Abstract
Peripheral nerve function depends on a regulated process of axon and Schwann cell development. Schwann cells interact with peripheral neurons to sort and ensheath individual axons. Ablation of laminin γ1 in the peripheral nervous system (PNS) arrests Schwann cell development prior to radial sorting of axons. Peripheral nerves of laminin-deficient animals are disorganized and hypomyelinated. In this study, sciatic nerves of laminin-deficient mice were treated with syngenic murine adipose-derived stem cells (ADSCs). ADSCs expressed laminin in vitro and in vivo following transplant into mutant sciatic nerves. ADSC-treatment of mutant nerves caused endogenous Schwann cells to differentiate past the point of developmental arrest to sort and myelinate axons. This was shown by 1) functional, 2) ultrastructural, and 3) immunohistochemical analysis. Treatment of laminin-deficient nerves with either soluble laminin or the immortalized laminin-expressing cell line 3T3/L1 did not overcome endogenous Schwann cell developmental arrest. In summary, these results indicate that 1) laminin-deficient Schwann cells can be rescued, 2) a cell-based approach is beneficial in comparison to soluble protein treatment, and 3) mesenchymal stem cells modify sciatic nerve function via trophic effects rather than transdifferentiation in this system.
Keywords: Laminin, Mesenchymal stem cell, Adipose, Myelin, Schwann cell, Sciatic nerve, CMD1A
INTRODUCTION
Schwann cells (SCs) are the glia of the PNS and originate from the neural crest. As they mature, SCs sort axons by inserting cytoplasmic protrusions between axons until all axons are ensheathed (Jessen and Mirsky 1997; Mirsky and Jessen 1996; Mirsky et al. 1996). Myelination occurs when SCs enwrap axons, forming myelin from multiple layers of cell membrane. Specific molecular cues regulate each stage of Schwann cell development (Mirsky et al. 2001). Laminin, an extracellular matrix (ECM) protein (Patton et al. 1997; Stewart et al. 1997; Uziyel et al. 2000), is one of these cues. Laminins are present in the ECM of most tissues, although the laminin subtype varies by tissue and developmental stage. In peripheral nerves, Schwann cell basal laminas contain laminin-211 (α2, β1, γ1) and -411 (α4, β1, γ1) during development (Aumailley et al. 2005; Feltri et al. 1999; Patton 2000; Yu et al. 2005) and only laminin-211 in adulthood (Patton 2000). Without laminin, SCs fail to radially sort and myelinate axons (Chen and Strickland 2003; Yu et al. 2005). Molecular markers of this include failure to upregulate the neural cell adhesion molecule L1 (Yu et al. 2009b), which is transiently expressed during early post-natal development in all SCs and many peripheral axons (Martini and Schachner 1986). In adulthood, L1 PNS expression is restricted to non-myelinating SCs (Haney et al. 1999), which also fail to form in laminin-deficient animals (Yu et al. 2009b).
Human laminin deficiency is also known as congenital muscular dystrophy type-1A (CMD1A). In CMD1A, mutations in LAMA2 encoding the laminin α2 subunit cause diminished levels of functional laminins containing the α2 subunit, which are primarily expressed in the peripheral nerve, skeletal muscle, and CNS (Kuang et al. 1998). Patients with LAMA2 mutations have a severe hypomyelinating peripheral neuropathy, muscular dystrophy, and CNS white matter changes (Mercuri et al. 2001).
In addition to CMD1A, other heritable peripheral neuropathies such as Charcot-Marie-Tooth 4F and neurofibromatosis have protein defects in critical pathways of which laminin is a component (Feltri and Wrabetz 2005). Defects in laminin or laminin-receptors have a profound impact on peripheral nerve development and myelination and suggest that strategies targeting these molecules may help re-establish normal peripheral nerve function for neuropathies in which normal Schwann cell development is disrupted.
In this report, ADSCs were transplanted into the sciatic nerves of mice that do not express laminins in their sciatic nerve. These animals model the peripheral neuropathy that accompanies human CMD1A. The Cre-LoxP system was used to generate Schwann cell-specific laminin-γ1 deficiency ((Lamγ1f/f * Tg(P0-cre); hereafter referred to as mutant mice) (Chen and Strickland 2003; Feltri et al. 1999; Yu et al. 2005). Mutant mice do not express α2, α4, β1, or γ1 subunits within the endoneurium in comparison to control animals as shown by immunofluorescence (Yu et al. 2005). Sciatic nerves of mutant mice treated with ADSCs showed improvement in axon sorting and myelination by endogenous SCs. These changes resulted in improvements in sciatic nerve and hind limb function and were secondary to trophic effects of the ADSCs and not to transdifferentiation of the ADSCs into SCs.
MATERIALS AND METHODS
Mutant Mouse Generation
Mutant mice were generated as described (Yu et al., 2005). Mice carrying a P0-Cre transgene, which activates Cre-mediated recombination specifically in SCs (Feltri et al. 1999), and heterozygous for the floxed laminin γ1 allele (fLamγ1) (Chen and Strickland 2003; Yu et al. 2005) were crossed with mice homozygous for the fLamγ1 allele. Genomic DNA from the resulting offspring was analyzed by PCR for the Cre transgene and the fLamγ1 allele. Mutant (P0Lamγ−/−) mice were homozygous for the fLAMγ1 allele and hemizygous for the P0-Cre transgene. Mice heterozygous for both the fLAMγ1 allele (Lamγ1f/+) and the P0-Cre transgene were used as controls in these experiments (hereafter referred to as control mice). Breeding and experimental protocols were approved by the Institutional Animal Care and Use Committee at The Rockefeller University. The initial mouse containing the P0-Cre transgene was generated on a FVB/N background (Feltri et al. 1999); however, all subsequent crosses were performed against mice with a C57/Bl6 background.
ADSC culture
ADSCs were isolated from C57/Bl6 wild type mice (Fujimura et al. 2005). Peri-inguinal fat pads were removed from adult mice and rinsed with sterile PBS (Gibco-Invitrogen; Carlsbad, CA). Fat pads were placed in DMEM (Gibco-Invitrogen; Carlsbad, CA) containing Pen-Strep antibiotics (Sigma; St. Louis, MO), 2 mg/mL collagenase (Sigma; St. Louis, MO), and 1% BSA (Gibco-Invitrogen; Carlsbad, CA), minced, and digested at 37°C for 45 min. Tissue homogenate was treated with FBS (Gibco-Invitrogen; Carlsbad, CA) and centrifuged at 200×g for 5 minutes. The stromal vascular fraction (pellet) was resuspended in DMEM with 10% FBS and antibiotics. Cell suspension was filtered and plated. Cultures were grown to 80% confluence prior to subculturing, and were used between passages 2 and 5.
Mesenchymal differentiation towards adipocytes and osteocytes was performed as described below. ADSCs were subcultured in a basal medium of alpha-MEM with 10% FBS, Pen-Strep, and 2 mM L-glutamine. For adipogenic differentiation, cells were plated at high density (7.5 × 104 cells/mL), and upon reaching 100% confluence, were subcultured in basal medium supplemented with Adipogenic Supplement (R&D Systems; Minneapolis, MN) for 12–14 days. ADSC-derived adipocytes were visualized by Oil Red O staining (Fisher Scientific; Pittsburg, PA) or FABP immunostaining (R&D Systems; Minneapolis, MN). For osteogenic differentiation, cells were plated at low density (1.5 × 104 cells/mL) on fibronectin (1μg/mL). At 50% confluence, cells were subcultured into medium supplemented with Osteogenic Supplement (R&D Systems; Minneapolis, MN) for 18–20 days. Differentiated mADSC-osteocytes were visualized by osteopontin immunostaining (R&D Systems; Minneapolis, MN).
3T3/L1 cells (ATCC; Manassas, VA) were grown as described (Niimi et al. 1997).
DiI labeling of cells
CM-DiI (Invitrogen; Carlsbad, CA) was diluted to 2 mg/ml in cell culture-grade DMSO and further diluted to 2 μg/ml in D-PBS. Adherent mADSCs and 3T3/L1 cells were rinsed with D-PBS and covered with CM-DiI. Flasks were incubated for five min at 37°C, followed by 15 min at 4°C. Excess CM-DiI was removed, and cells were dissociated with 0.25% trypsin-EDTA and resuspended in serum-free DMEM prior to transplantation.
Stem cell transplant
Adult mutant mice were anesthetized, and nerves were blunt-dissected from the adjacent fascia. Nerves were exposed from several millimeters distal to the dorsal root ganglion (DRG) to the point of nerve division in the distal leg. The indicated treatment was pipetted into the single pocket generated by exposure of the nerve. The area of sciatic nerve used for EM and IF analysis is the same area that was surgically treated. Surgical wounds were closed with a 5-O SofSilk suture and vet-bond tissue adhesive (3M; Minneapolis, MN).
Sciatic nerve function
The minimum stimulus current that needed to be applied to the sciatic nerve to trigger a muscle twitch (MCST) was used to quantify overall sciatic nerve function. There was minimal variance between limbs of the same mutant or control mouse. Mice were anesthetized with 2.5% avertine and atropine and placed on a plexiglass platform affixed to a stereotaxic apparatus. A blunt tip stimulating electrode was placed on the proximal sciatic nerve. MCST was determined by applying 0.15 msec current pulses starting at 100 μA and increasing them until a muscle twitch was detected. Exposed muscle was observed under a dissecting microscope for evidence of muscle twitch. This technique was performed in triplicate for each nerve. The velocity of neuromuscular current transmission was determined by recording the action potential from the muscle at the site of maximal muscle twitch. The velocity was calculated by dividing the latency time from stimulus administration to recording of the action potential by the distance between stimulating (nerve) and recording (muscle) electrodes.
Electron Microscopy (EM)
Twenty-one days after surgery, sciatic nerves were collected and fixed in 2.5% paraformaldehyde/ glutaraldehyde and 2% osmium tetroxide solution (EM Sciences; Hatfield, PA)(Chen and Strickland 2003; Yu et al. 2005). Nerve sections were collected from the area of nerve that was treated with cells or media. Nerves were embedded in resin, cut into ultra-thin sections, and visualized by EM as described (Chen and Strickland 2003; Yu et al. 2005).
Immuno-EM
Twenty-one days after surgery, mice were perfused with 4% paraformaldehyde. Sciatic nerves were collected, post-fixed, and cryoprotected in 30% sucrose for 48 hrs. Nerves sections (60 μm) were collected and affixed to glass slides. Sections were immunostained with rabbit antibody against beta-galactosidase (ABCam; Cambridge MA). Biotinylated secondary antibody against rabbit IgG (Vector labs; Burlingame, CA) was used, and sections were developed using Vectastain Elite ABC (Vector labs; Burlingame, CA) and DAB. Development time was adjusted so that adequate color developed on positive nerve sections from mice in which all tissues express beta-galactosidase (Z/eG mouse strain) and no color was evident on negative control sections (C57/Bl6 wild type). Sections were rinsed with water and fixed with 2.5% glutaradehyde in 0.1 M sodium cacodylate buffer pH 7.4. Samples were processed as described (Yu et al. 2009b).
Nerve Morphometry
At least 20 digitized electron micrographic images were taken from each limb of media- or ADSC-treated nerves. A minimum of 200 randomly-chosen axons from each nerve were manually categorized according to the presence of glial cytoplasmic process separating at least two sides of the axon from adjacent axolemmas, and for presence of at least one layer of myelin ensheathing the axon. Statistical analysis was performed as described.
Immunostaining
At the indicated time-point following transplant, groups of at least three mice were anesthetized and perfused. For nerves subsequently stained for L1, nerves were dissected and frozen immediately in OCT on dry ice. For samples used for DiI imaging, mice were perfused with 4% paraformaldehyde. Sciatic nerves were removed and post-fixed. Tissues were cryoprotected in 30% sucrose prior to sectioning (10 μm). Sectioned nerves (Chen and Strickland 2003; Yu et al. 2005) or cell cultures grown on Poly-D-lysine coated cover slips (BD Biosciences; San Jose, CA) were processed for immunostaining as described (Yu et al. 2005; Yu et al. 2009b).
Antibodies
Primary antibodies were rat anti-laminin γ1 (Chemicon, Temecula, CA), rabbit anti-L1 (Chemicon, Temecula, CA), and anti-laminin-1 (Sigma, St. Louis, MO). Samples were incubated with the appropriate secondary antibodies: Alexa 647-labeled anti-rabbit, Alexa 488-labeled anti-rabbit, and Alexa 488-labeled anti-rat IgG (Invitrogen; Eugene, OR). Samples were visualized using a Zeiss LSM 510 confocal microscope at The Rockefeller University’s BioImaging Resource Center.
Statistical Methods
All values are expressed as mean and standard error (SEM) as indicated. Analyses of significance were performed using two-tailed Student’s t-test. We considered P <0.05 (indicated * in figures) as significant and P < 0.01 (**) as highly significant. All analysis was performed using Graph Pad Prism software.
RESULTS
ADSC treatment improved mutant hind limb function
The mutant animals used in this study have severe hind limb paresis. Prior studies (Yu et al. 2005) showed that partial rescue with laminin peptide was possible during early postnatal development at the time of Schwann cell differentiation. However, beyond this point, there is increased Schwann cell apoptosis and decreased Schwann cell proliferation in mutant animals. Peripheral axons are maintained in unsorted bundles in which axolemmas are immediately adjacent to each other. There are no insulating Schwann cell cytoplasmic processes to ensheath axons and facilitate current transmission.
We hypothesized that restoration of function in the mutant animals would require reestablishment of normal axon: Schwann cell interactions. It was not known whether: 1) the adult mutant SCs could re-enter normal development, or 2) if exogenous glia were necessary. Therefore, we decided on a stem cell-based strategy that could potentially act by either mechanism to attempt functional repair in the mutant animals.
ADSCs were used to determine the potential for adult mesenchymal stem cells to rescue function of laminin-deficient sciatic nerves. The sciatic nerves of mutant mice were surgically exposed. One side was treated with ADSCs, and the other with stem cell growth media to allowed direct comparison between limbs of the same animal. In comparison to media-treated limbs, the ADSC-treated limbs were actively used by the mouse for walking (Fig 1A and Supplemental video 1), although motor function was not completely normalized in ADSC-treated limbs. Improvement was evident at 21 days post-injection. Longer time points were not examined because the life-span of the mutant animals is limited, and passed this point, there is significant mortality in both treated and untreated animals. Therefore, all measurements of functional recovery were taken 21 days after ADSC-treatment.
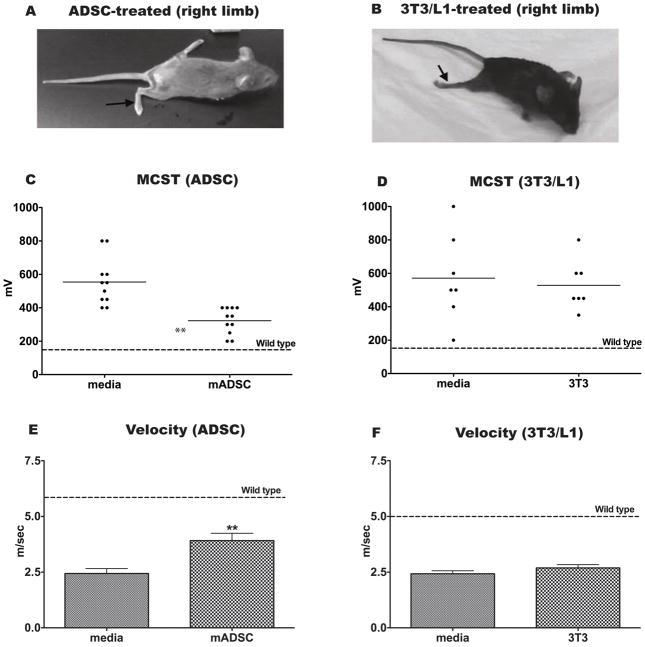
Figure 1. ADSC but not 3T3/L1 treatment of mutant sciatic nerves improves hind limb function.
ADSCs (A, C, E) or 3T3/L1 cells (B, D, F) were injected around sciatic nerves of adult mutant animals. Arrow in A and B indicates the limb that received cells; the contralateral limb was a surgical control (DMEM treatment). Following ADSC (A) but not 3T3/L1 (B) or DMEM treatment, hind limbs were used for ambulation by mutant mice. (C, D) Sciatic nerve function was characterized by determination of the MCST. ADSC-treatment (p = 0.0001) but not 3T3/L1-treatment (p = 0.714) resulted in a significant improvement in MCST of mutant hind limbs compared to media-treated limbs, (MCST of wild type control animals is indicated by dashed line). (E, F) Velocity of signal transduction from sciatic nerve to muscle was measured for ADSC-treated and 3T3/L1-treated hind limbs and compared to DMEM treatment. There was a significant increase in velocity of signal transduction in ADSC-treated (p = 0.0019) but not 3T3/L1-treated (p = 0.21) hind limbs when compared to contralateral DMEM-treated limbs. Wild type measurement is shown by dashed line.
The function of any nerve is the efficient relay of current. For motor fibers, transmission of electrical impulses along myelinated motor fibers stimulates muscle contraction. Schwann cell insulation of individual axons is necessary for current propagation. If the motor fibers of a nerve are improperly insulated, a greater amount of stimulus current is needed to induce attendant muscle contraction. The sciatic nerve MCST was measured in control and mutant animals. Control animals required less current than mutant mice to induce a muscle twitch (Table 1, dashed line in Figures 1, C and D). MCST was measured in media (DMEM and conditioned)- and ADSC- treated limbs to quantify the change in hind limb motor function with ADSC-treatment that was seen by gross observation (Table 1 and Figure 1C). A similar strategy was used to observe sciatic nerve function following hair follicle stem cell injection (Amoh et al. 2005), although Amoh et al. measured the length of contracted muscle with a set current stimulus rather than the amount of current needed to stimulate a minimal muscle twitch as presented here.
Table 1.
Analysis of MCST.
| Mean MCST ± SEM (μA)Control (n = 12) | Mean MCST ± SEM (μA)Mutant (n = 11) | Significance of difference | |
|---|---|---|---|
| 150 ± 30 | 680 ± 50 | p = 0.0026 | |
| P0Lamγ1−/− Mice + [Treatment] | Mean MCST ± SEM (μA) of [Treatment] limb | Mean MCST± SEM (μA) of control-limb | Significance of difference |
| + 3T3/L1 (n = 7) | 530 ± 60 | 570 ± 100 | p= 0.714 |
| + ADSCs (n = 11) | 320 ± 20 | 550 ± 40 | p= 0.0001 |
| + Laminin (soluble) (n = 12) | 580 ±40 | 580 ± 50 | p= 0.947 |
| + Conditioned media (n = 4) | 330 ±40 | 290 ±40 | P=0.440 |
Treatment of mutant nerves with ADSCs (Table 1 and Figure 1C) significantly improved MCST and thus motor function in comparison to surgical control limbs, but did not decrease the MCST to the level of wild type animals. There was no significant difference in MCST between media-treated and untreated mutant nerves (Table 1), indicating that the surgical procedure itself was not responsible for this effect. The velocity of neuromuscular current transmission from the sciatic nerve to the enervated muscle was also measured, and a similar trend was noted. Current was transmitted faster from the sciatic nerve to the hind limb musculature in the ADSC-treated limbs in comparison to contralateral media-treated limbs. As with the MCST, velocity was not increased to the speed of wild type animals (Table 2 and Figure 1E).
Table 2.
Analysis of mean velocity.
| Mean velocity ± SEM (m/s)Control (n = 16) | Mean velocity ± SEM (m/s)Mutant (n = 17) | ||
|---|---|---|---|
| 5.05 ± 0.21 | 1.84 ± 0.12 | p <0.0001 | |
| P0Lamγ1−/− Mice + [Treatment] | Mean velocity ± SEM (m/s)of [Treatment] limb | Mean NCV ± SEM (m/s) of control limb | Significance of difference |
| + 3T3 (n = 7) | 2.69 ± 0.15 | 2.42 ± 0.13 | p= 0.21 |
| + ADSCs (n = 9) | 3.91 ± 0.33 | 2.44 ± 0.22 | p= 0.0019 |
| + Laminin (soluble) (n = 12) | 2.41 ± 0.19 | 2.31 ± 0.19 | p= 0.627 |
To determine whether a related but more fate-restricted cell line would induce the same changes in sciatic nerve function, the 3T3/L1 fibroblast/pre-adipocyte cell line was used in place of ADSCs. 3T3/L1 cells produce laminins but are more fate-restricted than ADSCs (Niimi et al. 1997). In contrast to ADSC-treated limbs, 3T3/L1-treated limbs did not show obvious functional changes and did not show statistically significant improvement in the MCST or the velocity of current transmission (Tables 1 and 2, and Figure 1, B, D, and F).
ADSCs did not differentiate into Schwann cells
Transplantation of ADSCs, but not 3T3/L1 cells, around the sciatic nerves of mutant mice significantly improved mutant hind limb function. There are several reports in the literature that mesenchymal stem cells can differentiate into glial-like or neuronal-like cells in vitro (Xu et al. 2008; Yang et al. 2004). If this was the case in our experimental paradigm, mesenchymal-to-glial transdifferentiation might explain the improvement in sciatic nerve function. For this to be relevant to hind-limb functional changes, ADSCs or their derivatives should be detectable within the parenchyma of the treated mutant sciatic nerves. To examine this question, ADSCs and 3T3/L1 cells were labeled with a cell-membrane specific fluorophore, CM-DiI, and transplanted around mutant animal sciatic nerves. At the same time point shown to result in ADSC-induced functional improvement by MCST measurements, sciatic nerves were collected and imaged for the presence of DiI.
CM-DiI labeled ADSCS were found in abundance around the mutant sciatic nerves 21 days after injection, in contrast to the labeled 3T3/L1 cells which were only minimally evident (Figure 2, A-C). Very few CM-DiI-labeled ADSCs were found within the sciatic nerve parenchyma. CM-DiI label was not detected in media-treated nerves. This suggested that the mechanism of ADSC-induced changes in sciatic nerve function was not by direct contact with the axons of the sciatic nerve or transdifferentiation into peripheral nerve glia.
Figure 2. ADSCs are localized outside the sciatic nerve parenchyma, but induce changes within the nerve parenchyma.
Mutant sciatic nerves were treated with media (DMEM, A) or 5 × 104 CM-DiI labeled 3T3/L1 cells (B) or ADSCs (C). Few DiI-labeled 3T3/L1 cells (red, arrows) were detected (B), whereas many DiI-labeled ADSCs (red, arrows) were detected throughout the perineurium (C). Nuclei are stained blue with DAPI. Media- and ADSC-treated mutant limbs were analyzed by immunfluoresence for expression of the neural cell adhesion molecule L1 (D–F) which is not expressed in mutant sciatic nerves (Yu et al. 2009b). DMEM-treated sciatic nerves minimally expressed L1 (white) (D). One day after ADSC-treatment, there was significant increase in L1 immunostaining (white, arrows) (E), which persisted through post-operative day 21 (F). Three mice at each time point were evaluated, and these images are representative of these results.
ADSC- treatment induces developmental progression of endogenous Schwann cells
Ablation of Schwann cell-derived laminin-γ1 results in developmental arrest of SCs in the mutant sciatic nerves. L1 is a neural adhesion molecule that is expressed throughout the PNS during early postnatal development, with eventual restriction of expression to non-myelinating SCs in adulthood (Nieke and Schachner 1985). Mutant SCs fail to undergo normal development, which is evidenced in part by failure to upregulate L1 during early postanatal development or to maintain expression in non-myelinating SCs in adulthood (Yu et al. 2009b).
We therefore performed immunohistochemical analysis of ADSC-treated nerves compared to the contralateral media-treated nerves to examine changes in L1 expression as a surrogate for Schwann cell development (Figure 2D–F). Media-treated nerves failed to show significant expression of L1 (Yu et al. 2009b). However, as early as post-surgical day 1, ADSC-treated mutant nerves showed discernable L1 expression that persists through the time frame of the experiment.
To determine whether changes in L1 expression corresponded to improvement in axon sorting and Schwann cell development, morphologic examination of media-treated and ADSC-treated nerves was performed. Ultrastructural analysis of media-treated limbs was identical to untreated mutant sciatic nerves (Figure 3A) (Chen and Strickland 2003; Yu et al. 2005). The majority of axons were in unsorted bundles. SCs failed to extend cytoplasmic projections or to radially sort individual axons.
Figure 3. ADSC-treated nerves show evidence of endogenous Schwann cell development and radial sorting and myelination of axons.
(A) Electron microscopy of DMEM-treated sciatic nerves revealed ultrastructure that was unchanged from untreated mutant mice. Axons remained in unsorted bundles with axolemmas in contact with each other. The Schwann cell in panel A is immature, without cytoplasmic extension into the adjacent axon bundle. (B) Twenty-one days after stem cell transplant, more axons were sorted and separated (arrow) from a predominantly unsorted bundle. There was nascent myelin (closed arrowheads) and a basal lamina surrounding the cell sorting axons (open arrowheads). The area marked by the white box is shown in higher magnification in the adjacent panel. EM was analyzed from DMEM-and ADSC-treated mutant sciatic nerves for the presence of axons within primarily unsorted bundles that had undergone radial sorting (C) or initiation of myelination (D). Immuno-electron microscopy was used to identify the source of the ensheathing cells as either ADSC-derived (LacZ positive) or host-derived (LacZ negative). Wild type (E), LacZ positive (F) and mutant nerves treated with LacZ positive ADSCs (G) were examined for expression of LacZ. Unlike the positive control SCs (arrow in panel F), the cytoplasm (arrow in panel G) of the cell ensheathing the axon (*, panel G) does not express LacZ, and is thus derived from the host nerve.
However, in ADSC-treated mutant nerves, we observed bundles in which individual axons were sorted (Figure 3B). Multiple axons had cytoplasmic extrusions from adjacent SCs fully encircling them, and some showed evidence of nascent myelination. Although this new myelin was not as thick as the myelin from a normal adult myelinated axon, several layers could be distinguished. Glial cells associated with sorted and myelinated axons also appeared to have an associated basal lamina. Thinly myelinated axons with an associated glial basal lamina were never observed in untreated (Chen and Strickland 2003; Yu et al. 2005) or media-treated mutant nerves. Examination of at least 800 axons within predominantly unsorted bundles from media- and ADSC-treated nerves showed a significantly increased number of sorted (1.4 ± 0.2% vs 5.6% ± 0.1%, p = 0.0028) and myelinated axons (0.25 ± 0.13% vs 1.3 ± 0.2%, p = 0.047) in the stem cell-treated nerves (Figure 3C and 3D). There were a few fully myelinated axons in both media and stem cell treated mutant sciatic nerves. Rare myelinated axons were seen in untreated mutant mice as well (Yu et al. 2005; Yu et al. 2009b). Theses SCs either escaped recombination of the laminin γ1 transgene or were immediately adjacent to an alternate laminin source such as a blood vessel. Therefore, to prevent overestimation of the extent of myelination in ADSC-treated nerves, only axons within predominantly unsorted bundles were analyzed. Direct comparison to wild type nerves could not be made as these unsorted bundles are not found in normal adult nerves. Changes in axon sorting and ensheathment should improve the current transmission of previously unsorted axons by improving individual axon insulation and is consistent with changes seen in MCST and current transmission velocity measurements (Tables 1 and 2, and Figure 1).
To determine whether the cells beginning to sort and ensheath axons in ADSC-treated mutant nerves were host-derived or ADSCs or their progeny, immuno-electron microscopy was used (Figure 3E–G). Transplanted ADSCs were derived from Z/eG mice (Novak et al. 2000) so that all ADSCs or their derivatives would express LacZ. If the cells sorting and ensheathing axons were derived from the transplanted cells, they should express LacZ and be evident by immuno-EM. Sciatic nerves from mice that express the Z/eG transgene were used as positive controls, and wild type sciatic nerves were used as a negative control. Schwann cell bodies were labeled by antibody against LacZ in the Z/eG sciatic nerves (Figure 3F), and wild type nerves failed to show LacZ positive staining (Figure 3E). The cells sorting axons in the ADSC-treated animals did not express LacZ, which indicated that they were host-derived cells that were responsive to an ADSC-initiated cue (Figure 3G).
Laminin is necessary but insufficient to induce ADSC-mediated functional improvement in mutant nerves
Based on the functional, immunohistochemical, and morphological studies detailed above, ADSCs induced improvement in sciatic nerve function by stimulation of endogenous mutant SCs to initiate sorting of previously un-ensheathed axons. One explanation for ADSC-mediated improvement in mutant nerves was that ADSCs supplied the nerve with laminin. Laminins are required for the assembly of a basal lamina (Miner et al. 2004; Murray and Edgar 2000; Smyth et al. 1998), so the presence of a basal lamina as observed by EM implies increased presence of laminin in the mutant nerve (Figure 3B).
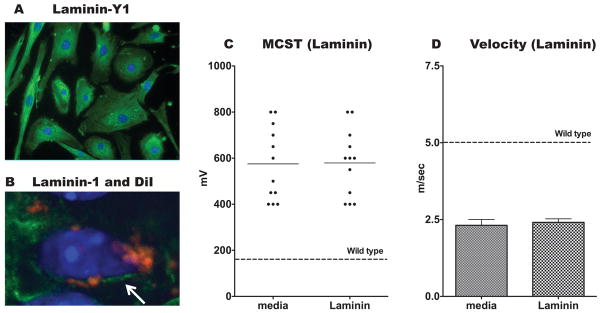
To verify expression of laminin, ADSCs were immunostained for laminin-γ1 and laminin-1 in vitro and in vivo (Figures 4A and B). ADSCs have been previously shown to express laminins (Hausman and Richardson 1998) in addition to other ECM proteins such as vitronectin, fibronectin, and collagens (Sugli et al. 2010; Tapp et al. 2008). Laminin-γ1 was produced by our ADSC population in vitro, and identifiable laminin-containing basal laminas were found surrounding CM-DiI-labeled ADSCs in the sciatic nerve in vivo. 3T3/L1 cells were selected as a cell-based control in part because they also express laminins (Niimi et al. 1997). Their failure to rescue function in the mutant nerves suggests that laminin expression alone was insufficient to improve nerve function in this model.
Figure 4. ADSCs produce laminin, yet soluble laminin alone is insufficient to mediate complete rescue of mutant nerves.
(A) ADSCs grown on Poly-D Lysine coated cover slips show evidence of laminin expression (green) by immunofluorescence. (B) Sections of mutant sciatic nerves that were treated with DiI-labeled ADSCs (red) were immunostained for laminin-1 (green). The arrow indicates the thin laminin-containing basal lamina surrounding a DiI-labeled cell. Nuclei for both A and B are labeled blue with DAPI. (C and D) A single dose of 23 μg of murine laminin-1 was injected around the sciatic nerve of mutant mice, while contralateral injections of DMEM were used as control. Laminin injection did not improve MCST (C) or velocity of current transmission (D) of mutant hind limbs as compared to DMEM treatment (p = 0.947 and 0.627 respectively). Wild type mouse recordings are represented by a dashed line.
To further explore the role of laminin in sciatic nerve repair in this genetic model of laminin-deficiency, we administered a local injection of murine-derived laminin to mutant nerves. MCST and current transmission velocities were compared between laminin-treated and media-treated contralateral limbs 21-days following injection (Figure 4C and D). As with the 3T3/L1-treated nerves, there was no evidence of significant improvement in hindlimb function following laminin-treatment. ADSCs deficient in laminin were generated, but were not viable (data not shown). Therefore, the specific role of ADSC-derived laminin could not be evaluated independently from ADSC-derived alternate trophic factors produced only in the presence of the mutant sciatic nerves.
DISCUSSION
There has been a recent surge of interest in the use of adult mesenchymal stem cells as biological therapy. This interest is related in part to the risk of unwanted teratoma formation with less differentiated cell types including embryonic stem cells, relative ease of ADSC isolation, immune-privileged status, and immunomodulatory effect of mesenchymal stem cells (Gotherstrom 2007; Le Blanc and Ringden 2006; Puissant et al. 2005), and their normal physiologic role as a stromal support cell. Mesenchymal stem cells isolated from white adipose tissue have been used in vitro to generate glia- and neuron-like cells (Xu et al. 2008), and in general, mesenchymal stem cells play a supportive role in their endogenous host tissue.
Our results showed that mesenchymal ADSCs induced functional and structural repair of developmentally dysregulated peripheral nerves. Treatment of congenitally laminin-deficient peripheral nerves with ADSCs but not with 3T3/L1 cells or soluble laminin resulted in functional improvement of the treated hindlimbs. The fundamental defect in the mutant sciatic nerves is in laminin expression, which results in developmental arrest during early post-natal development at a point in which axons are found in unsorted bundles (Yu et al. 2009a; Yu et al. 2005; Yu et al. 2009b). Ultrastructural evidence of developmental dysregulation coincides with absence of epitope expression characteristic of peripheral axon sorting and development of myelinating and non-myelinating Schwann cell: axon interactions such as the adhesion molecule L1 (Yu et al. 2009b).
There was negligible contribution of the ADSCs to the internal parenchyma of the target sciatic nerves. However, there was still significant functional improvement that occurred as a result of the endogenous SCs progressing past their developmental arrest. The supportive role for ADSCs has been seen when transplanted into other model injury systems. For instance, administration of ADSCs following myocardial infarction in a rat model curtails the extent of cardiac dysfunction (Schenke-Layland et al. 2009). Although ADSC-derivatives could be found within the target tissues many weeks after their injection, there was not significant engraftment of the ADSCs into the cardiac musculature despite improvement in cardiac function compared to untreated controls (Schenke-Layland et al. 2009). A similar trend has been seen in CNS models of injury; systemic administration of neurospheres (Pluchino et al. 2005) improved neuronal survival in a model of chronic CNS inflammation without the infused cells establishing themselves as nascent neurons or glia. Li et al showed that bone marrow stromal cells provided neurotrophic support in a rat model of stroke leading to functional recovery (Li et al. 2002), as did Mahmood et al with both systemic and directly transplanted bone marrow stromal cells in a model of traumatic brain injury (Mahmood et al. 2003; Mahmood et al. 2005). Human bone marrow stromal cells have similarly been used to promote neurite outgrowth from rat DRG-explants in culture without generation of neurons or glia derived from the stromal cells (Crigler et al. 2006). The same group has begun to investigate the transcriptome of bone marrow MSCs, and has shown that subpopulations of these cells produce the neurotrophins brain-derived neurotrophic factor (BDNF) and beta-nerve growth factor (β-NGF), both of which under the proper circumstances can promote neuronal survival. It is worth considering this as a potential mechanism by which ADSCs are inducing functional changes in our model system of mesenchymal stem cell-induced peripheral nerve repair. Further experiments will be needed to determine whether this is in fact the case.
Conditioned media from ADSCs grown on tissue culture plastic failed to induce improvement in sciatic nerve function as characterized by MCST measurements (Table 1). It would not be surprising for ADSCs to produce different trophic factors in the presence of the sciatic nerve than they express grown on tissue culture plastic. In addition, the local concentration of these factors could be expected to be higher and maintained for longer periods of time in ADSC-treated nerves, in which numerous DiI-labeled ADSCs were detected as long as 21-days following transplant encircling the mutant nerves. By comparison, conditioned-media treated animals received only a single injection.
Endogenous SCs began to sort and myelinate axons following ADSC-treatment as evidenced by ultrastructural and biochemical analyses and concordant functional changes. This was at least partially related to ADSC production of laminin, the primary deficiency in the mutant sciatic nerves. However, 3T3 control cells, which also express laminin, failed to induce functional changes in the mutant sciatic nerves. This was in part a result of their decreased persistence in the mutant sciatic nerves following injection, and highlighted a significant advantage that the ADSCs have over a control non-stem cell population.
One possible explanation for the lack of improvement after injection of soluble laminin could be that soluble laminin-1 (α1, β1, γ1) was used, while the predominant laminin found in adult sciatic nerves is laminin-2 (α2, β1, γ1). However, soluble laminin-1 has been used in vitro to completely rescue axon myelination defects in dorsal root ganglia co-cultures derived from the laminin-deficient mouse strain used in the experiments presented here (Yu et al. 2009a). It therefore seems unlikely that this is the reason that laminin injection did not rescue sciatic nerve function. Alternatively, transplanted ADSCs were a longer-lived continuous source of laminin, and may have expressed trophic factors in addition to laminins.
In summary, this study shows that adult ADSCs induced structural, biochemical, and functional improvement in a mouse model of laminin-deficient peripheral neuropathy. This report is consistent with the mechanism of mesenchymal stem cell-mediated cardioprotection in a model of cardiac ischemia, and with studies of mesenchymal stem cell-mediated changes in CNS models of injury. Trophic support, rather than tissue repopulation is becoming a common theme with respect to mesenchymal-stem cell mediated neural repair, and this study shows that the same trend continues in peripheral nerve models of injury.
Supplementary Material
Acknowledgments
We thank Eleana Sphicas from The Rockefeller University Bio-Imaging Resource Center for her work on the electron microscopy. We also thank Drs. Erin Norris, and Rajani Maiya and the members of the Strickland lab for their discussion of this project and critical review of this manuscript. This work was supported by grants from the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the NIH (NS038472), the Tri-Institutional/STARR Foundation, and the Muscular Dystrophy Association (MDA4066). KBC was supported in part by the F. M. Kirby fellowship for Sensory Neuroscience. PSD was supported by the Paul and Daisy Soros Fellowship and the Medical Scientist Training Program (GM07739). MMF was supported by the National Science Foundation Graduate Research Fellowship Program.
References
- Amoh Y, Li L, Campillo R, Kawahara K, Katsuoka K, Penman S, Hoffman RM. Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc Natl Acad Sci U S A. 2005;102(49):17734–8. doi: 10.1073/pnas.0508440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, et al. A simplified laminin nomenclature. Matrix Biol. 2005;24(5):326–32. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Chen ZL, Strickland S. Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J Cell Biol. 2003;163(4):889–99. doi: 10.1083/jcb.200307068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006;198(1):54–64. doi: 10.1016/j.expneurol.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Feltri ML, D’Antonio M, Previtali S, Fasolini M, Messing A, Wrabetz L. P0-Cre transgenic mice for inactivation of adhesion molecules in Schwann cells. Ann N Y Acad Sci. 1999;883:116–23. [PubMed] [Google Scholar]
- Feltri ML, Wrabetz L. Laminins and their receptors in Schwann cells and hereditary neuropathies. J Peripher Nerv Syst. 2005;10(2):128–43. doi: 10.1111/j.1085-9489.2005.0010204.x. [DOI] [PubMed] [Google Scholar]
- Fujimura J, Ogawa R, Mizuno H, Fukunaga Y, Suzuki H. Neural differentiation of adipose-derived stem cells isolated from GFP transgenic mice. Biochem Biophys Res Commun. 2005;333(1):116–21. doi: 10.1016/j.bbrc.2005.05.096. [DOI] [PubMed] [Google Scholar]
- Gotherstrom C. Immunomodulation by multipotent mesenchymal stromal cells. Transplantation. 2007;84(1 Suppl):S35–7. doi: 10.1097/01.tp.0000269200.67707.c8. [DOI] [PubMed] [Google Scholar]
- Haney CA, Sahenk Z, Li C, Lemmon VP, Roder J, Trapp BD. Heterophilic binding of L1 on unmyelinated sensory axons mediates Schwann cell adhesion and is required for axonal survival. J Cell Biol. 1999;146(5):1173–84. doi: 10.1083/jcb.146.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman GJ, Richardson RL. Newly recruited and pre-existing preadipocytes in cultures of porcine stromal-vascular cells: morphology, expression of extracellular matrix components, and lipid accretion. J Anim Sci. 1998;76(1):48–60. doi: 10.2527/1998.76148x. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Embryonic Schwann cell development: the biology of Schwann cell precursors and early Schwann cells. J Anat. 1997;191 ( Pt 4):501–5. doi: 10.1046/j.1469-7580.1997.19140501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang W, Xu H, Vachon PH, Liu L, Loechel F, Wewer UM, Engvall E. Merosin-deficient congenital muscular dystrophy. Partial genetic correction in two mouse models. J Clin Invest. 1998;102(4):844–52. doi: 10.1172/JCI3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc K, Ringden O. Mesenchymal stem cells: properties and role in clinical bone marrow transplantation. Curr Opin Immunol. 2006;18(5):586–91. doi: 10.1016/j.coi.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59(4):514–23. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53(3):697–702. doi: 10.1227/01.neu.0000079333.61863.aa. discussion 702–3. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Qu C, Goussev A, Chopp M. Human marrow stromal cell treatment provides long-lasting benefit after traumatic brain injury in rats. Neurosurgery. 2005;57(5):1026–31. doi: 10.1227/01.neu.0000181369.76323.50. discussion 1026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R, Schachner M. Immunoelectron microscopic localization of neural cell adhesion molecules (L1, N-CAM, and MAG) and their shared carbohydrate epitope and myelin basic protein in developing sciatic nerve. J Cell Biol. 1986;103(6 Pt 1):2439–48. doi: 10.1083/jcb.103.6.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri E, Rutherford M, De Vile C, Counsell S, Sewry C, Brown S, Bydder G, Dubowitz V, Muntoni F. Early white matter changes on brain magnetic resonance imaging in a newborn affected by merosin-deficient congenital muscular dystrophy. Neuromuscul Disord. 2001;11(3):297–9. doi: 10.1016/s0960-8966(00)00190-5. [DOI] [PubMed] [Google Scholar]
- Miner JH, Li C, Mudd JL, Go G, Sutherland AE. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development. 2004;131(10):2247–56. doi: 10.1242/dev.01112. [DOI] [PubMed] [Google Scholar]
- Mirsky R, Jessen KR. Schwann cell development, differentiation and myelination. Curr Opin Neurobiol. 1996;6(1):89–96. doi: 10.1016/s0959-4388(96)80013-4. [DOI] [PubMed] [Google Scholar]
- Mirsky R, Parkinson DB, Dong Z, Meier C, Calle E, Brennan A, Topilko P, Harris BS, Stewart HJ, Jessen KR. Regulation of genes involved in Schwann cell development and differentiation. Prog Brain Res. 2001;132:3–11. doi: 10.1016/S0079-6123(01)32060-5. [DOI] [PubMed] [Google Scholar]
- Mirsky R, Stewart HJ, Tabernero A, Bradke F, Brennan A, Dong Z, Jessen KR. Development and differentiation of Schwann cells. Rev Neurol (Paris) 1996;152(5):308–13. [PubMed] [Google Scholar]
- Murray P, Edgar D. Regulation of programmed cell death by basement membranes in embryonic development. J Cell Biol. 2000;150(5):1215–21. doi: 10.1083/jcb.150.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieke J, Schachner M. Expression of the neural cell adhesion molecules L1 and N-CAM and their common carbohydrate epitope L2/HNK-1 during development and after transection of the mouse sciatic nerve. Differentiation. 1985;30(2):141–51. doi: 10.1111/j.1432-0436.1985.tb00525.x. [DOI] [PubMed] [Google Scholar]
- Niimi T, Kumagai C, Okano M, Kitagawa Y. Differentiation-dependent expression of laminin-8 (alpha 4 beta 1 gamma 1) mRNAs in mouse 3T3-L1 adipocytes. Matrix Biol. 1997;16(4):223–30. doi: 10.1016/s0945-053x(97)90011-1. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28(3–4):147–55. [PubMed] [Google Scholar]
- Patton BL. Laminins of the neuromuscular system. Microsc Res Tech. 2000;51(3):247–61. doi: 10.1002/1097-0029(20001101)51:3<247::AID-JEMT5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Patton BL, Miner JH, Chiu AY, Sanes JR. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J Cell Biol. 1997;139(6):1507–21. doi: 10.1083/jcb.139.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436(7048):266–71. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129(1):118–29. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- Schenke-Layland K, Strem BM, Jordan MC, Deemedio MT, Hedrick MH, Roos KP, Fraser JK, Maclellan WR. Adipose tissue-derived cells improve cardiac function following myocardial infarction. J Surg Res. 2009;153(2):217–23. doi: 10.1016/j.jss.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth N, Vatansever HS, Meyer M, Frie C, Paulsson M, Edgar D. The targeted deletion of the LAMC1 gene. Ann N Y Acad Sci. 1998;857:283–6. doi: 10.1111/j.1749-6632.1998.tb10133.x. [DOI] [PubMed] [Google Scholar]
- Stewart HJ, Turner D, Jessen KR, Mirsky R. Expression and regulation of alpha1beta1 integrin in Schwann cells. J Neurobiol. 1997;33(7):914–28. doi: 10.1002/(sici)1097-4695(199712)33:7<914::aid-neu4>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Sugii S, Kida Y, Kawamura T, Suzuki J, Vassena R, Yin YQ, Lutz MK, Berggren WT, Izpisua Belmonte JC, Evans RM. Human and mouse adipose-derived cells support feeder-independent induction of pluripotent stem cells. PNAS. 2010;107(8):3558–63. doi: 10.1073/pnas.0910172106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapp H, Deepe R, Ingram J, Kuremsky M, Hanley EN, Gruber HE. Adipose-derived mesenchymal stem cells from the sand rat: transforming growth factor beta and 3D co-culture with human disc cells stimulate proteoglycan and collagen type I rich extracellular matrix. Arthritis Research and Therapy. 2008;10(R89):1–10. doi: 10.1186/ar2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uziyel Y, Hall S, Cohen J. Influence of laminin-2 on Schwann cell-axon interactions. Glia. 2000;32(2):109–21. doi: 10.1002/1098-1136(200011)32:2<109::aid-glia10>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Xu Y, Liu Z, Liu L, Zhao C, Xiong F, Zhou C, Li Y, Shan Y, Peng F, Zhang C. Neurospheres from rat adipose-derived stem cells could be induced into functional Schwann cell-like cells in vitro. BMC Neurosci. 2008;9:21. doi: 10.1186/1471-2202-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LY, Liu XM, Sun B, Hui GZ, Fei J, Guo LH. Adipose tissue-derived stromal cells express neuronal phenotypes. Chin Med J (Engl) 2004;117(3):425–9. [PubMed] [Google Scholar]
- Yu WM, Chen ZL, North AJ, Strickland S. Laminin is required for Schwann cell morphogenesis. J Cell Sci. 2009a;122(Pt 7):929–36. doi: 10.1242/jcs.033928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WM, Feltri ML, Wrabetz L, Strickland S, Chen ZL. Schwann cell-specific ablation of laminin gamma1 causes apoptosis and prevents proliferation. J Neurosci. 2005;25(18):4463–72. doi: 10.1523/JNEUROSCI.5032-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WM, Yu H, Chen ZL, Strickland S. Disruption of laminin in the peripheral nervous system impedes nonmyelinating Schwann cell development and impairs nociceptive sensory function. Glia. 2009b;57(8):850–9. doi: 10.1002/glia.20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.