Abstract
It is unknown whether smoking confers similar mortality risk in HIV-positive as in HIV-negative patients. We compared overall mortality stratified by HIV and smoking of 1,034 HIV-positive block-matched to 739 HIV-negative veterans, enrolled 2001–2002 in the Veterans Aging Cohort 5 Site Study. Adjusted incidence rate ratios (IRR) for mortality were calculated using Poisson regression. Mortality was significantly increased in HIV-positive veterans according to both smoking status and pack-years in unadjusted and adjusted analyses (adjusted IRR 2.31, 95% confidence interval [CI] 1.53–3.49 for HIV-positive current smokers and IRR 1.32, 95% CI 0.67–2.61 for HIV-negative current smokers). Comorbid diseases were also significantly increased according to smoking status and pack-years. Current smoking is associated with poor outcomes; even lower levels of exposure appear to be detrimental in HIV-infected veterans. These findings support the need for improvements in smoking cessation and for studies of mechanisms and diseases underlying increased mortality in smokers with HIV.
Cigarette smoking is a leading cause of morbidity and mortality in HIV-negative persons (Ezzati & Lopez, 2003) and is highly prevalent in HIV-positive populations. Approximately 40–70% of HIV-infected people smoke (Burns et al., 1996; Page-Shafer, Delorenze, Satariano, & Winkelstein, 1996; Galai et al., 1997; Niaura et al., 2000; Turner et al., 2001; Diaz et al., 2003). Although studies conducted before the era of highly active antiretroviral therapy (HAART) had conflicting results regarding the impact of smoking on mortality for HIV-positive patients (Burns et al., 1996; Galai et al., 1997; Page-Shafer et al., 1996;), we have shown in the HAART era that HIV-positive veterans who currently smoke have substantially increased mortality compared with those who have never smoked (Crothers et al., 2005).
Smoking is also associated with substantial morbidity among HIV-positive patients. Indeed, smoking and HIV infection are independent risk factors for many of the same comorbid illnesses. These include bacterial pneumonia (Hirschtick et al., 1995; Kohli et al., 2006), chronic obstructive pulmonary disease (Crothers et al., 2006; Diaz et al., 2000) and lung cancer (Kirk et al., 2007), as well as coronary artery disease (Sudano et al., 2006). Furthermore, HIV-positive patients who smoke have an increased risk of oral candidiasis (Burns et al., 1996) and Pneumocystis pneumonia (Miguez-Burbano et al., 2003).
Such findings suggest that cigarette smoking may be especially deleterious in HIV-positive patients and raise questions as to whether the mechanisms by which smoking influences the development of these diseases are exacerbated by HIV infection. However, it is unknown whether HIV-positive patients are more susceptible to the negative health effects of smoking than HIV-negative patients. Previous studies have not included an HIV-negative comparison group and have also not examined how degree of exposure to cigarettes, as measured by pack-years of smoking, influences outcomes (Burns et al., 1996; Galai et al., 1997; Page-Shafer et al., 1996).
Therefore, to begin to address the question of whether patients with HIV infection have a greater susceptibility to injury related to smoking, we sought to compare the impact of cigarette smoking on mortality among HIV-positive patients matched to HIV-negative patients in a cohort study of veterans receiving care within the Veterans Affairs Healthcare System. In addition, we sought to understand the relationship between smoking status and pack-years of smoking on outcomes.
METHODS
The Veterans Aging Cohort 5 Site Study (VACS 5) is an observational cohort study of 1,034 HIV-positive veterans block-matched to 739 HIV-negative veterans by age, race, and site of care. Block matching was achieved by targeting the demographics of the HIV-negative veterans to be similar to the HIV-positive veterans, utilizing 5-year blocks of age, race (White, Black, or other), and sex. Subjects were enrolled between 2001 and 2002 from the outpatient infectious disease and general medicine clinics at five U.S. Veterans Affairs (VA) Medical Centers in Atlanta, Bronx, Houston, Los Angeles, and Manhattan. The institutional review boards approved the study at all locations, and participants provided written informed consent. At study entry, all subjects completed a self-administered questionnaire (available at the VACS web site, www.vacohort.org). Additional data sources included electronic laboratory and pharmacy records, and ICD-9 diagnostic codes and mortality from the national administrative VA database.
As in other studies, smoking status was based on self-report at study entry on the baseline survey (Patrick et al., 1994). Current smokers were defined as those who reported current or any use within the last 4 weeks and former smokers as those who quit more than 4 weeks ago. Subjects were asked how many cigarettes per day and years they had smoked, from which we calculated pack-years. Cigarettes per day were truncated at 5 or more packs (for 10 subjects) and years of smoking truncated such that individuals could not have been smoking longer than they were alive (for five subjects who gave implausible responses on the survey when asked to report for how many years they had been smoking). Seventy-nine (6%) subjects who were current or former smokers did not report sufficient data to calculate pack-years. Because pack-years was missing in less than 10%, we used a single regression model to predict the missing pack-years value based on patients with complete data (Shrive, Stuart, Quan, & Ghali 2006). The missing value was considered the outcome variable with other available data points for an individual used as the predictor variables.
From the baseline survey, data were also obtained on respiratory symptoms, substance abuse, and quality of life. Dyspnea or cough was determined from a validated symptom index (Justice et al., 2001). Subjects were asked if they had “cough or trouble catching your breath,” and how burdensome the symptom was on a 4-point Likert scale. Subjects were considered to have bothersome cough or dyspnea if they scored 2 or higher on the Likert scale. Subjects were classified as injection drug users if they had ever used injection drugs or if they reported their HIV risk factor as injection drug use. We defined “hazardous alcohol abuse” based on a score of 8 or higher on the Alcohol Use Disorders Identification Test (AUDIT) (Isaacson, Butler, Zacharek, & Tzelepis 1994). Quality of life was measured using the physical component summary (PCS) of the Short Form 12 (SF-12); scaled to a score from 1 to 100 such that the mean score in the U.S. population was designed to be 50, lower scores reflect poorer quality of life (Ware, Kosinski, & Keller, 1995; Ware, Kosinski, & Keller, 1996).
Comorbid conditions were diagnosed using ICD-9 codes from hospitalizations and outpatient visits occurring within 12 months prior to and up to 6 months after study enrollment using methods previously described (Justice & Lasky et al., 2006); inpatient codes had to occur at least once and outpatient codes at least twice, as this improves the accuracy of ICD-9 codes when compared with other sources of clinical data. We focused on general medical illnesses and HIV associated conditions that have been related to cigarette smoking (Ezzati & Lopez, 2003; Centers for Disease Control and Prevention, 2005; Hirschtick et al., 1995). Because of the low prevalence of cancers at baseline study enrollment, we grouped together cancers that have been causally related to smoking to generate a composite list of smoking-attributable cancers (Centers for Disease Control and Prevention, 2005).
Laboratory data at study enrollment (CD4 cell count [cells/µl], HIV viral load [copies/ml], and hemoglobin [g/dl]) were obtained through the electronic medical record (Justice & Dombrowski et al., 2006; Justice & Erdos et al., 2006). Use of at least three antiretroviral medications (ART) at baseline enrollment was determined from electronic pharmacy records. Mortality data were obtained from inpatient files and the VA Beneficiary Identification Records Locator System Death File, which records 95% of all veteran deaths (Smola et al., 2001).
ANALYSIS
Our primary outcome was all-cause mortality. A secondary goal was to determine the association of cigarette smoking with quality of life, comorbid illnesses, and respiratory symptoms using data obtained at study entry. All analyses were performed using Stata, Version 10.0 (StataCorp, College Station, TX), with p values of ≤.05 considered statistically significant.
Parallel analyses were conducted stratifying subjects by HIV and smoking status, and by HIV and pack-years of smoking. Smokers were categorized as current, former or never smokers. For analyses stratified by pack-years, we divided subjects into three groups: 0 (never smokers), >0 to <20, and 20 or more. As most continuous variables including pack-years, HIV viral load, and CD4 were not normally distributed, median values are presented for all continuous variables for consistency, and medians were compared between groups using the Kruskal-Wallis test. Categorical variables were compared using chi-squared or Fisher’s exact tests.
We conducted survival analysis using a Poisson regression model. The model is similar to the Cox proportional hazards regression model in that both account for censored data and assume the rates between groups will be proportional; however, the Poisson regression allows us to report actual adjusted rates (McNutt, Wu, Xue, & Hafner 2003). To illustrate unadjusted survival, we used Kaplan-Meier survival curves, comparing groups with the log-rank test. Then, the overall incidence rate ratios for mortality were calculated in Poisson models that included all HIV-positive and HIV-negative subjects. Rates were adjusted for age, race/ethnicity and hemoglobin, as well as hepatitis C infection, hazardous alcohol use and history of injection drug use. To test the hypothesis that HIV-positive patients are more susceptible to deleterious effects of cigarettes, we tested interaction terms for HIV and smoking in the full model. To obtain rates separately in HIV-positive and HIV-negative patients, we stratified the models according to HIV status. In models restricted to HIV-positive subjects, we also adjusted for baseline CD4 cell count, HIV RNA level, and use of ART. The square root of the CD4 cell count and the log10 HIV RNA level were used to approximate normally distributed variables.
RESULTS
CLINICAL CHARACTERISTICS ACCORDING TO SMOKING AND HIV STATUS
Clinical characteristics and smoking histories differed between HIV-positive and HIV-negative subjects. HIV-positive subjects were significantly younger when compared with HIV-negative subjects (median overall age of 50 years for HIV-positive vs. 55 years for HIV-negative, p < .0001). When stratified by smoking status, former smokers were significantly older compared with current and never smokers (Table 1) for both the HIV-positive subjects and HIV-negative subjects. Most subjects in the cohort were male, and the predominant race was Black.
TABLE 1.
Subject Characteristics and Outcomes According to HIV and Smoking Status
| HIV-positive subjects, n = 1034 | HIV-Negative Subjects, n = 739 | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics and Outcomes | Current Smoker n = 475 |
Former Smoker n = 306 |
Never Smoker n = 253 |
p value | Current Smoker n = 262 |
Former Smoker n = 297 |
Never Smoker n = 180 |
p value |
| Baseline characteristics | ||||||||
| Age | 50 (44–54) | 51 (46–56) | 48 (39–55) | <0.001 | 54 (48–59) | 57 (53–65) | 54 (45–60) | <0.001 |
| Gender, male | 99% | 98% | 99% | 0.7 | 97% | 97% | 94% | 0.3 |
| Race | 0.06 | 0.07 | ||||||
| Black | 57% | 53% | 55% | 51% | 40% | 43% | ||
| White | 23% | 29% | 33% | 31% | 44% | 37% | ||
| Hispanic | 13% | 13% | 8% | 11% | 10% | 13% | ||
| Other | 7% | 6% | 5% | 7% | 6% | 6% | ||
| Pack-years | 17 (7–30) | 14 (5–28) | — | <0.001 | 20 (8–37) | 16 (5–40) | — | 0.7 |
| >20 pack-years | 45% | 38% | — | <0.001 | 51% | 45% | — | |
| Injection drug use, ever | 38% | 36% | 14% | <0.001 | 20% | 9% | 4% | <0.001 |
| AUDIT ≥8 | 14% | 6% | 4% | <0.001 | 17% | 6% | 10% | <0.001 |
| Hemoglobin, g/dl | 14 (13–15) | 14 (13–15) | 14 (13–15) | 0.3 | 15 (14–15) | 14 (13–15) | 14 (13–15) | 0.1 |
| CD4 cells | 370 (225–564) | 360 (207–530) | 372 (220–504) | 0.7 | — | — | — | |
| Log10 HIV RNA | 2.9 (1.7–4.0) | 2.6 (1.7–4.1) | 2.8 (1.7–4.0) | 0.7 | — | — | — | |
| HIV viral load <400 | 46% | 50% | 46% | 0.5 | — | — | — | |
| On ART | 80% | 81% | 87% | 0.07 | — | — | — | |
| Respiratory symptoms | ||||||||
| Bothersome cough/dyspnea | 38% | 32% | 27% | 0.01 | 36% | 30% | 28% | 0.1 |
| Comorbid conditions | ||||||||
| Hepatitis C | 49% | 43% | 24% | <0.001 | 27% | 17% | 6% | <0.001 |
| Obstructive lung disease | 8% | 8% | 3% | 0.02 | 10% | 7% | 6% | 0.2 |
| Cardiovascular disease | 8% | 11% | 4% | 0.02 | 21% | 26% | 18% | 0.02 |
| Smoking-attributable cancer | 3% | 3% | 0.4% | 0.02 | 3% | 4% | 1% | 0.1 |
| Bacterial pneumonia | 6% | 6% | 6% | 0.9 | 0.8% | 0.7% | 0 | 0.2 |
| Quality of life | ||||||||
| SF-12 physical component summary score | 38 (31–45) | 38 (32–45) | 41 (35–51) | 0.002 | 35 (26–42) | 35 (28–43) | 39 (30–46) | 0.2 |
| Mortality rate per 100 | 5.48 | 3.41 | 2.45 | <0.001 | 2.35 | 1.93 | 1.76 | 0.6 |
| person-years | (4.58–6.56) | (2.59–4.51) | (1.72–3.49) | (1.63–3.38) | (1.33–2.80) | (1.06–2.91) | ||
Note. P-values represent comparisons by smoking status within HIV-positive patients or within HIV-negative patients. Continuous variables are presented as medians (interquartile range). Mortality rate is expressed per 100 person-years with 95% confidence intervals.
HIV-positive subjects were significantly more likely to be current smokers compared with HIV-negative subjects (46% HIV-positive vs. 35% HIV-negative, p < .001). Fewer HIV-positive subjects were former smokers (30% HIV-positive vs. 40% HIV-negative, p < .001). Only 24% of both HIV-positive and HIV-negative subjects had never smoked. Among smokers, the median pack-years for HIV-positive subjects was 15 compared with 17.7 for HIV-negative subjects (p = .01). Similarly, the median number of years smoked was lower in HIV-positive compared with HIV-negative smokers (21 vs. 23 years, p = .02).
Both HIV-positive and HIV-negative current smokers were substantially more likely to report hazardous alcohol use, with AUDIT scores ≥ 8 (see Table 1). Overall, HIV-positive subjects were more likely to have a history of injection drug use (32% vs. 12%, p < .001) and hepatitis C (41% vs. 18%, p < .001) when compared with HIV-negative subjects. Injection drug use was also significantly associated with smoking status among HIV-positive and HIV-negative subjects (see Table 1). Quality of life was significantly lower among HIV-positive current and former smokers compared with never smokers (see Table 1).
ASSOCIATION OF SMOKING WITH INCREASED RESPIRATORY SYMPTOMS, COMORBID ILLNESS, AND QUALITY OF LIFE
The prevalence of comorbidity was higher in current smokers and among patients with greater pack-years of exposure. Cardiovascular diseases, chronic obstructive lung diseases, and smoking-attributable cancers were more likely in smokers (see Table 1), with highest prevalence of these conditions tending to occur in patients with ≥20 pack-years of smoking; this was consistent for both HIV-positive and HIV-negative subjects (data not otherwise shown). Likewise, bothersome dyspnea or cough was more common among both HIV-positive and HIV-negative smokers, with over 35% of current smokers and over 40% of those with ≥20 pack-years complaining of this symptom. Quality of life was also substantially decreased in current compared with former and never smokers.
UNADJUSTED MORTALITY ACCORDING TO SMOKING
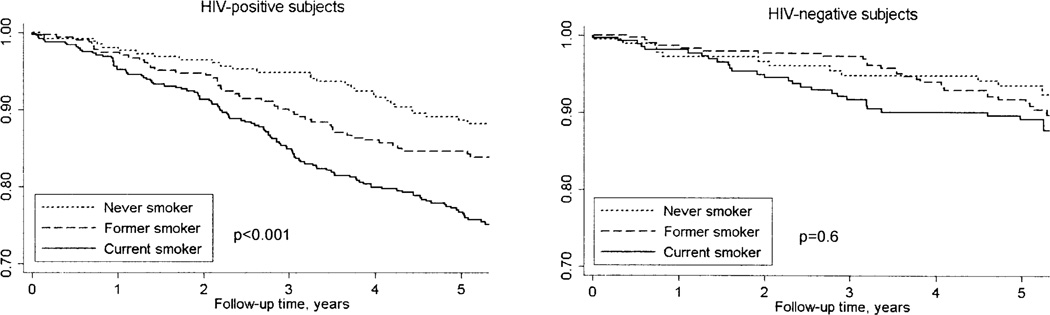
Subjects were followed for a median of 5.3 years during which there were 272 deaths, for an overall mortality rate of 3.2 per 100 person-years (PY) of observation. Of these, 200 deaths occurred in the HIV-positive and 72 deaths in the HIV-negative group, resulting in mortality rates of 4.1 and 2.0 per 100 PY, respectively. Kaplan-Meier survival curves illustrate a lower survival overall among HIV-positive subjects (Figure 1), particularly for current and former smokers.
FIGURE 1.
Kaplan-Meier survival curves for HIV-positive and HIV-negative subjects stratified by smoking status. P-values shown on graphs represent overall comparison between current, former, and never smokers. In pair-wise comparisons, HIV-positive current smokers have significantly decreased survival compared to never (p < 0.001) and former smokers (p = 0.004). Survival is not significantly different between HIV-positive former and never smokers (p = 0.15). Survival across groups was compared with the log-rank test.
Smoking status was significantly related to mortality among HIV-positive subjects (p < .001 for the overall comparison). HIV-positive subjects who were current smokers had the highest mortality rates, with mortality per 100 PY of 5.48 for current, 3.41 for former, and 2.45 for never smokers (see Table 1). Mortality was significantly greater for HIV-positive current compared with never smokers (p < .001) and to former smokers (p = .004). Although mortality rates were higher among HIV-negative subjects who were current smokers, the difference in mortality did not reach statistical significance between HIV-negative current, former, or never smokers (mortality of 2.35, 1.93, and 1.76 per 100 PY, respectively).
When stratified by pack-years of smoking, survival was also decreased among HIV-positive subjects if they had any degree of smoking exposure. Mortality rates were significantly higher in HIV-positive patients with <20 pack-years of exposure (mortality of 4.38 per 100 PY, p = 0.004) and ≥20 pack-years of exposure (5.01 per 100 PY, p = .0006), compared with HIV-positive patients with no smoking exposure (2.45 per 100 PY). Although mortality was also increased with prior exposure to cigarettes among the HIV-negative subjects (rates of 1.76 for no exposure, 2.10 for <20 pack-years, and 2.15 per 100 PY for ≥20 pack-years), these differences were not statistically significant.
ADJUSTED MORTALITY ACCORDING TO SMOKING
The incidence rate ratios for smoking remained significantly associated with mortality in multivariate Poisson regression in the combined population of HIV-positive and HIV-negative subjects, controlling for HIV infection, race/ethnicity, age, injection drug use, hepatitis C, and hazardous alcohol use (Table 2). Overall, cigarette smoking was significantly associated with increased mortality, with smoking status conferring a greater impact on mortality compared with pack-years of smoking. Namely, current smoking was associated with a higher rate ratio for mortality (incidence rate ratios 1.82, 95% CI 1.28–2.58) compared with the rate ratios for smoking <20 or ≥20 pack-years. Interaction terms for HIV and current smoking, or HIV and ≥20 pack-years were not statistically significant (data not otherwise shown).
TABLE 2.
Multivariate Poisson Regression Model of Mortality in All Subjects
| Incidence Rate Ratios | 95% CI | p value | |
|---|---|---|---|
| 2A: Incidence Rate Ratios For Mortality According To Smoking Status | |||
| Smoking | |||
| Former smoking | 1.02 | 0.70–1.49 | .9 |
| Current smoking | 1.82 | 1.28–2.58 | .001 |
| HIV infection | 1.90 | 1.41–2.57 | <.001 |
| Race/ethnicity | |||
| White | 1.61 | 1.21–2.14 | .001 |
| Hispanic/other | 1.09 | 0.78–1.54 | .6 |
| Age, per year | 1.04 | 1.02–1.05 | <.001 |
| Hepatitis C | 1.80 | 1.32–2.45 | <.001 |
| Hemoglobin, g/dl | 0.71 | 0.66–0.76 | <.001 |
| Injection drug use | 1.10 | 0.80–1.51 | .5 |
| AUDIT ≥8 | 1.11 | 0.75–1.64 | .6 |
| 2B: Incidence Rate Ratios For Mortality According To Pack-Years Of Smoking Strata | |||
| Smoking | |||
| >0 to <20 pack-years | 1.44 | 1.01–2.04 | .04 |
| ≥20 pack-years | 1.38 | 0.96–1.99 | .08 |
| HIV infection | 1.97 | 1.46–2.66 | <.001 |
| Race/ethnicity | |||
| White | 1.58 | 1.18–2.12 | .002 |
| Hispanic/other | 1.11 | 0.79–1.55 | .6 |
| Age, per year | 1.03 | 1.02–1.04 | <.001 |
| Hepatitis C | 1.90 | 1.40–2.58 | <.001 |
| Hemoglobin, g/dl | 0.71 | 0.66–0.76 | <.001 |
| Injection drug use | 1.11 | 0.81–1.53 | .5 |
| AUDIT ≥8 | 1.21 | 0.81–1.79 | .6 |
Note. The comparator group for smoking is never smokers (0 pack-years). For race/ethnicity the comparator is Black.
When stratified by HIV status, the incidence rate ratios for current smoking and for any pack-years of smoking remained significantly elevated in multivariate Poisson regression models (Table 3). These rate ratios correspond to adjusted mortality rates per 100 PY for HIV-positive subjects of 2.75 for current smokers, 1.53 for former smokers, and 1.19 for never smokers. Adjusted mortality rates for HIV-negative patients are 1.39 for current smokers, 0.87 for former smokers, and 1.05 for never smokers. When stratified by pack-years of exposure, adjusted mortality rates per 100 PY for HIV-positive subjects are 2.15 for ≥20 pack-years, 2.10 for <20 pack-years, and 1.15 for no exposure to cigarettes. Adjusted mortality rates per 100 PY for HIV-negative subjects are 0.98 for ≥20 pack-years, 1.13 for <20 pack-years, and 1.10 for no exposure to cigarettes.
TABLE 3.
Unadjusted and Adjusted Incidence Rate Ratios of Mortality According to Smoking Stratified by HIV Status
| HIV-Positive Patients | HIV-Negative Patients | |||
|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Smoking status | ||||
| Former smoker | 1.39 (0.89–2.18) | 1.29 (0.81–2.04) | 1.10 (0.59–2.06) | 0.83 (0.43–1.61) |
| Current smoker | 2.23 (1.51–3.31)* | 2.31 (1.53–3.49)* | 1.34 (0.72–2.49) | 1.32 (0.67–2.61) |
| Pack-years of smoking | ||||
| >0 to <20 | 1.79 (1.19–2.68)* | 1.82 (1.20–2.76)* | 1.19 (0.64–2.23) | 1.03 (0.53–2.00) |
| ≥20 | 2.04 (1.34–3.10)* | 1.87 (1.21–2.89)* | 1.23 (0.65–2.29) | 0.99 (0.51–1.91) |
Note. The comparator groups for smoking status and for pack-years of smoking are never smokers (0 pack-years). Models in both HIV-positive and HIV-negative patients are adjusted for: age, race/ethnicity, hemoglobin, hepatitis C, hazardous alcohol use and injection drug use. Models in HIV-positive patients are also adjusted for baseline CD4, HIV viral load, and use of ART.
p < 0.05 compared to HIV-positive never smokers (0 pack-years).
CONCLUSIONS
In this study, we sought to compare the mortality associated with smoking in HIV-positive and HIV-negative veterans and to examine how smoking status versus pack-years of smoking influenced outcomes. We found that current smoking at study enrollment was significantly associated with mortality when considering all patients. When stratified by HIV status, smoking status and pack-years were significantly associated with mortality among those with HIV. After adjusting for predictors known to be associated with mortality and potential confounders, current smoking and pack-years remained significantly associated with increased mortality among HIV-positive subjects. Additionally, we found that smoking was associated with increased comorbid diseases and respiratory symptoms, and with decreased quality of life. These associations were consistent when examined according to pack-years as well as smoking status, with greatest prevalence of comorbid diseases encountered in the heaviest smokers (≥20 pack-years).
Although current smoking was associated with mortality in our cohort, the increase in mortality that we observed in the subgroup of HIV-negative current smokers was not statistically significant, most likely owing to decreased statistical power in this group for such stratified analyses. The HIV-negative group had a smaller sample size and fewer deaths during the average of 5.2 years of follow-up, with only 72 of the 272 deaths occurring in the HIV-negative veterans. The Kaplan-Meier survival curves in Figure 1 are nonetheless suggestive of a trend towards greater mortality among HIV-negative current smokers when compared with other groups.
Of note, we found that mortality rates were substantially increased in White veterans compared with Black veterans. Previous studies have also demonstrated increased mortality among hospitalized white veterans compared with black veterans (Deswal, Petersen, Souchek, Ashton, & Wray, 2004; Jha, Shlipak, Hosmer, Frances, & Browner, 2001) Reasons for these differences are not clear from our analyses, and further studies are needed to clarify these findings.
Our results demonstrate the significant negative consequences of cigarette smoking on outcomes for HIV-positive patients. The greatest estimates for increased mortality were associated with current smoking rather than reported pack-years among HIV-positive subjects. These findings suggest that any amount of smoking may be hazardous to HIV-positive patients, and that smoking cessation should be prioritized for patients regardless of their pack-year history.
Improved smoking cessation among HIV-positive patients is needed. Although the number of studies are limited, data suggest that smoking cessation interventions may be effectively applied in HIV-positive populations (Cummins, Trotter, Moussa, & Turham 2005; Vidrine, Arduino, Lazev, & Gritz 2006; Wewers, Neidig, & Kihm 2000). One study combining nicotine patch and counseling found a cessation rate of 50% after 8 months among HIV-positive smokers (Wewers et al., 2000). Another study using cellular telephones for counseling among HIV-positive smokers increased cessation rates to 37% compared with 10% in the usual care group (Vidrine et al., 2006), results that are very similar to a study of HIV-negative patients utilizing telephone intervention (An et al., 2006). Further studies are needed to determine whether smoking cessation at any point results in improved outcomes in HIV-positive as in HIV-negative patients (Doll, Peto, Boreham, & Sutherland, 2004; Kawachi et al., 1993).
Our study has certain limitations. Whether smoking has greater attributable mortality in HIV-positive than in HIV-negative patients remains unclear, as our results may be due to fewer events in our HIV-negative sample and interaction terms between HIV and smoking were not significant in our models. Further analyses with longer follow-up time and greater statistical power are needed to address this question. Also, although we adjusted for baseline smoking status, CD4 count, HIV viral load, and use of ART, we did not have data to adjust for changes in these or other measures over time.
This study does not allow us to assess how mortality rates may vary in more recent quitters compared with those who quit in the distant past, as we did not collect data to adjust for the length of time since quitting. Those who have quit more recently may have worse outcomes in the short term, consistent with an “ill-quitter” phenomenon described previously (Kawachi et al., 1993). However, if we misclassified some subjects who had quit recently as former smokers when they were more representative of current smokers, this potential misclassification would likely have biased us away from finding an association between current smoking and increased mortality. This may also be a potential factor in the increased mortality observed among White subjects, as we do not know whether a greater proportion of Whites might be recent quitters compared with other racial/ethnic groups.
Our findings warrant further study. What mechanisms and diseases account for the increased mortality in smokers with HIV infection are not known. Although we adjusted for potential confounders and measured differences between groups, such as the greater prevalence of hepatitis C among HIV-positive smokers, residual confounding or other unmeasured behaviors that co-vary with smoking may be possible. Whether the excess mortality is due to smoking-related, AIDS-related or non-AIDS related diseases is currently under investigation. Additional investigations from the Women’s Interagency HIV Study suggest that women smokers who are HIV-positive may experience a poorer viral and immunologic response to HAART (Feldman et al., 2006). It is unclear whether a decreased virologic response to HAART among current smokers might be contributing to the increased mortality in our predominantly male population. We plan further longitudinal studies to assess the relationship between smoking status, HIV viral load, and other time-varying covariates with outcomes.
In summary, we found that current cigarette smoking at study enrollment was associated with significantly increased mortality for HIV-positive patients. Mortality was also increased in HIV-positive patients according to any pack-years of exposure to cigarette smoking. The impact of smoking on mortality was accentuated in the HIV-positive patients when contrasted to the HIV-negative patients. These findings underscore the need to devise effective strategies to promote successful smoking cessation among HIV-positive populations and for studies of mechanisms and diseases underlying increased mortality in smokers with HIV.
Acknowledgments
This study was funded by NIH/NCRR 1KL2 RR024138, NIH/NHLBI 1R01 HL090342 (KC); NIMH MH058984, AHRQ U18-HS016097 (SC); NIAAA 3U01AA13566 (ACJ). Funding for this publication was made possible in part by CTSA Grant KL2 RR024138 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
REFERENCES
- Centers for Disease Control. Annual smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 1997–2001. Morbidity and Mortality Weekly Report. 2005;54(25):625–628. [PubMed] [Google Scholar]
- An LC, Zhu SH, Nelson DB, Arikian NJ, Nugent S, Partin, et al. Benefits of telephone care over primary care for smoking cessation: A randomized trial. Archives of Internal Medicine. 2006;166(5):536–542. doi: 10.1001/archinte.166.5.536. [DOI] [PubMed] [Google Scholar]
- Burns DN, Hillman D, Neaton JD, Sherer R, Mitchell T, Capps L, et al. Cigarette smoking, bacterial pneumonia, and other clinical outcomes in HIV-1 infection. Terry Beirn Community Programs for Clinical Research on AIDS. Journal of Acquired Immune Deficiency Syndrome and Human Retrovirology. 1996;13(4):374–383. doi: 10.1097/00042560-199612010-00012. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. The health consequences of smoking: A report of the surgeon general. Atlanta, GA: US Department of Health and Human Services, Author; 2004. [PubMed] [Google Scholar]
- Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas MC, Crystal S, Justice AC. Increased COPD among HIV-positive compared with HIV-negative veterans. Chest. 2006;130(5):1326–1333. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- Crothers K, Griffith TA, McGinnis KA, Rodriguez-Barradas MC, Leaf DA, Weissman S, et al. The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV-positive veterans. Journal of General Internal Medicine. 2005;20(12):1142–1145. doi: 10.1111/j.1525-1497.2005.0255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins D, Trotter G, Moussa M, Turham G. Smoking cessation for clients who are HIV-positive. Nursing Standard. 2005;20(12):41–47. doi: 10.7748/ns2005.11.20.12.41.c4016. [DOI] [PubMed] [Google Scholar]
- Deswal A, Petersen NJ, Souchek J, Ashton CM, Wray NP. Impact of race on health care utilization and outcomes in veterans with congestive heart failure. Journal of the American College of Cardiology. 2004;43(5):778–784. doi: 10.1016/j.jacc.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Diaz PT, King MA, Pacht ER, Wewers MD, Gadek JE, Nagaraja HN, et al. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Annals of Internal Medicine. 2000;132(5):369–372. doi: 10.7326/0003-4819-132-5-200003070-00006. [DOI] [PubMed] [Google Scholar]
- Diaz PT, Wewers MD, Pacht E, Drake J, Nagaraja HN, Clanton TL. Respiratory symptoms among HIV-seropositive individuals. Chest. 2003;123(6):1977–1982. doi: 10.1378/chest.123.6.1977. [DOI] [PubMed] [Google Scholar]
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. British Medical Journal. 2004;328(7455):1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati M, Lopez AD. Estimates of global mortality attributable to smoking in 2000. Lancet. 2003;362(9387):847–852. doi: 10.1016/S0140-6736(03)14338-3. [DOI] [PubMed] [Google Scholar]
- Feldman JG, Minkoff H, Schneider MF, Gange SJ, Cohen M, Watts DH, et al. Association of cigarette smoking with HIV prognosis among women in the HAART era: A report from the Women’s Interagency HIV Study. American Journal of Public Health. 2006;96(6):1060–1065. doi: 10.2105/AJPH.2005.062745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galai N, Park LP, Wesch J, Visscher B, Riddler S, Margolick JB. Effect of smoking on the clinical progression of HIV-1 infection. Journal of Acquired Immune Deficiency Syndrome and Human Retrovirology. 1997;14(5):451–458. doi: 10.1097/00042560-199704150-00009. [DOI] [PubMed] [Google Scholar]
- Hirschtick RE, Glassroth J, Jordan MC, Wilcosky TC, Wallace JM, Kvale PA, et al. Bacterial pneumonia in persons infected with the human immunodeficiency virus: Pulmonary complications of HIV Infection Study Group. New England Journal of Medicine. 1995;333(13):845–851. doi: 10.1056/NEJM199509283331305. [DOI] [PubMed] [Google Scholar]
- Isaacson JH, Butler R, Zacharek M, Tzelepis A. Screening with the Alcohol use Disorders Identification Test (AUDIT) in an inner-city population. Journal of General Internal Medicine. 1994;9(10):550–553. doi: 10.1007/BF02599279. [DOI] [PubMed] [Google Scholar]
- Jha AK, Shlipak MG, Hosmer W, Frances CD, Browner WS. Racial differences in mortality among men hospitalized in the Veterans Affairs health care system. Journal of the American Medical Association. 2001;285(3):297–303. doi: 10.1001/jama.285.3.297. [DOI] [PubMed] [Google Scholar]
- Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T. Veterans Aging Cohort Study (VACS): Overview and description. Medical Care. 2006;44(8) Suppl. 2:S13–S24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC, Erdos J, Brandt C, Conigliaro J, Tierney W, Bryant K, et al. The Veterans Affairs Healthcare System: A unique laboratory for observational and interventional research. Medical Care. 2006;44(8) Suppl. 2:S7–S12. doi: 10.1097/01.mlr.0000228027.80012.c5. [DOI] [PubMed] [Google Scholar]
- Justice AC, Holmes W, Gifford AL, Rabeneck L, Zackin R, Sinclair G, et al. Development and validation of a self-completed HIV symptom index. Journal of Clinical Epidemiology. 2001;54 Suppl. 1:S77–S90. doi: 10.1016/s0895-4356(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Justice AC, Lasky E, McGinnis KA, Skanderson M, Conigliaro J, Fultz SL, et al. Medical disease and alcohol use among veterans with human immunodeficiency infection: A comparison of disease measurement strategies. Medical Care. 2006;44(8) Suppl. 2:S52–S60. doi: 10.1097/01.mlr.0000228003.08925.8c. [DOI] [PubMed] [Google Scholar]
- Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Rosner B, et al. Smoking cessation in relation to total mortality rates in women: A prospective cohort study. Annals of Internal Medicine. 1993;119(10):992–1000. doi: 10.7326/0003-4819-119-10-199311150-00005. [DOI] [PubMed] [Google Scholar]
- Kirk GD, Merlo C, O’Driscoll P, Mehta SH, Galai N, Vlahov D, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clinical Infectious Diseases. 2007;45(1):103–110. doi: 10.1086/518606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli R, Lo Y, Homel P, Flanigan TP, Gardner LI, Howard AA, et al. Bacterial pneumonia, HIV therapy, and disease progression among HIV-infected women in the HIV epidemiologic research (HER) study. Clinical Infectious Diseases. 2006;43(1):90–98. doi: 10.1086/504871. [DOI] [PubMed] [Google Scholar]
- McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. American Journal of Epidemiology. 2003;157(10):940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- Miguez-Burbano MJ, Burbano X, Ashkin D, Pitchenik A, Allan R, Pineda L, et al. Impact of tobacco use on the development of opportunistic respiratory infections in HIV seropositive patients on antiretroviral therapy. Addiction Biology. 2003;8(1):39–43. doi: 10.1080/1355621031000069864. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Morrow K, Tashima K, Flanigan T, Abrams DB. Human immunodeficiency virus infection, AIDS, and smoking cessation: The time is now. Clinical Infectious Diseases. 2000;31(3):808–812. doi: 10.1086/314048. [DOI] [PubMed] [Google Scholar]
- Page-Shafer K, Delorenze GN, Satariano WA, Winkelstein W., Jr Comorbidity and survival in HIV-infected men in the San Francisco Men’s Health Survey. Annals of Epidemiology. 1996;6(5):420–430. doi: 10.1016/s1047-2797(96)00064-6. [DOI] [PubMed] [Google Scholar]
- Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: A review and meta-analysis. American Journal of Public Health. 1994;84(7):1086–1093. doi: 10.2105/ajph.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrive FM, Stuart H, Quan H, Ghali WA. Dealing with missing data in a multi-question depression scale: A comparison of imputation methods. BMC Medical Research Methodology. 2006;6:57. doi: 10.1186/1471-2288-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smola S, Justice AC, Wagner J, Rabeneck L, Weissman S, Rodriguez-Barradas M. Veterans aging cohort three-site study (VACS 3): Overview and description. Journal of Epidemiology. 2001;54 Suppl. 1:S61–S76. doi: 10.1016/s0895-4356(01)00448-6. [DOI] [PubMed] [Google Scholar]
- Sudano I, Spieker LE, Noll G, Corti R, Weber R, Luscher TF. Cardiovascular disease in HIV infection. American Heart Journal. 2006;151(6):1147–1155. doi: 10.1016/j.ahj.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Turner J, Page-Shafer K, Chin DP, Osmond D, Mossar M, Markstein L, et al. Adverse impact of cigarette smoking on dimensions of health-related quality of life in persons with HIV infection. AIDS Patient Care and STDs. 2001;15(12):615–624. doi: 10.1089/108729101753354617. [DOI] [PubMed] [Google Scholar]
- Vidrine DJ, Arduino RC, Lazev AB, Gritz ER. A randomized trial of a proactive cellular telephone intervention for smokers living with HIV/AIDS. AIDS. 2006;20(2):253–260. doi: 10.1097/01.aids.0000198094.23691.58. [DOI] [PubMed] [Google Scholar]
- Ware J, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Ware JEJ, Kosinski M, Keller SD. SF-12: How to score the SF-12 physical and mental health summary scales. Boston: The Health Institute, New England Medical Center; 1995. [Google Scholar]
- Wewers ME, Neidig JL, Kihm KE. The feasibility of a nurse-managed, peer-led tobacco cessation intervention among HIV-positive smokers. Journal of the Association of Nurses in AIDS Care. 2000;11(6):37–44. doi: 10.1016/S1055-3290(06)60353-1. [DOI] [PubMed] [Google Scholar]