Abstract
The proper development and patterning of axons, dendrites, and synapses is essential for the establishment of accurate neuronal circuits in the brain. A major goal in neurobiology is to identify the mechanisms and principles that govern these fundamental developmental events of neuronal circuit formation. In recent years, exciting new studies have suggested that ubiquitin signaling pathways may play crucial roles in the control of neuronal connectivity. Among E3 ubiquitin ligases, Cdh1-anaphase promoting complex (Cdh1-APC) and Cdc20-APC have emerged as key regulators of diverse aspects of neuronal connectivity, from axon and dendrite morphogenesis to synapse differentiation and remodeling.
Introduction
The anaphase promoting complex (APC) is a large protein complex that is composed of at least 12 subunits [1, 2]. Among the subunits of the APC, APC2 represents a Cul1-related scaffold protein, and the RING finger protein APC11 encodes the catalytic E3 core subunit [1, 2]. The ubiquitin ligase activity of the APC is stimulated by interaction with one of two key co-activator subunits, Cdh1 or Cdc20, which also targets the APC to distinct substrates [1, 2] (Figure 1). Substrates of Cdc20-APC or Cdh1-APC contain a peptide sequence termed the destruction-box (D-box), which serves as the recognition motif for Cdc20 or Cdh1 [3]. Additional Cdh1 peptide recognition motifs, including the KEN box, A-box, and CRY box, have been identified within substrates of Cdh1-APC [4-6]. Although advances have been made in understanding the structure of the APC using electron microscopy studies [7-10], the precise molecular basis of APC-induced ubiquitination of substrates and the function of the numerous subunits in the complex remains a mystery.
Figure 1. The structure of the APC.

The APC is composed of at least 12 core subunits including the Cul1-related scaffold protein APC2 and the RING finger protein APC11 shown in red. The APC associates with one of two co-activators, Cdh1 or Cdc20 (shown in yellow), which confer substrate specificity and stimulate the ubiquitin ligase activity of the APC. The tetratricopeptide repeat (TPR) containing core subunits Cdc27, Cdc23, and Cdc16 (shown in blue) act as a scaffold to promote the interaction of Cdh1 and Cdc20 with the APC.
The characterization of the APC in proliferating cells has provided invaluable clues for studies of the APC in postmitotic neurons. A major concept that has emerged from studies of the APC in proliferating cells is that Cdc20-APC and Cdh1-APC control distinct temporal phases of the cell cycle [1]. Cdh1-APC operates during mitotic exit and G1 phase of the cell cycle, while Cdc20-APC drives anaphase in early mitosis. Cdh1 and Cdc20 are dynamically controlled during distinct phases of the cell cycle by several modes of posttranslational modifications, including phosphorylation, ubiquitination, and interactions with APC inhibitors. An additional layer of regulation is provided by transcription of Cdc20 in proliferating cells. The functions and regulation of the APC during the cell cycle in dividing cells have been reviewed [1, 2, 11-14]. In this review, we will focus on studies implicating the two distinct APC ubiquitin ligase subtypes, Cdh1-APC and Cdc20-APC, in neuronal patterning and connectivity.
The APC orchestrates axon and dendrite morphogenesis
Nearly a decade after the APC was identified in cycling cells [15, 16], its function in postmitotic neurons first came into view in studies of neuronal morphogenesis [17••]. Earlier evidence had revealed that Cdh1 and the APC core subunits are expressed in mammalian brain neurons [18]. Later, Cdc20 was also found to be expressed in neurons in the developing brain [19••]. Functional analyses of Cdh1-APC and Cdc20-APC have uncovered critical roles for these two distinct APC complexes in the regulation of axon and dendrite morphogenesis, respectively [17••, 19••]. These studies suggest the key concept that the temporally distinct activities of Cdh1-APC and Cdc20-APC during the cell cycle appear to have been transposed to distinct subcellular compartments in postmitotic neurons to coordinate the growth and patterning of axons and dendrites.
A nuclear Cdh1-APC ubiquitin signaling pathway regulates axon growth and patterning
A series of investigations have led to the identification of a Cdh1-APC ubiquitin signaling pathway that restricts the growth of axons and controls their patterning in the mammalian brain [17••, 20, 21, 22•, 23-25, 26•]. Using granule neurons of the rat cerebellar cortex as a model system for studies of neuronal morphogenesis [27-30], Konishi et al. discovered that knockdown of Cdh1 in neurons specifically stimulates the growth of axons but not dendrites [17••] (Figure 2). Granule neurons expressing the APC inhibitor Emi1 or a dominant interfering form of APC11 display longer axons than control neurons. These results suggest that the ubiquitin ligase activity of Cdh1-APC inhibits axon growth.
Figure 2. Cdh1-APC and Cdc20-APC govern the spatially distinct processes of axon and dendrite morphogenesis.

Cdh1-APC operates in the nucleus to regulate axon growth and patterning. Cdh1-APC restricts axon growth by targeting the transcriptional regulators SnoN and Id2 for degradation. SnoN and Id2 regulate the transcription of target genes enriched at the axon growth cone including Ccd1 and NgR. The TGFβ/Smad2 and Cdk signaling pathways converge upon Cdh1-APC to regulate axon morphogenesis. Upon activation by TGFβ, Smad2 interacts with Cdh1 and induces Cdh1-APC-dependent ubiquitination of SnoN. Conversely, Cdk inhibits Cdh1-APC activity by phosphorylating Cdh1 at nine conserved sites, leading to the accumulation of an inactive form of Cdh1 in the cytoplasm. Cdc20-APC operates at the centrosome to drive dendrite growth and elaboration. Polyubiquitination of Cdc20 enhances Cdc20-APC activity and dendrite growth. HDAC6 promotes the polyubiquitination of Cdc20, while USP44 deubiquitinates Cdc20. The centrosomally-localized protein Id1 represents a substrate of neuronal Cdc20-APC that inhibits dendrite growth.
Knockdown experiments in cerebellar slices and in postnatal rat pups in vivo revealed that beyond the control of axon growth, Cdh1-APC controls the layer-specific pattern of axon morphogenesis in the cerebellar cortex [17••]. Loss of Cdh1 also endows granule neurons with the ability to overcome the axon growth-inhibitory signals imposed by myelin, raising the prospect that inhibition of Cdh1-APC may represent a novel therapeutic strategy to stimulate axon regeneration following injury and disease.
Recent studies have explored the two major questions of how neuronal Cdh1-APC is regulated in neurons and the mechanisms by which Cdh1-APC controls axon morphogenesis [22•, 23-25, 26•]. An important clue relevant to these studies is the finding that neuronal Cdh1-APC remarkably operates in the nucleus to control the growth of axons [22•]. Regulation of the subcellular localization of Cdh1 by phosphorylation appears to bear important consequences on Cdh1-APC control of axon growth [23]. These studies suggest that Cdk-induced phosphorylation of Cdh1 at nine conserved sites stabilizes an inactive form of Cdh1 that accumulates in the cytoplasm, which fails to inhibit axon growth [23].
Extrinsic cues that may regulate Cdh1-APC activity in neurons are just beginning to be characterized (Figure 2). TGFβ enhances Cdh1-APC activity in proliferating cells by inducing the translocation of the signaling protein Smad2 or Smad3 to the nucleus, where Smad2/3 binds to Cdh1 and stimulates Cdh1-APC activity [31, 32]. In agreement with this model, inhibition of TGFβ/Smad2 signaling stimulates axon growth in granule neurons [24].
The nuclear site of action of Cdh1-APC in neurons has also provided important insight in the study of the mechanism by which Cdh1-APC restrains axon growth [22•]. The transcription modulator SnoN has been identified as a substrate of neuronal Cdh1-APC in the control of axon growth (Figure 2). Knockdown of SnoN in granule neurons inhibits axon growth in primary granule neurons and profoundly impairs the development of parallel fiber axons in vivo. Importantly, SnoN knockdown suppresses the ability of Cdh1 knockdown to stimulate axon growth [22•]. These results define Cdh1-APC and SnoN as components of a nuclear ubiquitin signaling pathway that orchestrates axon growth in the mammalian brain.
SnoN is widely believed to repress transcription [33, 34]. Surprisingly, the landscape of gene expression changes triggered by SnoN knockdown in gene profiling experiments suggests an important transcriptional activating role for SnoN in neurons [25]. Consistent with this conclusion, the transcriptional coactivator p300 interacts with SnoN, and p300 is required for the axon growth-promoting function of SnoN in neurons [25]. These results support the concept that SnoN may activate or repress transcription in a cell- and gene-specific manner [34-36]. Among SnoN targets in neurons, the gene encoding the signaling protein Ccd1 represents a physiologically relevant target gene in the control of axon growth. Ccd1 is enriched in axon growth terminals and appears to be necessary for SnoN-dependent axon growth [25]. Thus, Ccd1 links the nuclear Cdh1-APC/SnoN ubiquitin pathway to the local control of axon growth.
In addition to SnoN, the transcriptional regulatory protein inhibitor of DNA binding 2 (Id2) has been identified as a critical substrate of Cdh1-APC in the regulation of axon growth [26•]. Like SnoN, Id2 contains a conserved D-box. Cdh1-APC stimulates the ubiquitination of Id2 in a D-box-dependent manner, leading to Id2 degradation in neurons. Expression of an Id2 mutant protein in which the D-box is mutated (Id2-DBM) promotes axon growth in granule neurons. Id2 regulates transcription by inhibiting the function of basic helix-loop-helix (bHLH) transcription factors [37]. Expression of the bHLH transcription factor E47 suppresses axon growth and is epistatic to both Cdh1 knockdown and Id2-DBM expression in axon growth assays [26•]. Microarray analyses reveal that the transcriptional targets of E47 include ligands and receptors important for the control of axon guidance and growth including Sema3F, Unc5a, and Nogo receptor (NgR) [26•]. Consistent with these results, expression of Id2-DBM suppresses the ability of myelin to inhibit axon growth. Together, these results define a nuclear Cdh1-APC/Id2 ubiquitin pathway in neurons that regulates axon growth. Whether the Cdh1-APC/SnoN and Cdh1-APC/Id2 signaling pathways act in parallel or converge to regulate axon growth remains to be determined.
A centrosomal Cdc20-APC pathway drives dendrite growth and elaboration
The identification of Cdh1-APC function in the regulation of axon growth and patterning led to the intriguing question of whether the APC co-activator Cdc20 might also play a role in neuronal morphogenesis. Kim et al. found that Cdc20 is expressed in postmitotic neurons in the developing brain, with protein levels increasing with neuronal maturation [19••]. Remarkably, the authors discovered that in contrast to knockdown of Cdh1, knockdown of Cdc20 in granule neurons profoundly impairs dendrite growth and arborization in primary granule neurons and in the cerebellar cortex in vivo but has little or no effect on axon growth.
The subcellular compartment in which Cdc20 operates to control dendrite growth is distinct from the nuclear locus of Cdh1’s axon regulatory function [19••]. Immunocytochemical and biochemical analyses suggest that a substantial pool of Cdc20 resides at the centrosome in granule neurons. Strikingly, structure-function analyses suggest that the centrosomal localization of Cdc20 is critical for Cdc20-dependent dendrite growth and elaboration. These findings suggest that Cdc20-APC operates at the centrosome to drive dendrite growth and elaboration.
A mechanism of Cdc20-APC regulation by the centrosomally-localized histone deacetylase HDAC6 has been reported in neurons (Figure 2). HDAC6 interacts directly with Cdc20 and promotes dendrite growth in a Cdc20-dependent manner [19••]. Surprisingly, HDAC6 regulates Cdc20-APC independently of its deacetylase activity. Rather, HDAC6, through its ubiquitin binding domain ZnF UBP, promotes the polyubiquitination of Cdc20 and thereby stimulates the ubiquitin ligase activity of Cdc20-APC activity. The polyubiquitination of Cdc20 activates Cdc20-APC activity during mitosis [38, 39]. Knockdown of the major Cdc20-specific deubiquitinase USP44 in neurons augments the ubiquitination status of Cdc20 and substantially increases the length and complexity of dendrite arbors, suggesting that the dynamic modulation of Cdc20 polyubiquitination per se is critical for dendrite morphogenesis.
Since the ubiquitin ligase activity of Cdc20-APC is critical for its function, Cdc20-APC is anticipated to promote dendrite growth by inducing the ubiquitination and consequent degradation of a centrosomal protein that inhibits dendrite development. Using this rationale, the centrosomally localized protein Id1 has been identified as a substrate of neuronal Cdc20-APC [19••]. Knockdown of Id1 stimulates dendrite growth, and epistasis experiments suggest that Id1 operates downstream of Cdc20 [19••]. It will be important in future studies to explore the mechanism by which Id1 inhibits the growth and elaboration of dendrites. While Id1 is localized at the centrosome, Id1 is also established to inhibit bHLH-dependent transcription in the nucleus [37]. Therefore, it will be interesting to determine if Id1 controls dendrite morphogenesis by regulating transcription or by a local mechanism at the centrosome.
The APC regulates synaptic connectivity
Although the functions of the APC in the regulation of axon and dendrite morphogenesis are expected to profoundly impact neuronal circuitry, APC functions have also been more directly linked to the control of synapse development and hence neuronal connectivity. The picture that is coming into view from these studies is that the APC plays a critical role in distinct aspects of synapse formation, though we are still in the early days of our understanding of the role and mechanisms of APC regulation of synapse differentiation and remodeling in the brain.
Cdh1-APC controls synaptic differentiation and transmission in the invertebrate nervous system
The critical role of Cdh1-APC in synapse differentiation was first recognized in invertebrate model systems [40••, 41••] (Figure 3). Core components of the APC in Drosophila are expressed in motor neurons and muscle cells that communicate at the neuromuscular junction (NMJ). Loss-of-function APC2 mutant flies exhibit an increase in the number of presynaptic boutons at the NMJ [40••]. Specific knockdown of APC2 in motor neurons, but not in muscle cells, recapitulated the increase in bouton density. Consideration of the potential targets of the APC in motor neurons led the investigators to examine the protein Liprinα, which harbors a D-box motif and augments synapse size in Drosophila [42]. Motor neurons in APC2 mutant flies have increased Liprinα expression compared to neurons in wild type controls [40••]. Importantly, in the background of Liprinα loss-of-function mutant flies, loss of APC2 fails to increase presynaptic bouton number, suggesting that Liprinα is genetically downstream of APC2 in the regulation of synapse size. The authors surmised that Cdh1-APC is the form of the APC that restrains presynaptic bouton number, because immunohistochemical analyses suggest that Cdh1 and APC2 are expressed at presynaptic sites at the NMJ [40••]. In view of the recent discovery that Cdc20-APC regulates presynaptic differentiation in mammalian brain neurons (see below), it will be interesting to also explore the role of Cdc20-APC in presynaptic differentiation at the fly NMJ.
Figure 3. Cdh1-APC regulates synapse size and glutamatergic neurotransmission in invertebrates.
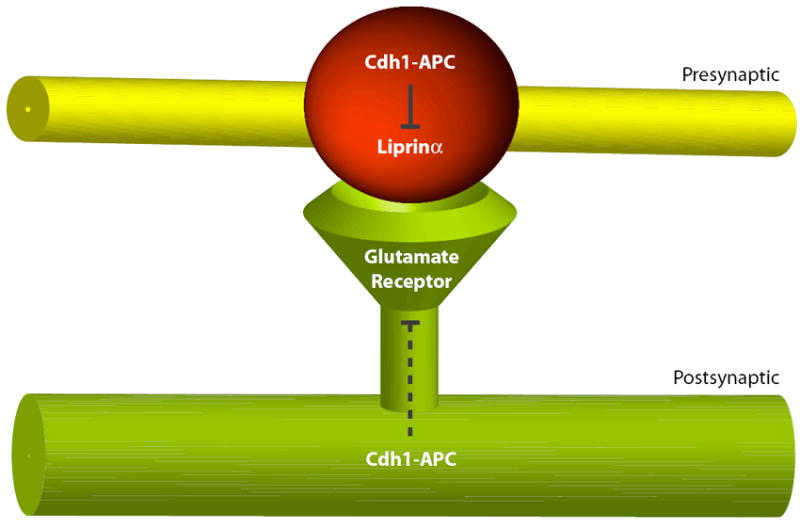
Cdh1 and core subunits of the APC are localized at the presynaptic bouton at the Drosophila NMJ. Presynaptic APC controls the abundance of the active zone protein Liprinα and restricts the number of presynaptic boutons contacting the postsynaptic muscle cell. On the postsynaptic side, Cdh1-APC limits the expression of glutamate receptor subunits in worms and flies. The mechanism of APC regulation of glutamate receptors awaits further study.
Evidence for a postsynaptic function of the APC in invertebrate nervous systems has also been identified. In C. elegans, loss-of-function mutants of several core APC subunits exhibit an increase in the abundance of the AMPA receptor subunit GLR-1 in ventral nerve cord neurons [41••]. Expression of APC subunits driven by the glr-1 promoter restored the normal width of GLR-1 synaptic puncta in APC mutant worms, suggesting that Cdh1-APC controls AMPA receptor levels in a cell-autonomous manner [41••]. However, GLR-1 lacks an obvious APC recognition motif, and GLR-1 ubiquitination appeared to be unchanged in APC subunit mutants compared to that in wild type worms. A postsynaptic locus of APC action may also regulate the fly NMJ [40••]. Just as in nematodes, however, the mechanism by which the APC regulates postsynaptic glutamate receptors at the fly NMJ remains elusive.
A Cdc20-APC ubiquitin signaling pathway orchestrates presynaptic differentiation in the brain
While Cdh1-APC has been implicated in synapse development in invertebrate systems, until recently the role of the APC in synapse differentiation in mammalian brain neurons remained to be identified. Yang et al. recently established assays that facilitate the study of presynaptic differentiation in the robust model system of cerebellar granule neurons [43••] (Figure 4). Using this system, knockdown of Cdh1 surprisingly does not appear to alter the number of presynaptic sites. Remarkably, the authors discovered that Cdc20-APC drives presynaptic differentiation in granule neurons [43••]. Knockdown of Cdc20 reduces the density of the synaptic vesicle and active zone proteins in granule neuron axons. Further, Cdc20 knockdown reduces the density of synapsin co-clusters with the postsynaptic protein PSD-95 and with sites of uptake of the dye FM4-64, suggesting that Cdc20-APC promotes the formation of functional synapses. Cdc20 knockdown also impairs the differentiation of presynaptic sites in the cerebellar cortex in postnatal rat pups in vivo.
Figure 4. Cdc20-APC drives presynaptic differentiation in mammalian brain neurons.

Top: during early axon differentiation, NeuroD2-dependent transcription of the target gene Cplx2 suppresses presynaptic differentiation. Bottom: As Cdc20 expression increases with neuronal maturation, Cdc20-APC targets NeuroD2 protein for degradation and thereby promotes the formation of functional presynaptic sites. Figure adapted from [43••].
The brain-enriched transcription factor NeuroD2, which contains a D-box motif, has been uncovered as a physiologically relevant substrate of Cdc20-APC in the control of presynaptic differentiation [43••] (Figure 4). Cdc20 interacts with NeuroD2 in a D-box-dependent manner and NeuroD2 is regulated by the ubiquitin-proteasome system in neurons. Knockdown of Cdc20 increases the levels of endogenous NeuroD2 protein in primary granule neurons. Depletion of NeuroD2 increases the density of presynaptic sites in primary granule neurons and in the cerebellar cortex in vivo in rat postnatal pups. Epistasis analyses show that NeuroD2 lies downstream of Cdc20 in the control of presynaptic number. Together, these results suggest that the Cdc20-APC-induced degradation of NeuroD2 drives presynaptic differentiation.
A NeuroD2 target gene has been identified that mediates the NeuroD2-dependent suppression of presynaptic differentiation [43••]. Among NeuroD2 targets, Complexin II (Cplx2) regulates synaptic vesicle membrane fusion and has been implicated in the control of presynaptic number in Drosophila [44, 45]. Expression of exogenous Cplx2 in granule neurons inhibits the increase in presynaptic site density elicited by NeuroD2 knockdown [43••]. In addition, Cplx2 knockdown increases the number of functional presynaptic sites both in primary granule neurons and in the cerebellar cortex in vivo in rat postnatal rat pups. Knockdown of Cplx2 also reverses the Cdc20 knockdown-triggered decrease in presynaptic density, suggesting that Cplx2 lies downstream of Cdc20. Collectively, these findings define a novel Cdc20-APC ubiquitin signaling pathway that orchestrates presynaptic differentiation in the mammalian brain.
Perspectives
With the rapid progress in our understanding of APC function in neuronal development, common themes have emerged. The recruitment of the two APC coactivators, Cdh1 and Cdc20, in different subcellular compartments allows for the specific targeting and degradation of APC substrates in distinct aspects of neuronal morphogenesis. Whereas Cdh1-APC controls axon growth from the nucleus [17••, 22•, 26•], Cdc20-APC operates at the centrosome to drive dendrite growth and arborization [19••]. In a similar vein, the two APC subtypes may regulate distinct temporal phases of neuronal maturation. While Cdh1-APC controls the first phases of axon development [17••, 22•, 26•], i.e. axon growth and patterning, Cdc20-APC coordinates the later phases of axon morphogenesis intimately linked to neuronal connectivity, i.e. presynaptic differentiation [43••].
An important question for future studies is the function of Cdh1-APC in postsynaptic differentiation in mammalian brain neurons. Inhibition of Cdh1-APC in rat hippocampal neurons has been reported to increase the levels of Liprinα in dendrites [46]. However, whether Cdh1-APC inhibition leads to alterations of dendritic spines or synapses in these neurons remains unexplored. In other studies, abnormalities in learning and memory and late phase of long-term potentiation (L-LTP) at the CA1 synapse in hippocampal slices have been observed in mice heterozygous for the Cdh1 gene [47]. However, whether and how impairment of synapse differentiation contributes to these phenotypes remains to be investigated. Together, these studies raise the interesting possibility that Cdh1-APC might control postsynaptic differentiation in the mammalian brain, though the nature of this function and its underlying mechanism are unknown.
Beyond the control of neuronal morphogenesis and connectivity, additional roles of the APC have been characterized in other aspects of neuronal development, from the control of neural stem cell proliferation to neuronal cell death to metabolic control of glycolysis in neurons [48-54]. These studies suggest that the APC may have pleiotropic roles in the brain, from development to maturity.
In each of the defined functions of Cdh1-APC and Cdc20-APC in neuronal connectivity, from axon and dendrite morphogenesis to presynaptic differentiation, several important questions remain to be addressed. It will be important to determine the regulatory mechanisms that control these pathways and to identify extrinsic cues that might interact with the cell-intrinsic functions of the APC in neuronal morphogenesis and connectivity. With advances in our understanding of the local cytoskeletal mechanisms that control neuronal morphology and development, it will be interesting to understand how the APC and its targets interface with regulators of cytoskeletal dynamics [55-59]. Identification of additional substrates of neuronal Cdh1-APC and Cdc20-APC should shed new light on the mechanisms of defined APC function as well as lead to uncovering novel functions of the APC in neurons. Finally, a major line of future research should elucidate the functions of the APC in the development and function of the brain using conditional knockout strategies. Such studies should unravel the range of behaviors that are controlled by the APC. This approach will also facilitate the determination of the role of the APC in the pathogenesis of neurological and psychiatric diseases. In view of the profound effects of the APC on neuronal connectivity, it is conceivable that manipulation of APC components and associated signaling pathways might have therapeutic implications for diverse neurological and psychiatric disorders, from developmental cognitive disorders of mental retardation and autism spectrum disorders to neurodegenerative diseases.
Acknowledgments
We thank members of the Bonni laboratory for helpful discussions. Supported by NIH grants NS051255 and NS041021 (A.B.); a NSF fellowship, the Lefler fellowship, and the Ryan foundation (Y.Y.); a Ruth L. Kirschstein National Research Service Award fellowship, National Cancer Institute, and Brain Science Foundation grant (A.H.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest have been highlighted as:
-
•
of special interest
-
••
of outstanding interest
- 1.Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 2.Harper JW, Burton JL, Solomon MJ. The anaphase-promoting complex: it’s not just for mitosis any more. Genes Dev. 2002;16:2179–2206. doi: 10.1101/gad.1013102. [DOI] [PubMed] [Google Scholar]
- 3.Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- 4.Pfleger CM, Kirschner MW. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 2000;14:655–665. [PMC free article] [PubMed] [Google Scholar]
- 5.Littlepage LE, Ruderman JV. Identification of a new APC/C recognition domain, the A box, which is required for the Cdh1-dependent destruction of the kinase Aurora-A during mitotic exit. Genes Dev. 2002;16:2274–2285. doi: 10.1101/gad.1007302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reis A, Levasseur M, Chang HY, Elliott DJ, Jones KT. The CRY box: a second APCcdh1-dependent degron in mammalian cdc20. EMBO Rep. 2006;7:1040–1045. doi: 10.1038/sj.embor.7400772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gieffers C, Dube P, Harris JR, Stark H, Peters JM. Three-dimensional structure of the anaphase-promoting complex. Mol Cell. 2001;7:907–913. doi: 10.1016/s1097-2765(01)00234-9. [DOI] [PubMed] [Google Scholar]
- 8.Dube P, Herzog F, Gieffers C, Sander B, Riedel D, Muller SA, Engel A, Peters JM, Stark H. Localization of the coactivator Cdh1 and the cullin subunit Apc2 in a cryo-electron microscopy model of vertebrate APC/C. Mol Cell. 2005;20:867–879. doi: 10.1016/j.molcel.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Passmore LA, Booth CR, Venien-Bryan C, Ludtke SJ, Fioretto C, Johnson LN, Chiu W, Barford D. Structural analysis of the anaphase-promoting complex reveals multiple active sites and insights into polyubiquitylation. Mol Cell. 2005;20:855–866. doi: 10.1016/j.molcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Herzog F, Primorac I, Dube P, Lenart P, Sander B, Mechtler K, Stark H, Peters JM. Structure of the anaphase-promoting complex/cyclosome interacting with a mitotic checkpoint complex. Science. 2009;323:1477–1481. doi: 10.1126/science.1163300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan DO. Regulation of the APC and the exit from mitosis. Nat Cell Biol. 1999;1:E47–53. doi: 10.1038/10039. [DOI] [PubMed] [Google Scholar]
- 12.Yu H. Cdc20: a WD40 activator for a cell cycle degradation machine. Mol Cell. 2007;27:3–16. doi: 10.1016/j.molcel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Pesin JA, Orr-Weaver TL. Regulation of APC/C activators in mitosis and meiosis. Annu Rev Cell Dev Biol. 2008;24:475–499. doi: 10.1146/annurev.cellbio.041408.115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 15.King RW, Peters JM, Tugendreich S, Rolfe M, Hieter P, Kirschner MW. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell. 1995;81:279–288. doi: 10.1016/0092-8674(95)90338-0. [DOI] [PubMed] [Google Scholar]
- 16.Irniger S, Piatti S, Michaelis C, Nasmyth K. Genes involved in sister chromatid separation are needed for B-type cyclin proteolysis in budding yeast. Cell. 1995;81:269–278. doi: 10.1016/0092-8674(95)90337-2. [DOI] [PubMed] [Google Scholar]
- 17••.Konishi Y, Stegmuller J, Matsuda T, Bonni S, Bonni A. Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science. 2004;303:1026–1030. doi: 10.1126/science.1093712. This paper reports the first characterization of a role for the APC outside the cell cycle in postmitotic neurons. The authors find that Cdh1-APC controls axon growth and patterning in the mammalian brain.
- 18.Gieffers C, Peters BH, Kramer ER, Dotti CG, Peters JM. Expression of the CDH1-associated form of the anaphase-promoting complex in postmitotic neurons. Proc Natl Acad Sci U S A. 1999;96:11317–11322. doi: 10.1073/pnas.96.20.11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Kim AH, Puram SV, Bilimoria PM, Ikeuchi Y, Keough S, Wong M, Rowitch D, Bonni A. A centrosomal Cdc20-APC pathway control dendritic morphogenesis in postmitoic neurons. Cell. 2009;136:322–336. doi: 10.1016/j.cell.2008.11.050. The authors demonstrate that Cdc20-APC operates at the centrosome in postmitotic neurons to drive dendrite morphogenesis, and elucidate a ubiquitin signaling pathway that controls Cdc20-APC-dependent dendrite development.
- 20.Stegmuller J, Bonni A. Moving past proliferation: new roles for Cdh1-APC in postmitotic neurons. Trends Neurosci. 2005;28:596–601. doi: 10.1016/j.tins.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Kim AH, Bonni A. Thinking within the D box: initial identification of Cdh1-APC substrates in the nervous system. Mol Cell Neurosci. 2007;34:281–287. doi: 10.1016/j.mcn.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 22•.Stegmuller JA, Konishi YA, Huynh MAA, Yuan ZA, Dibacco SA, Bonni AA. Cell-intrinsic regulation of axonal morphogenesis by the Cdh1-APC target SnoN. Neuron. 2006;50:389–400. doi: 10.1016/j.neuron.2006.03.034. In this paper, the authors discover that Cdh1-APC operates in the nucleus to target the transcription factor SnoN in the regulation of axon growth.
- 23.Huynh MA, Stegmuller J, Litterman N, Bonni A. Regulation of Cdh1-APC function in axon growth by Cdh1 phosphorylation. J Neurosci. 2009;29:4322–4327. doi: 10.1523/JNEUROSCI.5329-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stegmuller J, Huynh MA, Yuan Z, Konishi Y, Bonni A. TGFbeta-Smad2 signaling regulates the Cdh1-APC/SnoN pathway of axonal morphogenesis. J Neurosci. 2008;28:1961–1969. doi: 10.1523/JNEUROSCI.3061-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeuchi Y, Stegmuller J, Netherton S, Huynh MA, Masu M, Frank D, Bonni S, Bonni A. A SnoN-Ccd1 pathway promotes axonal morphogenesis in the mammalian brain. J Neurosci. 2009;29:4312–4321. doi: 10.1523/JNEUROSCI.0126-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Lasorella A, Stegmuller J, Guardavaccaro D, Liu G, Carro MS, Rothschild G, de la Torre-Ubieta L, Pagano M, Bonni A, Iavarone A. Degradation of Id2 by the anaphasepromoting complex couples cell cycle exit and axonal growth. Nature. 2006;442:471–474. doi: 10.1038/nature04895. In this paper, the authors uncover the HLH protein Id2 as a novel substrate of neuronal Cdh1-APC in the regulation of axon growth and responsiveness to myelin inhibtion.
- 27.Altman J, Bayer SA. Development of the cerebellar system : in relation to its evolution, structure, and functions. Boca Raton: CRC Press; 1997. [Google Scholar]
- 28.Palay SL, Chan-Palay V. Cerebellar cortex: cytology and organization. New York: Springer; 1974. [Google Scholar]
- 29.Hatten ME, Heintz N. Mechanisms of neural patterning and specification in the developing cerebellum. Annu Rev Neurosci. 1995;18:385–408. doi: 10.1146/annurev.ne.18.030195.002125. [DOI] [PubMed] [Google Scholar]
- 30.Mason CA, Morrison ME, Ward MS, Zhang Q, Baird DH. Axon-target interactions in the developing cerebellum. Perspect Dev Neurobiol. 1997;5:69–82. doi: 10.1080/0907676x.1997.9961300. [DOI] [PubMed] [Google Scholar]
- 31.Wan Y, Liu X, Kirschner MW. The anaphase-promoting complex mediates TGF-beta signaling by targeting SnoN for destruction. Mol Cell. 2001;8:1027–1039. doi: 10.1016/s1097-2765(01)00382-3. [DOI] [PubMed] [Google Scholar]
- 32.Stroschein SL, Bonni S, Wrana JL, Luo K. Smad3 recruits the anaphase-promoting complex for ubiquitination and degradation of SnoN. Genes Dev. 2001;15:2822–2836. doi: 10.1101/gad.912901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo K. Ski and SnoN: negative regulators of TGF-beta signaling. Curr Opin Genet Dev. 2004;14:65–70. doi: 10.1016/j.gde.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Pot I, Bonni S. SnoN in TGF-beta signaling and cancer biology. Curr Mol Med. 2008;8:319–328. doi: 10.2174/156652408784533797. [DOI] [PubMed] [Google Scholar]
- 35.Sarker KP, Wilson SM, Bonni S. SnoN is a cell type-specific mediator of transforming growth factor-beta responses. J Biol Chem. 2005;280:13037–13046. doi: 10.1074/jbc.M409367200. [DOI] [PubMed] [Google Scholar]
- 36.Sarker KP, Kataoka H, Chan A, Netherton SJ, Pot I, Huynh MA, Feng X, Bonni A, Riabowol K, Bonni S. ING2 as a novel mediator of transforming growth factor-beta-dependent responses in epithelial cells. J Biol Chem. 2008;283:13269–13279. doi: 10.1074/jbc.M708834200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- 38.Reddy SK, Rape M, Margansky WA, Kirschner MW. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature. 2007;446:921–925. doi: 10.1038/nature05734. [DOI] [PubMed] [Google Scholar]
- 39.Stegmeier F, Rape M, Draviam VM, Nalepa G, Sowa ME, Ang XL, McDonald ER, 3rd, Li MZ, Hannon GJ, Sorger PK, et al. Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature. 2007;446:876–881. doi: 10.1038/nature05694. [DOI] [PubMed] [Google Scholar]
- 40••.van Roessel P, Elliott DA, Robinson IM, Prokop A, Brand AH. Independent regulation of synaptic size and activity by the anaphase-promoting complex. Cell. 2004;119:707–718. doi: 10.1016/j.cell.2004.11.028. This paper identifies a role for the APC in presynaptic size and glutamatergic neurotransmission at the fly neuromuscular junction. The authors also report that the APC controls the abundance of the presynpatic target gene Liprinα.
- 41••.Juo P, Kaplan JM. The anaphase-promoting complex regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Curr Biol. 2004;14:2057–2062. doi: 10.1016/j.cub.2004.11.010. The authors describe a novel function for Cdh1-APC in the regulation of AMPA receptor levels and the locomotion behavior of worms.
- 42.Kaufmann N, DeProto J, Ranjan R, Wan H, Van Vactor D. Drosophila liprin-alpha and the receptor phosphatase Dlar control synapse morphogenesis. Neuron. 2002;34:27–38. doi: 10.1016/s0896-6273(02)00643-8. [DOI] [PubMed] [Google Scholar]
- 43••.Yang Y, Kim AH, Yamada T, Wu B, Bilimoria PM, Ikeuchi Y, de la Iglesia N, Shen J, Bonni A. A Cdc20-APC ubiquitin signaling pathway regulates presynaptic differentiation. Science. 2009;326:575–578. doi: 10.1126/science.1177087. In this paper, the authors uncover a function for Cdc20-APC in presynaptic differentiation in mammalian brain neurons. The authors also identify the brain-enriched transcription factor NeuroD2 as a novel substrate of neuronal Cdc20-APC and demonstrate that Cdc20-APC-induced degradation of NeuroD2 drives presynaptic differentiation.
- 44.Reim K, Mansour M, Varoqueaux F, McMahon HT, Sudhof TC, Brose N, Rosenmund C. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell. 2001;104:71–81. doi: 10.1016/s0092-8674(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 45.Huntwork S, Littleton JT. A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nat Neurosci. 2007;10:1235–1237. doi: 10.1038/nn1980. [DOI] [PubMed] [Google Scholar]
- 46.Hoogenraad CC, Feliu-Mojer MI, Spangler SA, Milstein AD, Dunah AW, Hung AY, Sheng M. Liprinalpha1 degradation by calcium/calmodulin-dependent protein kinase II regulates LAR receptor tyrosine phosphatase distribution and dendrite development. Dev Cell. 2007;12:587–602. doi: 10.1016/j.devcel.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Li M, Shin YH, Hou L, Huang X, Wei Z, Klann E, Zhang P. The adaptor protein of the anaphase promoting complex Cdh1 is essential in maintaining replicative lifespan and in learning and memory. Nat Cell Biol. 2008 doi: 10.1038/ncb1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harmey D, Smith A, Simanski S, Moussa CZ, Ayad NG. The anaphase promoting complex induces substrate degradation during neuronal differentiation. J Biol Chem. 2009;284:4317–4323. doi: 10.1074/jbc.M804944200. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Higuera I, Manchado E, Dubus P, Canamero M, Mendez J, Moreno S, Malumbres M. Genomic stability and tumour suppression by the APC/C cofactor Cdh1. Nat Cell Biol. 2008;10:802–811. doi: 10.1038/ncb1742. [DOI] [PubMed] [Google Scholar]
- 50.Herrero-Mendez A, Almeida A, Fernandez E, Maestre C, Moncada S, Bolanos JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- 51.Maestre C, Delgado-Esteban M, Gomez-Sanchez JC, Bolanos JP, Almeida A. Cdk5 phosphorylates Cdh1 and modulates cyclin B1 stability in excitotoxicity. EMBO J. 2008;27:2736–2745. doi: 10.1038/emboj.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cuende J, Moreno S, Bolanos JP, Almeida A. Retinoic acid downregulates Rae1 leading to APC(Cdh1) activation and neuroblastoma SH-SY5Y differentiation. Oncogene. 2008;27:3339–3344. doi: 10.1038/sj.onc.1210987. [DOI] [PubMed] [Google Scholar]
- 53.Almeida A, Bolanos JP, Moreno S. Cdh1/Hct1-APC is essential for the survival of postmitotic neurons. J Neurosci. 2005;25:8115–8121. doi: 10.1523/JNEUROSCI.1143-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaplow ME, Korayem AH, Venkatesh TR. Regulation of glia number in Drosophila by Rap/Fzr, an activator of the anaphase-promoting complex, and Loco, an RGS protein. Genetics. 2008;178:2003–2016. doi: 10.1534/genetics.107.086397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- 56.Creppe C, Malinouskaya L, Volvert ML, Gillard M, Close P, Malaise O, Laguesse S, Cornez I, Rahmouni S, Ormenese S, et al. Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell. 2009;136:551–564. doi: 10.1016/j.cell.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 57.Kwiatkowski AV, Rubinson DA, Dent EW, Edward van Veen J, Leslie JD, Zhang J, Mebane LM, Philippar U, Pinheiro EM, Burds AA, et al. Ena/VASP Is Required for neuritogenesis in the developing cortex. Neuron. 2007;56:441–455. doi: 10.1016/j.neuron.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Lowery LA, Van Vactor D. The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol. 2009;10:332–343. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yokota Y, Ring C, Cheung R, Pevny L, Anton ES. Nap1-regulated neuronal cytoskeletal dynamics is essential for the final differentiation of neurons in cerebral cortex. Neuron. 2007;54:429–445. doi: 10.1016/j.neuron.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


