.1042/bj2220157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Varnai P, Rother KI, Balla T. Phosphatidylinositol 3-kinase-dependent membrane association of the Bruton’s tyrosine kinase pleckstrin homology domain visualized in single living cells. J Biol Chem. 1999;274:10983–10989. doi: 10.1074/jbc.274.16.10983. [DOI] [PubMed] [Google Scholar]